Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Screening
- Inclusion Criteria
- Study design
- Participants
- Exposure
- Outcomes
- Exclusion Criteria
- Conference abstracts, reviews or editorials;
- Studies evaluating the effect of interventions, e.g., Randomised Controlled Trials;
- Studies using ‘posterior’ methods to evaluate the diet pattern;
- Studies evaluating the effect of the traditional Mediterranean diet in Mediterranean populations (as this has been studied elsewhere). If a study reported results involving a mixture of populations, only those relevant to the non-Mediterranean populations were included;
- Studies assessing the effect of dietary patterns on osteoporotic fractures by DII (its application has been widely studied).
2.3. Data Extraction
2.4. Descriptive Synthesis
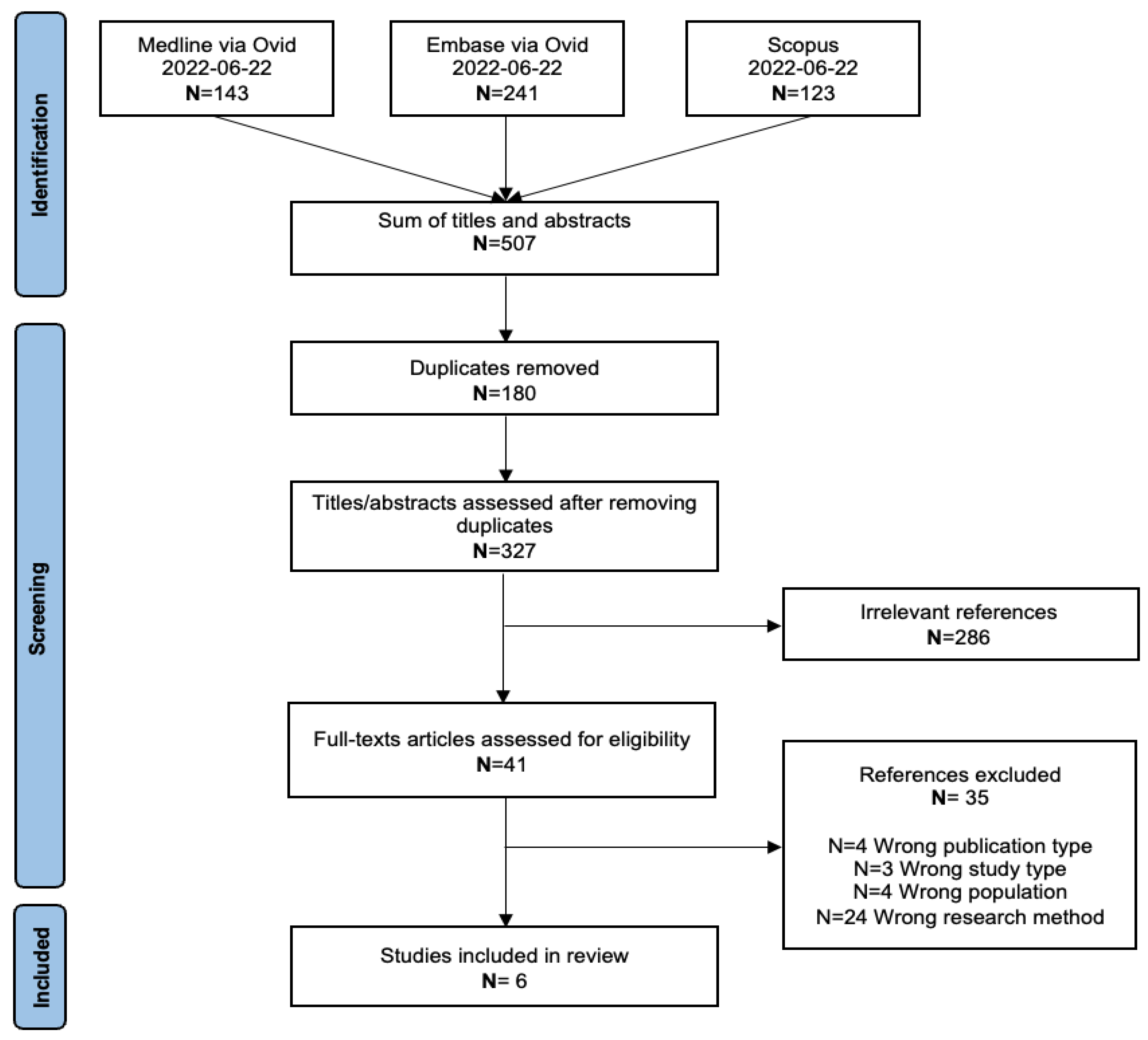
3. Results
3.1. Study Selection
3.2. Characteristics of Included Studies
| First Author, Year | Country (No.) | Gender (F/M) and Population | Mean Age (Age Range) (Years) | Study Design | Dietary Pattern | Diet Score | Outcome | Main Associations Studies | Statistical Measure of Effect |
|---|---|---|---|---|---|---|---|---|---|
| Erkkilä et al., 2017 [36] | Finland | F 554 | 67.9 (65–71) | Prospective cohort | BSD | BSD score | Bone Mineral Density (BMD) | Association between quartiles of BSD (Q1-Q2-Q3-Q4) score and BMD (Femoral, lumbar, or total body). | Lumbar BMD: p = 0.428 (NS) * |
| Femoral neck BMD: p = 446 (NS) * | |||||||||
| Total body BMD: p = 0.294 (NS) * | |||||||||
| Shahriarpour et al., 2020 [37] | Iran | F 151 | 61.2 (50–85) | Cross sectional | DASH diet | DASH score | Bone Mineral Density (BMD) | 1. Association between tertiles of DASH score (T1-T2-T3) and BMD (Femoral neck or lumbar spine). | Lumbar spine BMD overall difference across tertiles: p = 0.068 ** (NS) |
| Lumbar spine BMD; pairwise difference between T3-T1: p = 0.594 ** (NS) | |||||||||
| Femoral neck BMD overall difference across tertiles: p = 0.323 ** (NS) | |||||||||
| Femoral neck BMD pairwise difference between T3-T1: p = 0.921 ** (NS) | |||||||||
| Lumbar spine osteoporosis: OR = 0.28 (95% CI 0.09–0.88) (p = 0.029) ** | |||||||||
| 2. Association between adherence to the DASH dietary pattern in different tertile divisions (T1-T2-T3) and risk of osteoporosis | Femoral neck osteoporosis: OR = 1.21 (95%CI 0.21–7.00) ** (NS) | ||||||||
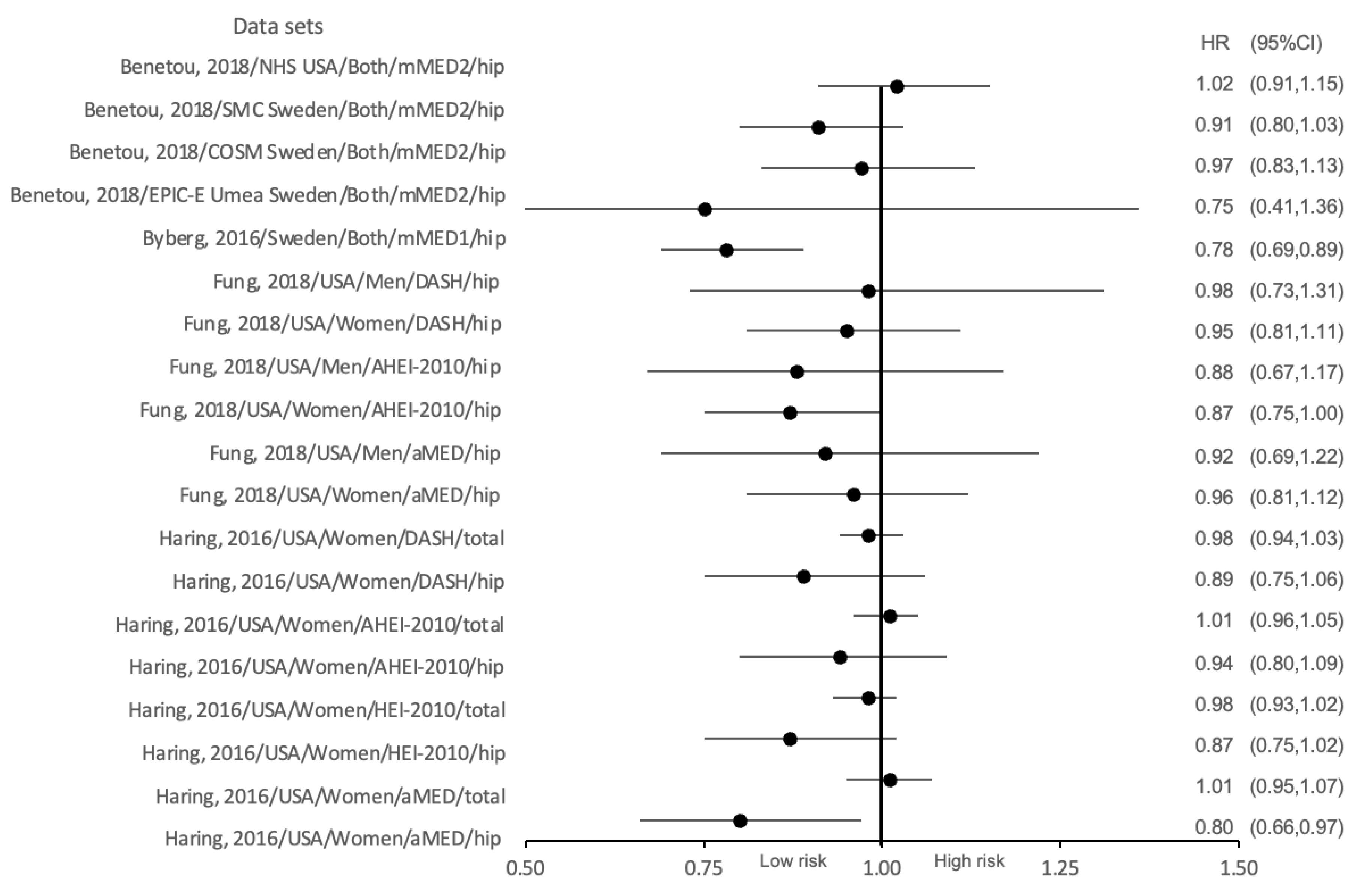
| Byberg et al., 2016 [40] | Sweden | Total 71,306 F (33,403) M (37,903) | 60 | Cross sectional | Mediterranean-like diet | mMED score | First incident hip fracture (main outcome) | Association between adherence to the mMED score in different tertile divisions (T1-T2-T3) and the incidence of osteoporotic fracture in both genders. | Comparing the highest quintile of adherence to the mMED (6 to 8 points) with the lowest (0 to 2 points): |
| First incident fracture of any type and first incident non-hip fracture (secondary outcomes) - | Both genders: hip fracture risk: HR = 0.78 (95%CI 0.69–0.89) ***** | ||||||||
| Haring et al., 2016 [38] | The United States | F 7916 | 63.6 (63.6 ± 7.4) | Prospective cohort | No prescribed eating patterns | aMED score, HEI-2010 score, AHEI-2010 score, DASH score | Incident hip and total fractures | Association between adherence to the aMED, HEI-2010, AHEI-2010, and DASH score in different quintile divisions (Q1-Q2-Q3-Q4-Q5) and the risk of osteoporotic fracture. | aMED: hip fracture: HR = 0.80 (95%CI 0.66–0.97) *** total fracture: HR = 1.01 (95%CI 0.95–1.07) *** (NS), |
| HEI-2010: hip fracture: HR = 0.87 (95%CI 0.75–1.02) *** (NS) total fracture: HR = 0.98 (95%CI 0.93–1.02) *** (NS), | |||||||||
| AHEI-2010: hip fracture: HR = 0.94 (95%CI 0.80–1.09) *** (NS) total fracture: HR = 1.01 (95% CI 0.96–1.05) *** (NS), | |||||||||
| DASH: hip fracture: HR = 0.89 (95%CI 0.75–1.06) *** (NS) total fracture: HR = 0.98 (95%CI 0.94–1.03) *** (NS). | |||||||||
| Fung et al., 2018 [39] | The United States | 111,048, F (74,446) M (36,602) | (50–75) | Prospective cohort | No prescribed eating patterns | aMED score, AHEI-2010 score, DASH score | Hip fracture (self-reported) | Association between adherence to the aMED, AHEI-2010, and DASH score in different quintile divisions (Q1-Q2-Q3-Q4-Q5) and the risk of osteoporotic fracture in different genders. | aMED Women: hip fracture: HR = 0.96 (95%CI 0.81–1.12) **** (NS) Men: hip fracture: HR = 0.92 (95%CI 0.69–1.22) **** (NS) |
| AHEI-2010 Women: hip fracture: HR = 0.87 (95%CI = 0.75–1.00) **** (NS) Men: hip fracture: HR = 0.88 (95%CI 0.67–1.17) **** (NS) | |||||||||
| DASH Women: hip fracture: HR = 0.95 (95%CI 0.815–1.11) **** (NS) Men: hip fracture: HR = 0.98 (95%CI 0.73–1.31) **** (NS). | |||||||||
| Benetou et al., 2018 [41] | Europe and the USA | Total 131,241 F (110,459) M (20,782) | ≥60 | Prospective cohort | Mediterranean diet | mMED score | Hip fracture (diagnosed or reported at follow-up or recorded as cause of death) | Association between adherence to the mMED score in different tertile divisions (T1-T2-T3) and the risk of osteoporotic fracture in both genders. | Both genders: EPIC-Umea Sweden cohort: hip fracture: HR = 0.75 (95%CI 0.41–1.36) ****** (NS) |
| NHS-USA cohort: hip fracture: HR = 1.02 (95%CI 0.91–1.15) ****** (NS) | |||||||||
| COSM-Sweden cohort: hip fracture: HR = 0.97 (95%CI 0.83–1.13) ****** (NS) | |||||||||
| SMC-Sweden cohort: hip fracture: HR = 0.91 (95% 0.82–1.03) ****** (NS). |
3.3. Summary of ‘a Priori’ Dietary Scores
3.4. The Effect of Diet Scores on BMD and Osteoporosis Diagnosis
3.5. The Influence of Diet Scores on Osteoporotic Fracture
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| BMD (g/cm2) | Quantiles of Diet Scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartiles of BSD Score (Mean 95%CI) (Erkkilä et al., 2017 [36]) | p Value | Tertiles of DASH Score (Mean 95%CI) (Shahriarpour et al., 2020 [37]) | p Value | ||||||
| Q1 | Q2 | Q3 | Q4 | T1 | T2 | T3 | |||
| Lumbar BMD | 1.136 (1.093–1.179) | 1.102 (1.057–1.146) | 1.112 (1.065–1.159) | 1.135 (1.087–1.184) | 0.446 | 0.83 (0.79–0.87) | 0.89 (0.86–0.93) | 0.87 (0.83–0.90) | 0.068 |
| Femoral neck BMD | 0.874 (0.846–0.901) | 0.886 (0.837–0.896) | 0.892 (0.862–0.922) | 0.873 (0.842–0.904) | 0.428 | 0.66 (0.63–0.68) | 0.68 (0.66–0.71) | 0.68 (0.65–0.70) | 0.323 |
| Total body BMD | 1.078 (1.056–1.101) | 1.073 (1.050–1.095) | 1.094 (1.071–1.118) | 1.088 (1.064–1.112) | 0.294 | - | - | - | - |
| Osteoporosis | |||||||||
| Lumbar spine | - | - | - | - | - | 1.0 | 0.21 (0.07–0.64) | 0.28 (0.09–0.88) | 0.020 |
| Femoral neck | - | - | - | - | - | 1.0 | 0.51 (0.10–2.71) | 1.21 (0.21–7.00) | 0.860 |
References
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Rizzoli, R. Management of the oldest old with osteoporosis. Eur. Geriatr. Med. 2010, 1, 15–21. [Google Scholar] [CrossRef]
- Cannarella, R.; Barbagallo, F.; Condorelli, R.A.; Aversa, A.; La Vignera, S.; Calogero, A.E. Osteoporosis from an Endocrine Perspective: The Role of Hormonal Changes in the Elderly. J. Clin. Med. 2019, 8, 1564. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2016, 17, 1726–1733. [Google Scholar] [CrossRef]
- Pisani, P.; Renna, M.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171. [Google Scholar] [CrossRef]
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2018, 67, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Alswat, K.A. Gender Disparities in Osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Ahlborg, H.; Center, J.; Eisman, J.; Nguyen, T. Residual Lifetime Risk of Fractures in Women and Men. J. Bone Miner. Res. 2007, 22, 781–788. [Google Scholar] [CrossRef]
- Kanis, J.A.; Odén, A.; McCloskey, E.V.; Johansson, H.; Wahl, D.A.; Cooper, C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012, 23, 2239–2256. [Google Scholar] [CrossRef]
- Cauley, J.A. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin. Orthop. Relat. Res. 2011, 469, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Cauley, J.A.; Palermo, L. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Osteoporos. Int. 1994, 4, 226–229. [Google Scholar] [CrossRef]
- Choi, E.; Park, Y. The Association between the Consumption of Fish/Shellfish and the Risk of Osteoporosis in Men and Postmenopausal Women Aged 50 Years or Older. Nutrients 2016, 8, 113. [Google Scholar] [CrossRef]
- Li, G.; Thabane, L.; Papaioannou, A.; Ioannidis, G.; Levine, M.A.H.; Adachi, J.D. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet. Disord. 2017, 18, 46. [Google Scholar] [CrossRef]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef]
- Singer, A.; McClung, M.R.; Tran, O.; Morrow, C.D.; Goldstein, S.; Kagan, R.; McDermott, M.; Yehoshua, A. Treatment rates and healthcare costs of patients with fragility fracture by site of care: A real-world data analysis. Arch. Osteoporos. 2023, 18, 42. [Google Scholar] [CrossRef]
- Kemmak, A.R.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Cao, W.T.; Tian, H.Y.; He, J.; Chen, G.D.; Chen, Y.M. Greater Intake of Fruit and Vegetables Is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef]
- Bian, S.; Hu, J.; Zhang, K.; Wang, Y.; Yu, M.; Ma, J. Dairy product consumption and risk of hip fracture: A systematic review and meta-analysis. BMC Public Health 2018, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Larijani, B.; Esmaillzadeh, A. Consumption of milk and dairy products and risk of osteoporosis and hip fracture: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1722–1737. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Fransen, H.P.; May, A.M.; Stricker, M.D.; Boer, J.M.A.; Henni, C.; Rosseel, Y.; Ocke, M.C.; Peeters, P.H.M.; Beulens, J.W.J. A Posteriori Dietary Patterns: How Many Patterns to Retain? J. Nutr. 2014, 144, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Khaled, K.; Hundley VAlmilaji, O.; Koeppen, M.; Tsofliou, F. A Priori and a Posteriori Dietary Patterns in Women of Childbearing Age in the UK. Nutrients 2020, 12, 2921. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Cashman, K.D.; Gillings, R.; Cassidy, A.; Tang, J.; Fraser, W.; Dowling, K.G.; Hull, G.; Berendsen, A.; de Groot, L.; et al. A Mediterranean-like dietary pattern with vitamin D3 (10 µg/d) supplements reduced the rate of bone loss in older Europeans with osteoporosis at baseline: Results of a 1-y randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 633–640. [Google Scholar] [CrossRef]
- Malmir, H.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2018, 57, 2147–2160. [Google Scholar] [CrossRef]
- Jennings, A.; Mulligan, A.A.; Khaw, K.T.; Luben, R.N.; Welch, A.A. A Mediterranean Diet Is Positively Associated with Bone and Muscle Health in a Non-Mediterranean Region in 25,450 Men and Women from EPIC-Norfolk. Nutrients 2020, 12, 1154. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A.; Whitehouse, M.R.; Blom, A.W. Adherence to a Mediterranean-style diet and incident fractures: Pooled analysis of observational evidence. Eur. J. Nutr. 2018, 57, 1687–1700. [Google Scholar] [CrossRef]
- Quattrini, S.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Brandi, M.L. The Mediterranean Diet in Osteoporosis Prevention: An Insight in a Peri- and Post-Menopausal Population. Nutrients 2021, 13, 531. [Google Scholar] [CrossRef]
- Kim, H.S.; Sohn, C.; Kwon, M.; Na, W.; Shivappa, N.; Hébert, J.R.; Kim, M.K. Positive Association between Dietary Inflammatory Index and the Risk of Osteoporosis: Results from the KoGES_Health Examinee (HEXA) Cohort Study. Nutrients 2018, 10, 1999. [Google Scholar] [CrossRef]
- Morimoto, M.; Shivappa, N.; de Souza Genaro, P.; Martini, L.A.; Schuch, N.J.; Hebert, J.R.; Pinheiro, M.M. Lack of association between dietary inflammatory index and low impact fractures in the Brazilian population: The Brazilian Osteoporosis Study (BRAZOS). Adv. Rheumatol. 2019, 59, 16. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, J.; Fan, J.; Sun, L.; Cai, S.; Fan, C.; Zhong, Y.; Li, Y. Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 633–643. [Google Scholar] [CrossRef]
- Kim, D.; Park, Y. Association between the Dietary Inflammatory Index and Risk of Frailty in Older Individuals with Poor Nutritional Status. Nutrients 2018, 10, 1363. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Erkkilä, A.T.; Sadeghi, H.; Isanejad, M.; Mursu, J.; Tuppurainen, M.; Kröger, H. Associations of Baltic Sea and Mediterranean dietary patterns with bone mineral density in elderly women. Public Health Nutr. 2017, 20, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Shahriarpour, Z.; Nasrabadi, B.; Shariati-Bafghi, S.E.; Karamati, M.; Rashidkhani, B. Adherence to the dietary approaches to stop hypertension (DASH) dietary pattern and osteoporosis risk in postmenopausal Iranian women. Osteoporos. Int. 2020, 31, 2179–2188. [Google Scholar] [CrossRef]
- Haring, B.; Crandall, C.J.; Wu, C.; Leblanc, E.S.; Shikany, J.M.; Carbone, L.; Orchard, T.; Thomas, F.; Jean, W.W.; Li, W.; et al. Dietary patterns and fractures in postmenopausal women: Results from the women’s health initiative. JAMA Intern. Med. 2016, 176, 645–652. [Google Scholar] [CrossRef]
- Fung, T.T.; Meyer, H.E.; Willett, W.C.; Feskanich, D. Association between Diet Quality Scores and Risk of Hip Fracture in Postmenopausal Women and Men Aged 50 Years and Older. J. Acad. Nutr. Diet. 2018, 118, 2269–2279.e4. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Larsson, S.C.; Orsini, N.; Wolk AMichaelsson, K. Mediterranean Diet and Hip Fracture in Swedish Men and Women. J. Bone Miner. Res. 2016, 31, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaëlsson, K.; Pettersson-Kymmer, U.; Byberg, L.; Eriksson, S.; Grodstein, F.; Wolk, A.; Jankovic, N.; et al. Mediterranean diet and hip fracture incidence among older adults: The CHANCES project. Osteoporos. Int. 2018, 29, 1591–1599. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Lotti, S.; Giangrandi, I.; Colombini, B.; Sofi, F. Adherence to Mediterranean Diet Measured through Medi-Lite Score and Obesity: A Retrospective Study. Nutrients 2021, 13, 2007. [Google Scholar] [CrossRef]
- Looker, A.C.; Borrud, L.G.; Dawson-Hughes, B.; Shepherd, J.A.; Wright, N.C. Osteoporosis or Low Bone Mass at the Femur Neck or Lumbar Spine in Older Adults: United States, 2005–2008; NCHS Data Brief no 93; National Center for Health Statistics: Hyattsville, MD, USA, 2012. [Google Scholar]
- Yoon, B.H.; Kim, D.Y. Discordance between Hip and Spine Bone Mineral Density: A Point of Care. J. Bone Metabolism. 2021, 28, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Sayón-Orea, C.; Bullón-Vela, V.; Bes-Rastrollo, M.; Rodríguez-Artalejo, F.; Yusta-Boyo, M.J.; García-Solano, M. Effect of olive oil consumption on cardiovascular disease, cancer, type 2 diabetes, and all-cause mortality: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 2659–2682. [Google Scholar] [CrossRef] [PubMed]
- García-Gavilán, J.F.; Bulló, M.; Canudas, S.; Martínez-González, M.A.; Estruch, R.; Giardina, S.; Fitó, M.; Corella, D.; Ros, E.; Salas-Salvadó, J. Extra virgin olive oil consumption reduces the risk of osteoporotic fractures in the PREDIMED trial. Clin. Nutr. 2018, 37, 329–335. [Google Scholar] [CrossRef]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary Acid Load, and Bone Effects-A Controversial Subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef]
- Moradi, S.; Khorrami-Nezhad, L.; Ali-Akbar, S.; Zare, F.; Alipour, T.; Dehghani Kari Bozorg, A.; Yekaninejad, M.S.; Maghbooli, Z.; Mirzaei, K. The associations between dietary patterns and bone health, according to the TGF-β1 T869→C polymorphism, in postmenopausal Iranian women. Aging Clin. Exp. Res. 2018, 30, 563–571. [Google Scholar] [CrossRef]
- Chen, G.D.; Ding, D.; Tian, H.Y.; Zhu, Y.Y.; Cao, W.T.; Wang, C.; Chen, Y.M. Adherence to the 2006 American Heart Association’s Diet and Lifestyle Recommendations for cardiovascular disease risk reduction is associated with bone mineral density in older Chinese. Osteoporos. Int. 2017, 28, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ni, S.; Zhang, H. Association between Healthy Eating Index-2015 total and component food scores with osteoporosis in middle-aged and older Americans: A cross-sectional study with U.S. National Health and Nutrition Examination Survey. Osteoporos. Int. 2022, 33, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Boschitsch, E.P.; Durchschlag, E.; Dimai, H.P. Age-related prevalence of osteoporosis and fragility fractures: Real-world data from an Austrian Menopause and Osteoporosis Clinic. Climacteric 2017, 20, 157–163. [Google Scholar] [CrossRef]
- Todd, J.A.; Robinson, R.J. Osteoporosis and exercise. Postgrad. Med. J. 2003, 79, 320–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; Papandreou, D. Exercise and Nutrition Impact on Osteoporosis and Sarcopenia; The Incidence of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef] [PubMed]
| Scoring System | BSD | DASH | aMED | HEI-2010 | AHEI-2010 | mMED (Byberg et al. [40]) | mMED (Benetou et al. [41]) | ||
|---|---|---|---|---|---|---|---|---|---|
| Scoring component | 1 | Fruits and berries | Fruits | Fruits | Total vegetables | Whole fruit | Fruits and vegetables | Fruits | |
| 2 | Vegetables | Vegetables | Vegetables | Total fruit | Vegetables | Legumes and nuts | Vegetables | ||
| 3 | Cereals | Whole grains | Whole grains | Whole fruit | Nuts and legumes | No refined or high fibre grains | Legumes | ||
| 4 | Low-fat milk | Low-fat dairy | Fish | Seafood proteins | Whole grains | Fermented dairy products | Cereals | ||
| 5 | Fish | Nuts and legumes | Nuts | Plant proteins | PUFAs | Fish | Fish | ||
| 6 | Meat products | Red and processed meats | Legumes | Total protein foods | Long-chain ω−3 polyunsaturated fatty acids | Use of olive or rapeseed oil (%) | Meat | ||
| 7 | Total fat | Sodium | Fat ratio | Whole grains | Red and processed meats | Red and processed meats | Dietary products | ||
| 8 | Fat ratio | Sweetened beverages | Red and processed meats | Low-fat dairy | Sugar-sweetened beverages and fruit juice | Alcohol | Fat ratio | ||
| 9 | Alcohol | Alcohol | Fatty acids ratio | Trans fat | Alcohol | ||||
| 10 | Refined grains | Sodium | |||||||
| 11 | Sodium | Alcohol | |||||||
| 12 | Empty calories | ||||||||
| Total | 9 | 8 | 9 | 12 | 11 | 8 | 9 | ||
| Scoring criteria | |||||||||
| Quantile segmentation | Quartiles | Tertile a | Quintile b | Quintile | Quintile | Quintile | Tertile | Tertile | |
| Detailed scores | Components (1–8) were scored according to sex-specific population consumption quartile points: the consumption of 1,2,3,4,5 and 8 was positively awarded for 0–3 points (0,1,2,3), while the scores of 6 and 7 were pointed vice versa. For 9, Men consume ≤ 20 g/d or women consume ≤ 10 g/d received 1 point; otherwise, received 0 points. | Components (1–8) were scored according to sex-specific population consumption quintile points: the consumption of 1–5 was awarded for 1–5 points (1,2,3,4,5), while the scores of 6–8 were pointed vice versa. | Components (1–8) were scored according to sex-specific population consumption median points: the consumption of 1–7 above the sex-specific median was awarded for 1 point, otherwise received 0, while the scores of 8 were pointed vice versa. For 9, Men consume 10–25 g/d or women consume 5–15 g/d received 1 point; otherwise, received 0 points. | Components (1–12) were scored according to sex-specific population consumption quintile points: the consumption of 1–6 was awarded for 0–5 points; the consumption of 7–11 was rewarded for 0–10 points; the consumption of 12 was rewarded for 0–20 points. | Components (1–11) were scored according to sex-specific population consumption quintile points: the component 1–11 was rewarded for 0–10 points. | Components (1–7) were scored according to sex-specific population consumption median points: the consumption of 1–6 above the sex-specific median was awarded for 1 point, while the scores of 7 were pointed vice versa. For 8, both genders consume 5–15 g/d 1 point; otherwise, received 0 points. | Components (1–7) were scored according to sex-specific population consumption median points: the consumption of 1,2,3,4,5 and 8 above the sex-specific median was awarded for 1 point, while the scores of 6–7 were pointed vice versa. For 9, Men consume 10–50 g/d or women consume 5–25 g/d received 1 point; otherwise, received 0 points. | ||
| Total scores | 25 | 40 | 40 | 9 | 100 | 110 | 8 | 9 | |
| Score interval for different quantiles | Q1 ≤ 9 | T1 10–22 | Q1< 20 | Q1 < 2 | Q1 < 53 | Q1 < 47 | T1 0–2 | T1 0–6 | |
| Q2 10–13 | T2 22–26 | Q2 20–23 | Q2 2–4 | Q2 53–60 | Q2 47–53 | T2 3–5 | T2 4–5 | ||
| Q3 14–15 | T3 27–35 | Q3 23–25 | Q3 4–5 | Q3 60–66 | Q3 53–59 | T3 6–8 | T3 6–9 | ||
| Q4 ≥ 16 | Q4 25–28 | Q4 5–6 | Q4 66–72 | Q4 59–65 | |||||
| Q5 >28 | Q5 > 6 | Q5 > 72 | Q5 > 65 | ||||||
| Notes | -8 = PUFA/(SFA + trans-fatty acids) | -7 = MUFA/SFA | -2 includes 100% fruit juice -3 includes all forms except juice -4 includes legumes (beans and peas) -9 = (PUFAs + MUFAs)/SFAs -13 includes energy from solid fats, added sugars, and any alcohol more than 13 g per 1000 kcal | -1 does not include fruit juice and potatoes | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Avgerinou, C. Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review. Nutrients 2023, 15, 4255. https://doi.org/10.3390/nu15194255
Chen H, Avgerinou C. Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review. Nutrients. 2023; 15(19):4255. https://doi.org/10.3390/nu15194255
Chicago/Turabian StyleChen, Huiyu, and Christina Avgerinou. 2023. "Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review" Nutrients 15, no. 19: 4255. https://doi.org/10.3390/nu15194255
APA StyleChen, H., & Avgerinou, C. (2023). Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review. Nutrients, 15(19), 4255. https://doi.org/10.3390/nu15194255





