Dietary Patterns in Pregnancy and the Postpartum Period and the Relationship with Maternal Weight up to One Year after Pregnancy Complicated by Gestational Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Sample
2.2. Recruitment
2.3. Postpartum Follow-Up
2.4. Food Consumption
2.5. Outcome
2.6. Covariates
2.7. Statistical Analysis
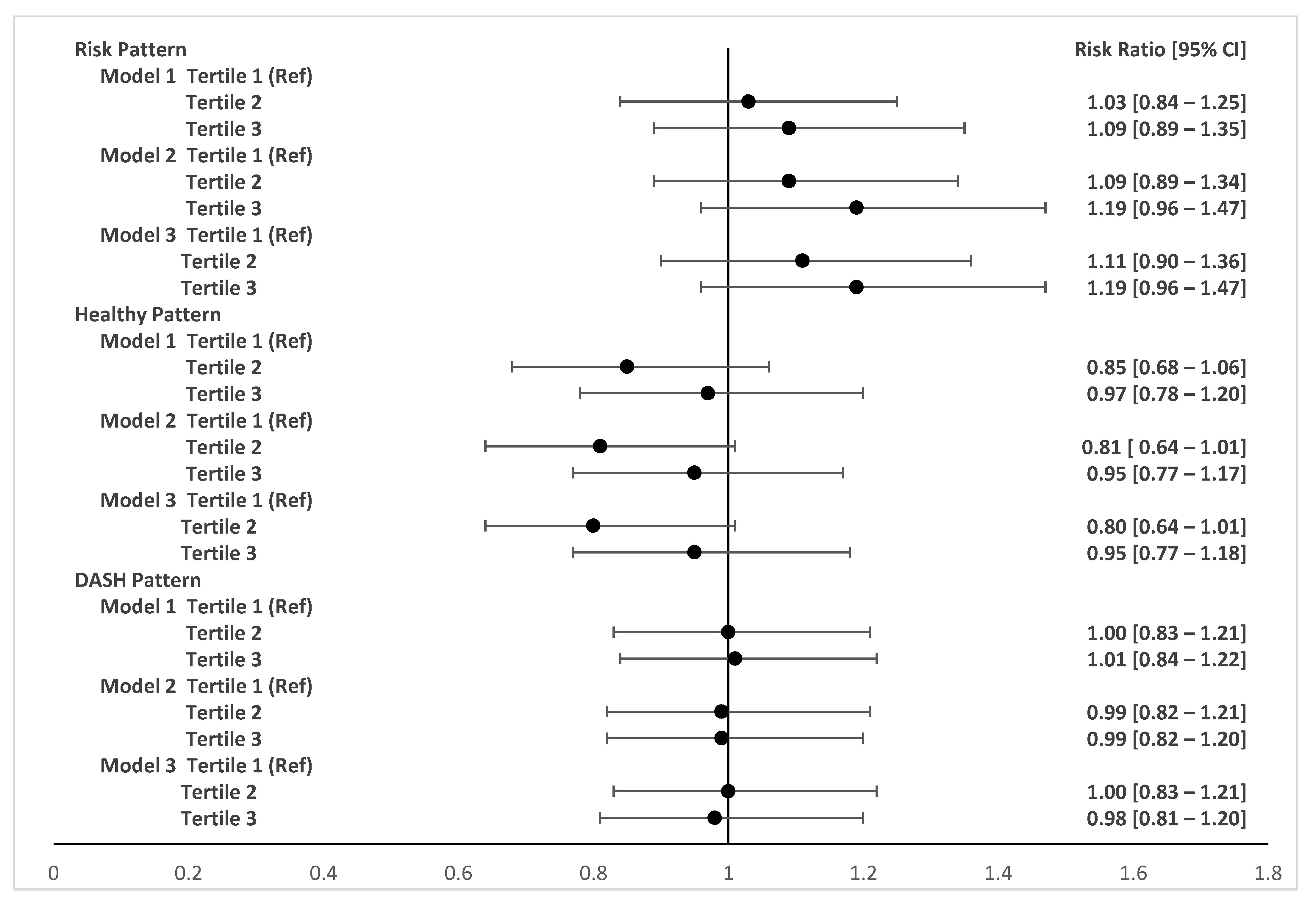
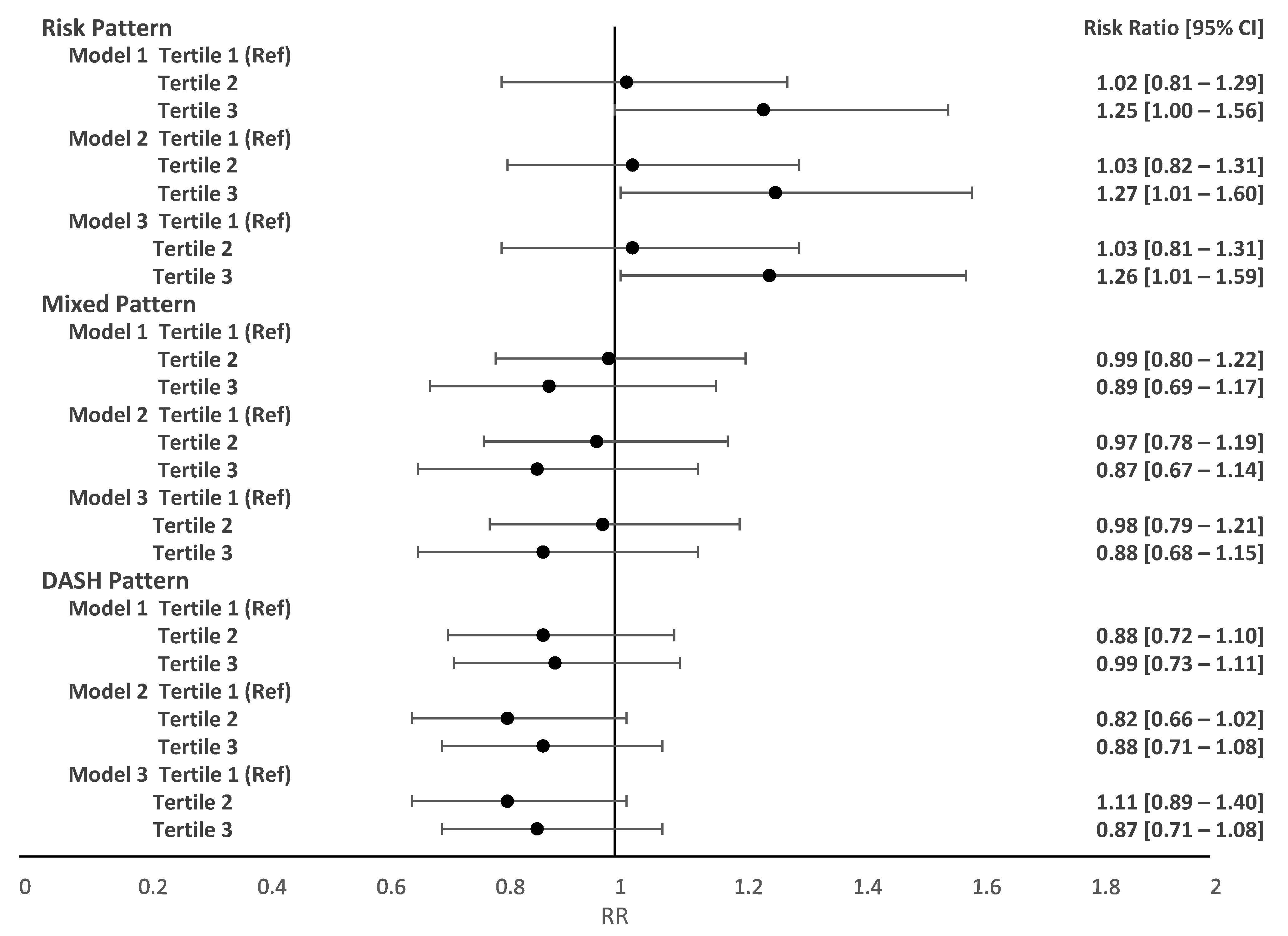
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef]
- Sauder, K.A.; Ritchie, N.D. Reducing intergenerational obesity and diabetes risk. Diabetologia 2021, 64, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Golab, B.P.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Leonard, S.A.; Rasmussen, K.M.; King, J.C.; Abrams, B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am. J. Clin. Nutr. 2017, 106, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Schneider, B.C.; Menezes, A.M.B.; Wehrmeister, F.C.; Gonçalves, H. Gestational weight gain and childhood body mass index across three generations: Results from the 1993 Pelotas (Brazil) Birth Cohort. Pediatr. Obes. 2021, 16, e12760. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Vigitel Brazil 2019: Surveillance of Risk and Protective Factors for Chronic Diseases by Telephone Survey: Estimates of Frequency and Sociodemographic Distribution of Risk and Protective Factors for Chronic Diseases in the Capitals of the 26 Brazilian States and the Federal District in 2019; Ministry of Health: Brasília, Brazil, 2020; 137p.
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Mamun, A.A.; Kinarivala, M.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M.; Callaway, L.K. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: Evidence from 21-year postpartum follow-up. Obstet. Gynecol. Surv. 2010, 65, 554–555. [Google Scholar] [CrossRef]
- Nehring, I.; Schmoll, S.; Beyerlein, A.; Hauner, H.; von Kries, R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1225–1231. [Google Scholar] [CrossRef]
- Phelan, S.; Clifton, R.G.; Haire-Joshu, D.; Redman, L.M.; Van Horn, L.; Evans, M.; Joshipura, K.; Couch, K.A.; Arteaga, S.S.; LIFE-Moms Research Group. One-year postpartum anthropometric outcomes in mothers and children in the LIFE-Moms lifestyle intervention clinical trials. Int. J. Obes. 2020, 44, 57–68. [Google Scholar] [CrossRef]
- Dabelea, D.; Snell-Bergeon, J.K.; Hartsfield, C.L.; Bischoff, K.J.; Hamman, R.F.; McDuffie, R.S. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM screening program. Diabetes Care 2005, 28, 579–584. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sartorelli, D.S.; Crivellenti, L.C.; Zuccolotto, D.C.C.; Franco, L.J. Relationship between minimally and ultra-processed food intake during pregnancy with obesity and gestational diabetes mellitus. Cad. Saude Publica 2019, 35, e00049318. [Google Scholar] [CrossRef] [PubMed]
- Tobias, K.D.; Zhang, C.; Chavaro, J.; Olsen, S.; Bao, W.; Bjjerregaard, A.A.; Fung, T.T.; Manson, J.E.; Hu, F.B. Healthful dietary patterns and long-term weight change among women with a history of gestational diabetes mellitus Deirdre. Int. J. Obes. 2016, 40, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and maternal outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. 7), 705S–728S. [Google Scholar] [CrossRef]
- Zadeh, S.H.; Boffetta, P.; Hosseinzadeh, M. Dietary patterns and risk of gestational diabetes mellitus: A systematic review and meta-analysis of cohort studies. Clin. Nutr. ESPEN 2020, 36, 1–9. [Google Scholar] [CrossRef]
- Leone, A.; Martínez González, M.A.; Craig, W.; Fresán, U.; Gómez-Donoso, C.; Bes-Rastrollo, M. Pre-Gestational Consumption of Ultra-Processed Foods and Risk of Gestational Diabetes in a Mediterranean Cohort. The SUN Project. Nutrients 2021, 13, 2202. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Castilhos, C.; Wendland, E.M.; Hallal, P.C.; Schaan, B.A.; Drehmer, M.; Forti, A.C.E.; Façanha, C.; Nunes, M.A. Lifestyle Intervention for Diabetes prevention After pregnancy (LINDA-Brasil): Study protocol for a multicenter randomized controlled trial. BMC Pregnancy Childbirth 2016, 16, 68. [Google Scholar] [CrossRef]
- Ministry of Health. Vigitel Brazil 2006–2020: Nutritional Status and Food Consumption. Surveillance of Risk and Protective Factors for Chronic Diseases by Telephone Survey: Estimates of Frequency and Sociodemographic Distribution of Nutritional Status and Food Consumption in the Capitals of the 26 Brazilian States and the Federal District between 2006 and 2020; Ministry of Health: Brasília, Brazil, 2022; 76p.
- Ministry of Health. Guidelines for Evaluation of Food Consumption Markers in Primary Health Care; Ministry of Health: Brasília, Brazil, 2015; 33p.
- Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- SPSS Incorporated. PASW Statistics for Windows; Version 21.0; SPSS Inc.: Chicago, IL, USA, 2009. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 15. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S232–S243. [Google Scholar] [CrossRef]
- Forbes, L.E.; Graham, J.E.; Berglund, C.; Bell, R.C. Dietary change during pregnancy and women’s reasons for change. Nutrients 2018, 10, 1032. [Google Scholar] [CrossRef]
- Van Ryswyk, E.; Middleton, P.; Shute, E.; Hague, W.; Crowther, C. Women’s views and knowledge regarding healthcare seeking for gestational diabetes in the postpartum period: A systematic review of qualitative/survey studies. Diabetes Res. Clin. Pract. 2015, 110, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Soh, S.E.; Tint, M.T.; Loy, S.L.; Yap, F.; Tan, K.H.; Lee, Y.S.; Shek, L.P.C.; Godfrey, K.M.; Gluckman, P.D.; et al. Combined analysis of gestational diabetes and maternal weight status from pre-pregnancy through post-delivery in future development of type 2 diabetes. Sci. Rep. 2021, 11, 5021. [Google Scholar] [CrossRef] [PubMed]
- Jardí, C.; Aparicio, E.; Bedmar, C.; Aranda, N.; Abajo, S.; March, G.; Basora, J.; Arija, V.; The Eclipses Study Group. Food consumption during pregnancy and post-partum. ECLIPSES study. Nutrients 2019, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Sui, Z.; Cramp, C.S.; Dodd, J.M. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post-partum. Int. J. Obes. 2013, 37, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Radwan, H.; Hashim, M.; Hasan, H.; Abbas, N.; Obaid, R.S.; Ghazal, H.A.; Naja, F. Adherence to the Mediterranean diet during pregnancy is associated with lower odds of excessive gestational weight gain and postpartum weight retention: Results of the MISC cohort. Br. J. Nutr. 2022, 128, 1401–1412. [Google Scholar] [CrossRef]
- Skreden, M.; Hillesund, E.R.; Wills, A.K.; Brantsæter, A.L.; Bere, E.; Øverby, N.C. Adherence to the New Nordic Diet during pregnancy and subsequent maternal weight development: A study conducted in the Norwegian Mother and Child Cohort Study (MoBa). Br. J. Nutr. 2018, 119, 1286–1294. [Google Scholar] [CrossRef]
- Cummings, J.R.; Lipsky, L.M.; Schwedhelm, C.; Liu, A.; Nansel, T.R. Associations of ultra-processed food intake with maternal weight change and cardiometabolic health and infant growth. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 61. [Google Scholar] [CrossRef]
- Gomes, C.B.; Malta, M.B.; Benício, M.H.D.A.; Carvalhaes, M.A.B.L. Consumption of ultra-processed foods in the third gestational trimester and increased weight gain: A Brazilian cohort study. Public Health Nutr. 2020, 24, 3304–3312. [Google Scholar] [CrossRef]
- Mahabamunuge, J.; Simione, M.; Hong, B.; Horan, C.; Ayala, S.G.; Davison, K.; Redline, S.; Taveras, E.M. Association of Sugar-Sweetened Beverage Intake with Maternal Postpartum Weight Retention. Public Health Nutr. 2021, 24, 4196–4203. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Chen, L.; Hu, F.B.; Yeung, E.; Willett, W.; Zhang, C. Prospective Study of Pre-Gravid Sugar- Sweetened Beverage Consumption and the Risk of Gestational Diabetes Mellitus. Diabetes Care 2009, 32, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Satija, A.; Fung, T.T.; Clinton, S.K.; Giovannucci, E.L. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J. Nutr. 2019, 149, 5. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Saunders, C.; Peres, W.; Folino, B.; Kamel, T.; Dos Santos, M.S.; Padilha, P. Effect of ultra-processed foods consumption on glycemic control and gestational weight gain in pregnant with pregestational diabetes mellitus using carbohydrate counting. PeerJ 2021, 9, e10514. [Google Scholar] [CrossRef] [PubMed]
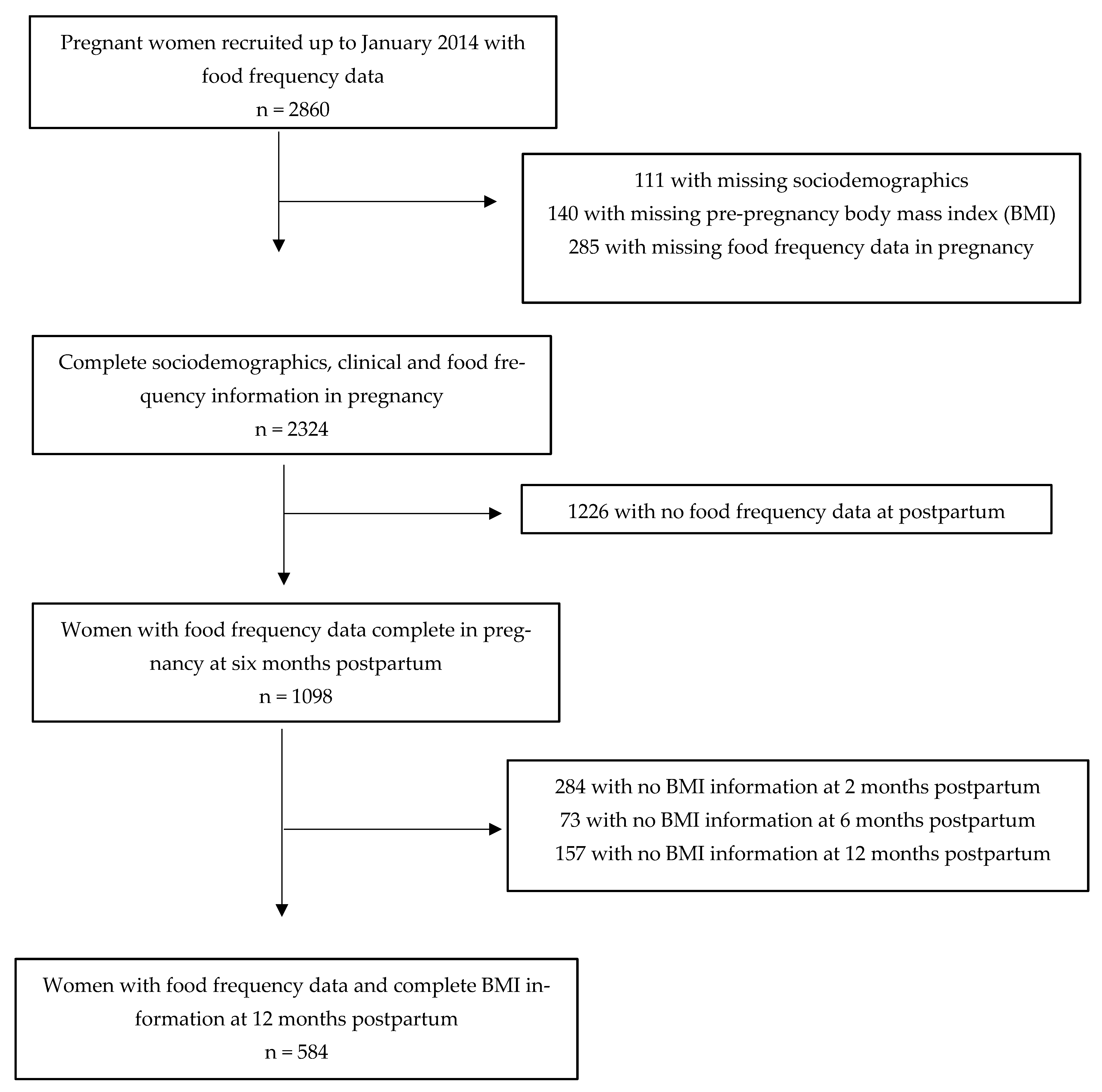


| Sample Characteristics | Initial Sample n = 2324 | With Complete Food Frequency Data n = 1098 | With Weight Information at Follow-Up n = 584 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age at enrollment (years) | |||
| 18–29 | 791 (34.0) | 395 (36.0) | 202 (34.6) |
| 30–39 | 1256 (54.0) | 586 (53.4) | 323 (55.3) |
| 40+ | 277 (11.9) | 117 (10.7) | 59 (10.1) |
| Self-declared skin color | |||
| White | 1392 (59.9) | 594 (54.2) | 295 (50.8) |
| Non-white | 932 (40.1) | 501 (45.8) | 286 (49.2) |
| Living with partner | |||
| No | 255 (11.0) | 102 (9.3) | 45 (7.7) |
| Yes | 2069 (89.0) | 996 (90.7) | 539 (92.3) |
| Family income (minimum wage) | |||
| <1 | 495 (21.3) | 207 (18.9) | 90 (15.4) |
| 1 to <2 | 908 (39.1) | 442 (40.3) | 218 (37.3) |
| 2 to <3 | 499 (21.5) | 244 (22.2) | 144 (24.7) |
| ≥3 | 422 (18.2) | 205 (18.7) | 132 (22.6) |
| Parity | |||
| 0 | 527 (22.7) | 241 (21.9) | 138 (23.6) |
| 1–2 | 1201 (51.7) | 573 (52.2) | 308 (52.7) |
| ≥3 | 596 (25.6) | 284 (25.9) | 138 (23.6) |
| Self-reported smoking before pregnancy | |||
| No | 1836 (79.0) | 849 (77.3) | 446 (76.4) |
| Yes | 487 (21.0) | 249 (22.7) | 138 (23.6) |
| Pre-pregnancy BMI (kg/m2) | |||
| Normal and underweight (<25) | 380 (16.4) | 200 (18.3) | 111 (19.1) |
| Overweight (25 to <30) | 890 (38.4) | 417 (38.1) | 228 (39.2) |
| Obesity (≥30) | 1048 (45.2) | 477 (43.6) | 243 (41.8) |
| Gestational weight gain (IOM 2009) | |||
| Insufficient | 1149 (54.4) | 556 (52.1) | 305 (53.2) |
| Adequate | 526 (24.9) | 262 (24.9) | 145 (25.3) |
| Excessive | 439 (20.8) | 235 (22.3) | 123 (21.5) |
| BMI at 2 months postpartum (kg/m2) | |||
| Normal and underweight (<25) | 203 (12.8) | 108 (13.3) | 83 (14.2) |
| Overweigh (25 to <30) | 633 (40.0) | 349 (42.9) | 257 (44.0) |
| Obesity (≥30) | 745 (47.1) | 357 (43.9) | 244 (41.8) |
| Breastfeeding at 6 months | |||
| No | 378 (34.8) | 378 (34.7) | 191 (32.8) |
| Yes | 709 (65.2) | 710 (65.3) | 391 (67.2) |
| Breastfeeding at 12 months | |||
| No | 592 (47.6) | 420 (47.0) | 279 (48.1) |
| Yes | 652 (52.4) | 474 (53.0) | 301 (51.9) |
| Food Items | Initial Sample n = 2324 | With Complete Food Frequency Data n = 1098 | |||||
|---|---|---|---|---|---|---|---|
| Pregnancy | Postpartum (6 Months) | ||||||
| 0–2 Days a Week | 3–4 Days a Week | 5–7 Days a Week | 0–2 Days a Week | 3–4 Days a Week | 5–7 Days a Week | p-Value * | |
| Vegetables, fruits and beans | |||||||
| Raw salad | 509 (21.9) | 711 (30.6) | 1104 (47.5) | 242 (22.0) | 439 (40.0) | 417 (38.0) | <0.001 |
| Cooked salad | 762 (32.8) | 1055 (45.4) | 507 (21.8) | 341 (31.1) | 568 (51.8) | 188 (17.1) | <0.001 |
| Fruits | 188 (8.1) | 489 (21.0) | 1647 (70.9) | 249 (22.7) | 362 (33.0) | 487 (44.4) | <0.001 |
| Natural fruit juice | 1082 (46.6) | 581 (25.0) | 660 (28.4) | 683 (62.3) | 211 (19.2) | 203 (18.5) | <0.001 |
| Beans | 177 (7.6) | 476 (20.5) | 1671 (71.9) | 89 (8.1) | 258 (23.5) | 751 (68.4) | <0.001 |
| Dairy products | |||||||
| Low-fat dairy | 1377 (59.3) | 196 (8.4) | 751 (32.3) | 845 (83.8) | 143 (14.2) | 20 (2.0) | <0.001 |
| Full-fat dairy | 1078 (46.4) | 353 (15.2) | 893 (38.4) | 529 (56.8) | 366 (39.3) | 37 (4.0) | <0.001 |
| Meat | |||||||
| Red meat with visible fat | 1738 (74.8) | 460 (19.8) | 125 (5.4) | 776 (70.7) | 264 (24.1) | 57 (5.2) | <0.001 |
| Red meat without visible fat | 742 (31.9) | 1193 (51.3) | 389 (16.7) | 350 (32.0) | 588 (53.7) | 157 (14.3) | <0.001 |
| Chicken | 292 (12.6) | 1468 (63.2) | 563 (24.2) | 98 (8.9) | 715 (65.3) | 282 (25.8) | <0.001 |
| Fish | 1882 (81.0) | 412 (17.7) | 30 (1.3) | 958 (87.2) | 134 (12.2) | 6 (0.5) | <0.001 |
| High caloric and ultra-processed foods | |||||||
| Fried foods | 1815 (78.1) | 393 (16.9) | 115 (5.0) | 889 (81.0) | 190 (17.3) | 19 (1.7) | <0.001 |
| Salty snacks | 1078 (46.4) | 793 (34.1) | 453 (19.5) | 561 (51.1) | 386 (35.2) | 150 (13.7) | <0.001 |
| Cookies and sweets | 1600 (68.8) | 456 (19.6) | 126 (11.5) | 713 (64.9) | 297 (27.0) | 88 (8.0) | <0.001 |
| Sweetened beverages | 1226 (52.8) | 502 (21.6) | 595 (25.6) | 439 (40.0) | 274 (25.0) | 385 (35.1) | <0.001 |
| Processed meat | 1436 (61.8) | 638 (27.5) | 250 (10.8) | 666 (60.7) | 317 (28.9) | 115 (10.5) | <0.001 |
| Food Patterns | Food Items | Factor Loading | % of Total Variance |
|---|---|---|---|
| Pregnancy | |||
| Risk | 15.938 | ||
| Fried foods | 0.712 | ||
| Cookies and sweets | 0.683 | ||
| Sweetened beverages | 0.671 | ||
| Processed meat | 0.604 | ||
| Red meat with visible fat | 0.416 | ||
| Healthy | 10.811 | ||
| Red meat without visible fat | 0.680 | ||
| Raw salad | 0.635 | ||
| Cooked salad | 0.541 | ||
| Fruits | 0.272 | ||
| DASH | 10.492 | ||
| Low-fat dairy | 0.826 | ||
| Raw salad | 0.193 | ||
| Cooked salad | 0.173 | ||
| Postpartum | |||
| Risk | 12.103 | ||
| Fried foods | 0.685 | ||
| Cookies and sweets | 0.613 | ||
| Processed meat | 0.521 | ||
| Red meat with visible fat | 0.510 | ||
| Sweetened beverages | 0.447 | ||
| Mixed | 11.148 | ||
| Natural fruit juice | 0.640 | ||
| Salty snacks | 0.461 | ||
| Fruits | 0.422 | ||
| Chicken | 0.338 | ||
| DASH | 10.752 | ||
| Cooked salad | 0.679 | ||
| Raw salad | 0.667 | ||
| Low-fat dairy | 0.503 | ||
| Fruits | 0.452 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.M.; Schmidt, M.I.; Vigo, Á.; Drehmer, M. Dietary Patterns in Pregnancy and the Postpartum Period and the Relationship with Maternal Weight up to One Year after Pregnancy Complicated by Gestational Diabetes. Nutrients 2023, 15, 4258. https://doi.org/10.3390/nu15194258
Dias LM, Schmidt MI, Vigo Á, Drehmer M. Dietary Patterns in Pregnancy and the Postpartum Period and the Relationship with Maternal Weight up to One Year after Pregnancy Complicated by Gestational Diabetes. Nutrients. 2023; 15(19):4258. https://doi.org/10.3390/nu15194258
Chicago/Turabian StyleDias, Letícia Machado, Maria Inês Schmidt, Álvaro Vigo, and Michele Drehmer. 2023. "Dietary Patterns in Pregnancy and the Postpartum Period and the Relationship with Maternal Weight up to One Year after Pregnancy Complicated by Gestational Diabetes" Nutrients 15, no. 19: 4258. https://doi.org/10.3390/nu15194258
APA StyleDias, L. M., Schmidt, M. I., Vigo, Á., & Drehmer, M. (2023). Dietary Patterns in Pregnancy and the Postpartum Period and the Relationship with Maternal Weight up to One Year after Pregnancy Complicated by Gestational Diabetes. Nutrients, 15(19), 4258. https://doi.org/10.3390/nu15194258






