Abstract
Acute leukaemia is probably one of the most recurrent cancers in children and younger adults, with an incidence of acute lymphoblastic leukaemia in 80% of cases and an incidence of acute myeloid leukaemia in 15% of cases. Yet, while incidence is common in children and adolescents, acute leukaemia is a rare disease whose aetiology still requires further analysis. Many studies have investigated the aetiology of acute leukaemia, reporting that the formation of gut microbiota may be modified by the start and development of many diseases. Considering that in patients affected by acute lymphoblastic leukaemia, there is an inherent disequilibrium in the gut microbiota before treatment compared with healthy patients, increasing evidence shows how dysbiosis of the gut microbiota provokes an inflammatory immune response, contributing to the development of cancer. Our analysis suggeststhe key role of gut microbiota in the modulation of the efficacy of leukaemia treatment as well as in the progress of many cancers, such as acute leukaemia. Therefore, in this paper, we present an examination of information found in literature regarding the role of dietary factors and gut microbiota alterations in the development of leukaemia and suggest possible future preventive and therapeutic strategies.
1. Introduction
Leukaemia, characterised by the unregulated clonal proliferation of haematopoietic stem cells and, after accidents, is the second leading cause of paediatric-aged mortality, with an increase of 0.9% in incidence every year, whose risk factors are currently a topic of study aimed at possible prevention and future therapeutic strategies [1,2,3]. Albeit the precise cause of the disease is not clear, with the exception of the recognised role of ionizing radiation, benzene exposure, some leukaemogenic gene fusions and translocations and that might induce some childhood cases of acute lymphoblastic leukaemia (ALL) with an in-utero origin, many studies are increasingly considering its pathogenetic, infectious and environmental risk factors [4,5,6,7].
Considering that costs related to leukaemia treatment have a major impact on individual patients and their families, the study of environmental and lifestyle risk factors may impact significantly in the incidence and management of the disease [8] (Figure 1).
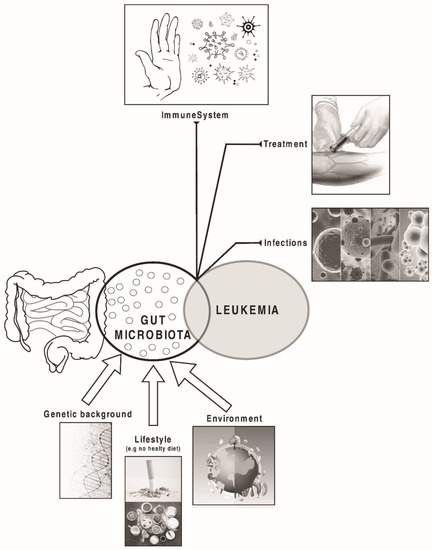
Figure 1.
Factors that may influence the composition of gut microbiota and the development and prognosis of leukaemia.
Gut microbiota is considered a complex ecosystem, which developed along with the gastrointestinal tract, that can be influenced by many genetic, environmental, and lifestyle factors, along with inherited and acquired genetic disorders [9,10]. In particular, regarding molecular abnormalities that could lead to the deregulation of signalling pathways involved in specific cell behaviour and, therefore, in the development of haematological malignancies, such as the LNK inhibitory adaptor protein encoded by the LNK/SH2B adaptor protein 3 (SH2B3) gene, which is the target of numerous genetic mutations, inherited or acquired in lymphoid leukaemia, high-resolution genome analysis using microarray and large-scale sequencing have both played a key role in the identification of many relevant acquired gene mutations [10].
The role of the microbiota also appears to be closely connected with the response to pharmacological therapies administered to patients with leukaemia, as alterations of the microbiota caused by therapy can influence the prognosis of patients [11,12].
In particular, in this paper, we would like to report our analysis regarding the possible connections among diet, microbiota and acute leukaemia, focusing on the underlying immunological pathways and therefore evaluating possible future therapeutic approaches for the treatment and prevention of leukaemia, raising points for reflection: whether the variability in terms of make-up of gut microbiota might impact the toxicity or efficacy r of drugs administered during the therapeutic management and how; whether allogeneic hematopoietic stem cell transplant (allo-HSCT) or chemotherapy can alter the gut microbiota and how; whether knowledge of gut microbiota can predict the complications due to therapy, such as diarrhoea and infections; and whether the regulation of gut microbiota can help therapy-related complications [1].
Moreover, we also examine the role of the microbiota, whose modulation may improve metabolic health and reduce the risk of cancer, thus indicating potential pathways for precision medicine research in this emerging field.
2. The Risk of Leukaemia and Diet: Possible Associations
Various dietary factors play a key role in human health through cellular metabolism, the regulation of gut microbiota and immunological processes [13].
Many studies have reported that the risk of leukaemia is increased in relation to maternal smoking and alcohol consumption or other dietary factors during pregnancy [14,15]. Additionally, the risk of childhood leukaemia can be influenced by maternal diet during pregnancy involving various mechanisms such as the synthesis and repair of DNA and epigenetic factors [16]. Various studies have analysed the link between taking maternal folic acid and the risk of leukaemia in children and have reported that maternal consumption of fruit and vegetables is inversely related to childhood ALL [16,17,18]. In accordance with this result, in California, in a case-control study, evaluating the link between the quality of maternal diet before pregnancy, considering a diet quality index, and risk of childhood ALL and acute myeloid leukaemia (AML), some authors highlighted that a lower risk of childhood ALL may be related with greater maternal consumption of vegetables and fruit and of micronutrients such as folic acid, which act on DNA synthesis and repair or epigenetic processes [19,20].
On the other hand, maternal malnutrition and low levels of micronutrients could cause elevated concentrations of maternal cortisol, influencing foetal immune system development, could interfere with the normal proliferation of immune cells and organogenesis and could modify the quantity and quality of immunological factors which transfer prenatally through the placenta or postnatally through breastfeeding [21,22].
In relation to the risk of ALL, research has also reported a low risk of this disease in relation to a diet that includes fish, seafood, beans and beef [18,23,24]. Instead, the risk of ALL may be increased when mothers eat various foods such as sugars or syrups [25].
As reported in a recent systematic review, there is a possible inverse association between a maternal diet rich in fruit and vegetables during pregnancy and the risk of ALL in children, while there is a positive association between the risk of this disease on drinking more coffee and/or caffeinated beverages [26]. However, while the inclusion of a diet rich in fruit and vegetables may have a preventive role in the pathogenesis of acute leukaemia, it is also reported that in the case of overt disease, therefore, in patients with acute leukaemia, a neutropenic diet, aimed at limitation of introducing bacteria into the gastrointestinal tract of the host through the limitation in particular of fresh fruit and vegetables, does not have a preventative role on the onset of infections, mortality or change in stool microbial flora [27].
Furthermore, apart from maternal diet, many studies have been conducted with children to evaluate the possible link of childhood leukaemia with dietary risk factors. At the Children’s Hospital, Pakistan Institute of Medical Sciences, Islamabad, Pakistan, in a case-control study carried out from January to December 2017, in which 2–12 year-old children were enrolled with recently diagnosed ALL or AML, including healthy children as a control group, the authors reported the intake of very high amounts of junk food and caffeinated drinks in the patients [28]. Considering that an unbalanced diet is associated with conditions such as obesity, one cannot fail to highlight the possible role of obesity as a main contributor to cancer risk, which can be prevented. Kinkaid JWR et al. reported that diet-induced obesity (DIO) hastens the progress of acute promyelocytic leukaemia (APL) in both male and female genetically predisposed mice [29].
Another important aspect to consider is the possible connection between breastfeeding and childhood leukaemia, for which an inverse relationship is reported that is explained by some biological mechanisms. Breastmilk has a prebiotic effect on the intestinal microbiota of the breastfed baby, in particular thanks to the secretory IgA antibodies it contains. Moreover, breastmilk contains lactoferrin, which plays a key role against microbes and a significant number of natural-killer cells, suggesting that formula-fed infants present a less mature immune system than breastfed children. Moreover, breastfed children reported a stomach pH level that is better in inducing the production of the protein-lipidα-lactalbumin (termed HAMLET), which is involved in apoptosis like death of tumour cells. Studies have reported that around 14–19% of all childhood leukaemia cases might be prevented if children are breastfed for at least 6 months [30].
3. Maternal Diet Quality and the Risk of Childhood Leukaemia: What Is the Possible Mechanism?
Gut microbiota is heavily modulated by diet: modulation during pregnancy is currently of great interest, considering the possible role it might play on maternal and neonatal health. Indeed, diet can alter the microbiota composition very quickly, in less than a week [31]. New evidence has reported that specific nutrients exert different actions on metabolic outcomes, depending on individual microbial patterns subject to specific individuals or conditions, suggesting the important role of a personalizing human nutrition treatment [32].
Throughout a healthy pregnancy, gut microbiota composition undergoes some alterations. In particular, decreased richness (intra-individual or α-diversity) and increased between-individual diversity (β-diversity) has been observed, with the presence in late pregnancy of microbial patterns that are comparable to those of non-pregnant women with metabolic syndrome. Moreover, during pregnancy an increase of Proteobacteria/Actinobacteria, a reduction of Roseburia intestinalis and Faecalibacterium praunitzii and α-diversity is typical of the microbiota composition [33]. The presence of dysbiosis induces metabolic abnormalities, for example abnormal gut permeability, which increases absorption of lipopolysaccharide (LPS) and abnormal SCFA production. There is also increased production of bacterial toxic substances (TMAO), leading to the activation of autoimmune and inflammatory pathways [31,32]. Therefore, alterations of the maternal microbiome would reflect on the neonatal gut microbiome. The neonatal gut microbiome primarily derives from the maternal gut, considering that vaginal microbiota and maternal skin only colonize the newborn baby in a transient way [34]. There are reports that intrapartum antibiotics, caesarean section, and formula feeding could disrupt microbiome establishment, inducing an increased risk of chronic inflammation and infectious exposures in a later stage, with the possible risk of ALL [34,35].
Maternal diet quality may play a key role in the possible risk of childhood leukaemia through various mechanisms, such as the impact of certain nutrients, such as folic acid, through epigenetic processes, DNA synthesis and repair, or mechanisms that impact children’s immune system development, before and after birth [36,37].
The immune response of the mother greatly impacts foetal immune development, and thus, incorrect maternal immune activation, which might be related to elevated levels of pro-inflammatory cytokines, can promote an augmented risk in the onset of many autoimmune diseases, neurodevelopmental disorders, and allergies, at a later stage [38,39]. Furthermore, an alteration in maternal cytokine production may induce foetal resorption [40]. Indeed, in mice models, the presence of increased levels of pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α (a T helper (Th)1 cytokine) and interferon (INF)-γ and have been related to miscarriages [41].
Instead, health during pregnancy is related to an increased expression of humoral immunity, Th2 cytokines (e.g., IL-10, IL-13), and reduced cell-mediated immunity (Th1-type cytokines) in response to both foetal allo-antigens and environmental antigens [42].
The balance between Th17 cells and regulatory Tcells (Tregs) also plays a major role in maintaining maternal immune tolerance. Tregs have the ability to suppress both Th1and Th2 responses, while the role of Th17 cells is fundamental in autoimmune diseases and in protection against infections [43,44].
The foetal immune system is above all sensitive to alterations induced by the environment with particular factors acting on the maternal immune system, for instance, malnutrition. Development of the foetal immune system may be influenced by maternal malnutrition through diverse mechanisms which have only recently been hypothesized: (1) maternal malnutrition may induce elevated levels of maternal cortisol with a negative role on foetal immune system development; (2) low levels of micronutrients may alter processes such the normal proliferation of immune cells and organogenesis; and (3) reduced maternal nutrition might induce an alteration of the quantity and quality of immune factors that are passed on to the foetus via the placenta or subsequently through the mammary gland [45,46].
4. Microbiome and Acute Leukaemia: A Possible Relation?
The human microbiome, or the collective genetic information of microorganisms, both symbiotic and pathogenic, that populate the body, in particular the gut, have a major part to play in disease and health conditions, such as cancer, making up the “second genome” [47]. Many factors impact the make-up of gastrointestinal microbiota, for instance, heredity, environmental factors and lifestyle [48]. Several functions are mediated by gut microbiota, for example, the development of the immune system, digestion and absorption of food, involvement in the regulation of immunological processes and pathogen resistance, and synthesis of vitamins [49]. Moreover, intestinal microbiota homeostasis is involved in drug efficacy and side effects [50]. In literature, it has been reported that, in the population, the make-up of gut microbiota, which presents some parallels, can be modified by the start and progress of various diseases. The study of metagenomic samples from patients affected by different forms of cancer highlighted how microbiota might be used as bacterial markers for diagnosis in various diseases, for instance, Fusobacterium nucleatum and Bacteroides fragilis in colorectal cancer, and Rikenellaceae, Akkermansia muciniphila and Bacteroides in non-small cell lung cancer [51,52]. The development of several forms of cancer has been seen in association with microbial dysbiosis. Some studies reported a disequilibrium in the gut microbiota of ALL patients before treatment in comparison with healthy patients [2,3,53,54]. Dysbiosis of the gut microbiota induces the obliteration of the intestinal epithelial barrier, and some gut microbiota enter into local lymph nodes or the blood. These processes induce an inflammatory immune response that activates immune cells and involves metabolic pathways, contributing to the development of cancer [55,56,57]. One study reported a relevant difference in bacterial composition between a control group and a xenotransplant paediatric ALL mouse model in the faeces and small intestine, such as a relevant prevalence of bacteria with a conversion function of dietary flavonoids in the mouse model of ALL [58]. Rajagopala SV. et al. evaluated the composition of the GI microbiota in ALL adolescent and paediatric patients throughout chemotherapy. In particular, the authors compared stool samples before the start of chemotherapy with samples collected at different moments during chemotherapy. In both groups, the microbiota compositions were represented above all by Faecalibacterium, Bacteroides and Prevotella, and, while the authors reported a higher microbiota diversity in the control group than in the patient group. It was possible to identify the most frequent as Coprococcus, Anaerostipes, and Ruminococcus2, and Roseburia which were lower in abundance in the study group. These microbiota alterations are due to various factors, such as an indirect action of chemotherapy on the immune system and direct action of therapeutic compounds on the gut flora [59]. In literature, it has been reported that the assumption of bioflavonoids induces mixed lineage leukaemia (MLL) gene cleavage by targeting topoisomerase II, which might cause leukaemia in children [60,61]. Therefore, it can be understood how an abnormal gut microbiota make-up is closely related to the pathogenesis of leukaemia and how, in addition, alterations in the gut microbiota may be linked with genetic susceptibility to ALL, in ALL mouse models. Following this line, alterations in gut microbiota make-up were highlighted between mice genetically predisposed to leukaemia and healthy mice [62].
The relationship between cancer and microbes requires a thorough evaluation; indeed, it is possible that the various microbes localized at mucosal sites become part of the tumour microenvironment once barriers are breached, inducing cancer growth and then spreading using proinflammatory or immunosuppressive programs [52,63].
5. Microbiota and Drug Pharmacokinetics in Leukaemia
A significant factor related to drug effectiveness and possible side effects is intestinal microbiota homeostasis [48,49,50]. The complications and prognosis of acute leukaemia after hematopoietic stem cell transplantation or chemotherapy may be impacted by gut microbiota make-up; in particular, the use of probiotics, prebiotics and changes in diet may reduce the incidence of side effects related to the leukaemia treatment, improving efficacy and prognosis [49,60].
The link between chemotherapeutic drugs and gut microbiota is a matter of debate; for instance, chemotherapy drugs may act on the gut microbiota directly or induce injury to intestinal epithelial cells, causing an imbalance of the microbiota. But at the same time, dysbiosis of gut microbiota may have various effects on both the metabolism and absorption of chemotherapeutic drugs, with consequent increased toxicity and reduced efficacy [64,65]. In ALL patients, chemotherapy may induce some alterations in the gut microbiota make-up, with, in particular, a decrease of the diversity of gut microbiota and an increased presence of certain bacteria, for instance Bacteroidetes, while there is a reduction of other bacteria, for example Streptococcaceae and Clostridiaceae, as reported in a study conducted with 199 children affected by ALL who had undergone intensive induction [66,67]. In addition, similar research on the alteration of the composition of gut microbiota indicates an association with complications after chemotherapy, such as organ toxicity, neutropenia, infections and gastrointestinal dysfunction, influencing the prognosis of patients affected by leukaemia. High morbidity and mortality are related to febrile neutropenia [68,69].
In literature, an increased presence of opportunistic pathogens has also been described, for example, Streptococcus, Staphylococcus, Lactococcus and Ralstonia, and in the faeces of ALL mice when compared with control mice [58]. Moreover, injury to the intestinal epithelial barrier brought on by anti-tumour treatment and the abundance of some pathogens promotes the possible development of bacteraemia and resistant bacterial infections [60]. Some studies reported a close connection between alterations in gut microbiota and the level of intestinal epithelial loss and systemic inflammation and during chemotherapy [68]. Moreover, an increased level of Proteobacteria before treatment can be considered a predictive factor of febrile neutropenia: an increased presence of Enterococcaceae or Streptococcaceae was related to a higher risk of later infection in ALL patients, a reduction in alpha and beta diversities was related to an augmented risk of infection in the 6 months after therapy, and higher temporal variability of gut microbiota was related to an increased risk of infection at 3 months after induction in AML patients [70].
Regarding the concept of drug efficacy, the mutual association between gut microbiota and non-antibiotic drugs should be highlighted: the gut microbiome make-up may be influenced by drugs and vice versa [71,72]. Gut microbiome also plays a key role in influencing patient response to a drug, inducing an alteration in its structure and modifying its bioactivity, bioavailability, or toxicity [71]. In particular, gut microbiota may influence patient response to chemotherapeutic drugs during ALL treatment. For example, one of the strongest anticancer drugs, methotrexate (MTX), very frequently used for the treatment of leukaemia for its ability to block folate metabolism, can induce atrophy of jejunal villi, an increase of goblet cells, a collapse of the muscularis mucosa, an increase in inflammatory processes such as a dysregulation of macrophages (with a rise in M1 phenotype and a reduction in M2 phenotype) in lymph nodes and spleen. In addition, MTX may cause a change in the gut microbiota make-up in mice, above all a significant reduction in Bacteroidales. Bacteroides fragilis may induce an improvement in the polarization disequilibrium of macrophages and inflammatory response induced by MTX [72].
Some studies reported that non-high dose Cyclophosphamide (CTX), another broad-spectrum anti-tumour drug, in mice can induce alterations in the mouse intestine such as shortening of villi, discontinuity of the epithelial barrier, focal accumulation of monocytes in the lamina propria, interstitial oedema, and a rise in Paneth cells and goblet cells causing an increase in intestinal permeability, facilitation of intestinal bacterial translocation, leading thus to bloodstream infection (BSI). In particular, two bacterial species, Barnesiella intestinihominis and Enterococcus hirae, are reported to be involved in CTX therapy [73,74]. Viaud et al. (2013) reported an alteration of the microbiota make-up in the small intestine and a substantial translocation of commensal bacteria in the majority of mice which were administered CTX, with the detection of some Gram-positive bacteria species (for example, Lactobacillus murinus, Lactobacillus johnsonii and Enterococcus hirae) in lymphoid organs, for instance, spleens and mesenteric lymph nodes. The inhibition of tumour growth mediated by CTX can be decreased by antibiotic treatment, whilst adoptive transfer of pathogenic Th17 (pTh17) cells may eradicate this harmful effect of antibiotics, highlighting how gut microbiota might have a key role contributing to CTX-mediated anticancer immune responses through pTh17 cells, and Gram-positive bacteria may have a key role in contributing to anticancer immunological responses by stimulating Th17 cells [73].
A study that evaluated 271 oral drugs and 76 diverse human gut bacteria reported that the metabolism of various drugs, such as corticosteroids, is chemically modified by microbiota [75]. The use of gene sequencing in combination with mass spectrometry can recognise drug metabolic activities of human gut bacteria and microbial gene products that metabolize drugs. In this way, microbiota may be envisaged by analysing their genomic contents. In a multicentre retrospective study, the authors reported that antibiotic exposure of patients receiving a chimeric type of antigen receptor (CAR) T cell therapy, one of the main immunotherapies for ALL, was linked with worse overall survival (OS). An analysis of stool samples from 48 patients with lymphoma or leukaemia highlighted that patients undergoing CAR-T cell therapy with a higher efficacy of this therapy have an unusually low alpha diversity and an elevated presence of Bacteroides, Ruminococcus and Faecalibacterium. Moreover, the various microbiota data at diverse treatment times (for instance, subsequent to CAR-T cell therapy and during follow-up) might help clinicians in better defining the role of gut microbiota in CAR-T cell therapy [76].
Therefore, a personalized analysis of the individual differences of microbiota is necessary to understand the possible disparities in drug responses and drug metabolism, which can be modulated through the adjustment of exogenous microorganisms. However, further studies are needed to evaluate specific mechanisms of the interaction of bacterial metabolites and bacterial taxa in the immune system, so as to define and advance patient outcomes after specific treatment.
6. Vitamin D and Acute Leukaemia
Bone metabolism and calcium homeostasis are the principal biological activities of vitamin D. Nonetheless, considering that the vitamin D receptor (VDR), a member of the family of nuclear receptors, is present in various diverse cells and tissues of the human body, such as dendritic cells (DCs), it is important to remember that this vitamin performs many actions that can be classified as “non-classical actions”. In particular, vitamin D has a major role in regulating immune cell function and hematopoietic cell differentiation and proliferation. Considering these effects, vitamin D might represent a therapeutic strategy hematologic malignancy treatment [77]. Moreover, as reported by Murdaca G. et al., reduced values of vitamin D influence the microbiome, inducing an alteration of the integrity of the gut epithelial barrier and the microbiome composition. Both dysbiosis and vitamin D deficiency are related to chronic inflammation and an augmented risk of various diseases, for example, cancer. Changes in vitamin D/VDR signalling with microbiome dysbiosis have been reported to be related to both intestinal inflammatory processes and extra-intestinal disorders [78]. In particular, the action mediated by vitamin D is related to its capacity to act on cell function in the gut by binding to its intracellular VDR, inducing the transcription of many genes. In the lumen, vitamin D has a key part in the maintenance of the correct level of antimicrobial peptides in the mucus and in maintaining epithelial integrity acting on the intercellular junctions. When bacteria infiltrate the epithelial layer and arrive at the interstitium, inflammation activates Th1/Th17 cells. In these conditions, the activation of vitamin D/VDR signalling allows the clearance of the bacteria in the affected cells with the suppression of Th1/Th17 cells and activation of Treg cells [79].
Many studies reported the use of vitamin D and its analogues in myeloid neoplasms, above all in AML and myelodysplastic syndrome (MDS) treatment. Some preclinical experiences with HL-60 and other leukemic lines, for example U-937 and THP-1, reported that vitamin D induces differentiation and apoptosis of blasts. In particular, Tanaka H. et al. showed that the vitamin D analogue induces an improvement in survival in leukemic mice [80]. Muto A. et al. highlighted the efficacy of calcitriol in inhibiting the cell cycle and inducing leukaemia cell differentiation through VDR [81]. Moreover, in literature, it has been reported that vitamin D induces growth inhibition and cell differentiation in leukaemic cell lines or AML blasts [82].
Numerous studies have tried to analyse the precise mechanism of action regarding the activation of VDR, theorizing the involvement of various pathways involving the MAPK pathway, P13 kinase, and possibly the upregulation of factors like p53. In a retrospective study, a correlation between VDR expression and prognosis was reported. In particular, higher VDR expression has been related to increased survival. Moreover, the authors reported a correlation between patient prognosis and expression of VDR-targeted genes, such as the finding that patients with higher cyclic adenosine monophosphate (cAMP) expression are associated with higher event-free survival (EFS), unlike patients with reduced cAMP expression levels [83].
Motomura et al., analysing 30 patients with myelodysplastic syndromes (MDS) undergoing treatment with 25(OH)D3 as opposed to supportive treatment, reported that just one of the fifteen vitamin D group patients reported progression to AML as against seven in the control group [84]. In some studies, the combination of vitamin D with other cytotoxic agents was evaluated. Siitonen et al., treating 19 MDSs patients using a combination of 1,25(OH)2D3 (1 mg/day),13-cis retinoic acid, and valproic acid, reported that three patients had a haematological response. However, due to 13-cis retinoic acid and valproic acid, some intolerance was highlighted in eight patients, [85].
Therefore, considering the capacity of vitamin D and its analogues to drive the differentiation of immature myeloid hematopoietic cells into mature monocytic cells, in literature, it has been reported that only in very few patients is there either a persistent or transient progress in blood counts using vitamin D and analogue therapy. However, some additional studies would be needed to evaluate this approach in the formal medical treatments for MDS and AML [86,87,88].
7. Possible Therapeutic Strategies for the Recovery of Microbiota in Leukaemia Patients
Probiotics, active microorganisms that modulate immunity or block intestinal inflammation, decreasing intestinal dysbiosis, have been amply adopted to manage intestinal alterations and adverse results induced by various treatments or conditions in patients affected by diseases such as leukaemia. In a randomized controlled trial conducted on 60 children affected by ALL treated with chemotherapy, a relevant decrease in many gastrointestinal side effects, such as nausea, vomiting, flatulence and abdominal distension/pain, was reported in some patients treated with oral probiotics (Lactobacillus rhamnosus), taken daily, and chemotherapy [89]. Faecal microbiota transplantation (FMT), consisting of the transfer of stool through medically or self-dispensed enemas, oral capsules, via nasogastric tube, or instilled into the duodenum by upper endoscopy or the right colon by colonoscopy or cecostomy, from a healthy donor into the colon of the recipient, has the aim to restore the microbiota composition according to different patients and conditions [90,91]. In a clinical trial conducted on four ALL patients undergoing allo-HSCT with refractory diarrhoea due to refractory intestinal infection or intestinal graft-versus-host disease, a complete remission induced by FMT in three patients was reported, while one was stable [92]. In literature, it was also reported that the use of prebiotics, such as Lactobacillus rhamnosus, during chemotherapy may help patients restore the altered microbiota after treatment, reducing the therapeutic side effects. A study reported how also a melatonin supplement can induce an augmented diversity of gut microbiota, regulating the make-up, that increases the presence of Lactobacillus in mice, suggesting that melatonin-enriched food can play a major part in remodulation of gut microbiota after chemotherapy in leukaemia patients [93].
Therefore, an appropriate diet and the assumption of prebiotics can be considered as alternative strategies of support for the management of altered microbiota in leukaemia patients.
However, it is important to highlight the concept of safety of probiotic products that is related to factors such as infectivity, pathogenicity, excessive immune stimulation in susceptible patients and virulence factors (e.g., toxicity, metabolic activity). Literature reports on side effects triggered by the use of probiotic bacteria that can be involved in systemic infections, in disturbing metabolism, in the stimulation of the immune system, and participating in horizontal gene transfer [94].
Moreover, it is important to highlight that amongst the various mechanisms through whereby an altered microbiota might induce the onset and progression of neoplastic diseases, there is the role of dysbiosis that can provoke lipid metabolism-related microRNA (miRNA) expression change [95].
Therefore, considering the existence of a bidirectional relationship between miRNAs, a novel group of small endogenous non-coding miRNA molecules that are involved in gene expression through base complementarity between the seed region of the miRNA and the 3′-untranslated region (UTR) of the target mRNA, and microbiota, it is more and more evident how miRNA modulation might become a significant therapeutic opportunity for the treatment of many diseases [96,97].
8. Conclusions
Our analysis suggests the key role of gut microbiota in the modulation of the efficacy of leukaemia treatment, and, more importantly, in the development of some cancers, such as acute leukaemia. Although an increasing number of studies are dealing with the possible role of the microbiota as a targeted therapy in many neoplastic diseases, there is scanty data on the relevance of microbiota and diet changes in leukaemia. In particular, as reported above and in Table 1, many studies investigated the role of diet on the development and prevention of acute leukaemia and the possible role of a diet high in vegetables and fruit in leukaemia development due its significant effects on altering microbiota and immunological pathways [13,19,26,27,28,29,61,98,99,100,101,102,103,104,105,106].

Table 1.
Main articles regarding the role of diet on the development and prevention of acute leukaemia.
However, while prevention represents one of the weapons on which we are increasingly trying to act to limit the development of neoplasms, particularly during adulthood, a different discussion could take place for childhood cancer, when talking about both leukaemia and solid tumours. Indeed, in these cases, the identification of causal factors is extremely difficult, given that neoplastic conditions are biologically diverse and rare in prevalence [107]. Furthermore, for most childhood cancers, there is the possibility of underlying stochastic developmental errors compounded by inherited susceptibility [2,53,108].
An exception to this pessimistic consideration is childhood ALL, for which a risk factor might be the lack of exposure to microbes at a very early age. In particular, protective factors against the appearance of ALL include daycare attendance and protracted breastfeeding, while among increased risk factors, there is caesarean section birth [34,107,108]. Evidence shows that the gut microbiome make-up at birth and during the early years of life have relevant, long-lasting effects on the immune system as a result of many mechanisms, for instance, direct microbial binding to toll receptors on innate immune cells, the role of metabolic products of bacteria, and the consequent activation of regulatory T cells [109,110,111].
Moreover, in this paper, considering that in literature it has been reported that vitamin D induces cell differentiation and growth inhibition on AML blasts or leukemic cell lines, we investigated the role of vitamin D use and its analogues in particular in myeloid neoplasms inducing, or not, a haematological response.
Much research on the mother’s diet and leukaemia risk in childhood hasalso analysed the function of diet quality in influencing childhood leukaemia risk. Indeed, as already reported, the risk of leukaemia in childhood may be influenced by maternal nutrition, both through taking maternal folic acid and maternal consumption of specific food groups during pregnancy, due to its role in foetal development, such as the synthesis and repair of DNA, inducing epigenetic mechanisms.
An important aspect to highlight is that most of the cited studies are performed on cell lines or mice, with a lack of studies on large groups of patients, thus supporting the need for further studies and analysis to ascertain evidence of the link between diet, microbiota and leukaemia and how to act with specific dietary interventions and therapeutic strategies. In particular, further studies are needed to confirm whether or not that in patients developing ALL the microbiome is lacking or deficient in diversity, considering, undoubtedly, possible potential confounding effects, such as the disease process itself or prior antibiotic use.
From our analysis we see that the impact of the microbiome and changes in diet through certain immunological pathways might be tumour-specific, and this may represent a future approach of precision medicine in the management and prevention of leukaemia.
Therefore, if improvement in the prognosis of leukaemia patients is a goal of the management of this disease, we can understand how restoring the altered microbiota can be considered a key tool for the patient following an approach of precision and personalized medicine.
Author Contributions
Conceptualization, F.F., S.G. and A.A.; investigation, S.G. and A.A.; writing—original draft preparation, F.F.; writing—review and editing, F.F., S.G., N.C. and A.A.; visualization, F.F. and N.C.; supervision, S.G. and A.A.; project administration, S.G. and N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable, as this study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wen, Y.; Jin, R.; Chen, H. Interactions Between Gut Microbiota and Acute Childhood Leukemia. Front. Microbiol. 2019, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Sakata-Haga, H.; Saikawa, Y.; Hatta, T. Influence of Immune System Abnormalities Caused by Maternal Immune Activation in the Postnatal Period. Cells 2023, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, H.-X.; Yan, H.-Y.; Wu, D.-M.; Ping, J. Developmental origins of inflammatory and immune diseases. Mol. Hum. Reprod. 2016, 22, 858–865. [Google Scholar] [CrossRef]
- Belson, M.; Kingsley, B.; Holmes, A. Risk factors for acute leukemia in children: A review. Environ. Health Perspect. 2007, 115, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; Lübbert, M. Acute myeloid leukaemia: Epidemiology and etiology. Cancer 2006, 107, 2099–2107. [Google Scholar] [CrossRef]
- Shu, X.O.; Potter, J.D.; Linet, M.S.; Severson, R.K.; Han, D.; Kersey, J.H.; Neglia, J.P.; Trigg, M.E.; Robison, L.L. Diagnostic X-rays and ultrasound exposure and risk of childhood acute lymphoblastic leukemia by immunophenotype. Cancer Epidemiol. Biomark. Prev. 2002, 11, 177–185. [Google Scholar]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Wakeford, R.; Borrego, D.; French, B.; Zablotska, L.B.; Adams, M.J.; Allodji, R.; de Vathaire, F.; Lee, C.; Brenner, A.V.; et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: A pooled analysis of nine historical cohort studies. Lancet Haematol. 2018, 5, 346–358. [Google Scholar]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; Bargiela, R.; Diez, M.S.; Friedrichs, A.; Perez-Cobas, A.E.; Gosalbes, M.J.; Knecht, H.; Martinez-Martinez, M.; Seifert, J.; von Bergen, M.; et al. Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes 2013, 4, 306–315. [Google Scholar] [CrossRef]
- Perez-Cobas, A.E.; Artacho, A.; Knecht, H.; Ferrus, M.L.; Friedrichs, A.; Ott, S.J.; Moya, A.; Latorre, A.; Gosalbes, M.J. Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS ONE 2013, 8, e80201. [Google Scholar] [CrossRef] [PubMed]
- Janakiraman, M.; Salei, N.; Krishnamoorthy, G. High salt diet does not impact the development of acute myeloid leukemia in mice. Cancer Immunol. Immunother. 2023, 72, 265–273. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, J. Paternal Smoking Before Conception and During Pregnancy Is Associated with an Increased Risk of Childhood Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis of 17 Case-Control Studies. J. Pediatr. Hematol. Oncol. 2020, 42, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Dessypris, N.; Thomopoulos, T.P.; Ntouvelis, E.; Kantzanou, M.; Diamantaras, A.A.; Moschovi, M.; Baka, M.; Hatzipantelis, E.; Kourti, M.; et al. Parental alcohol consumption and risk of leukemia in the offspring: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2017, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Milne, E.; Dockerty, J.D.; Clavel, J.; Pombo-de-Oliveira, M.S.; Wesseling, C.; Spector, L.G.; Schuz, J.; Petridou, E.; Ezzat, S.; et al. Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: A Childhood Leukemia International Consortium study. Epidemiology 2014, 25, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.D.; Block, G.; Buffler, P.; Ma, X.; Selvin, S.; Month, S. Maternal dietary risk factors in childhood acute lymphoblastic leukemia (United States). Cancer Causes Control 2004, 15, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Jensen, C.D.; Block, G.; Hudes, M.L.; Chu, L.W.; Buffer, P.A. Maternal diet and risk of childhood acute lymphoblastic leukemia. Public. Health Rep. 2009, 124, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.W.; Carmichael, S.L.; Selvin, S.; Fu, C.; Block, G.; Metayer, C. Maternal diet quality before pregnancy and risk of childhood leukaemia. Br. J. Nutr. 2016, 116, 1469–1478. [Google Scholar] [CrossRef]
- Fenech, M. The role of folic acid and vitamin B12 in genomic stability of human cells. Mutat. Res. 2001, 475, 57–67. [Google Scholar] [CrossRef]
- Marques, A.H.; O’Connor, T.G.; Roth, R.; Susser, E.; Bjørke-Monsen, A.L. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front. Neurosci. 2013, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C. Nutritionally mediated programming of the developing immune system. Adv. Nutr. 2011, 2, 377–395. [Google Scholar] [CrossRef]
- Petridou, E.; Ntouvelis, E.; Dessypris, N.; Terzidis, A.; Trichopoulos, D.; The Childhood Hematology-Oncology Group. Maternal diet and acute lymphoblastic leukemia in young children. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Larson, R.A. Acute leukemia in adults: Recent developments in diagnosis and treatment. CA Cancer J. Clin. 1994, 44, 326–352. [Google Scholar] [CrossRef]
- Bonaventure, A.; Rudant, J.; Goujon-Bellec, S.; Orsi, L.; Leverger, G.; Baruchel, A.; Bertrand, Y.; Nelken, B.; Pasquet, M.M.; Michel, G. Childhood acute leukemia, maternal beverage intake during pregnancy, and metabolic polymorphisms. Cancer Causes Control 2013, 24, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Lopez, J.; Iguacel, I.; Pisanu, S.; Almeida, C.C.B.; Steliarova-Foucher, E.; Sierens, C.; Gunter, M.J.; Ladas, E.J.; Barr, R.D.; Van Herck, K.; et al. Role of Maternal Diet in the Risk of Childhood Acute Leukemia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5428. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, V.; Lagudu, P.B.B.; Gangopadhyay, D.; Vijaykumar, V.; Rajaraman, S.; PerumalKalaiyarasi, J.; Ganesan, P.; Ganesan, T.S. Neutropenic versus regular diet for acute leukaemia induction chemotherapy: Randomised controlled trial. BMJ Support. Palliat. Care 2022, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.; Ahmad, Q.M.; Javed, T.; Hayat, M.Z.; Farooq, M.A.; Anwar, H.M.Z.; Khan, M.A. Evaluation of diet as a risk factor in the development of childhood leukaemia: A case control study. J. Pak. Med. Assoc. 2020, 70, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, J.W.R.; Weiss, G.; Hill-Baskin, A.E.; Schmidt, H.M.; Omoijuanfo, O.; Thompson, C.L.; Beck, C.R.; Berger, N.A. Obesity accelerates acute promyelocyticleukemia in mice and reduces sex differences in latency and penetrance. Obesity 2022, 30, 1420–1429. [Google Scholar] [CrossRef]
- Amitay, E.L.; Keinan-Boker, L. Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review. JAMA Pediatr. 2015, 169, e151025. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedeli, D.; Gozzo, I.; Leone, F.; Lezzo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-GutMicrobiotaInteractions and GestationalDiabetesMellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, S.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018, 24, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Peppas, I.; Ford, A.M.; Furness, C.L.; Greaves, M.F. Gut microbiome immaturity and childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2023, 23, 565–576. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Cantarella, C.D.; Ragusa, D.; Giammanco, M.; Tosi, S. Folate deficiency as predisposing factor for childhood leukaemia: A review of the literature. Genes Nutr. 2017, 12, 14. [Google Scholar] [CrossRef]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Aberg, K.A.; Liu, Y.; Bukszár, J.; McClay, J.L.; Khachane, A.N.; Andreassen, O.A.; Blackwood, D.; Corvin, A.; Djurovic, S.; Gurling, H.; et al. A comprehensive family-based replication study of schizophrenia genes. JAMA Psychiatry 2013, 70, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Tangri, S.; Wegmann, T.G.; Lin, H.; Raghupathy, R. Maternal anti-placental reactivity in natural, immunologically-mediated fetalresorptions. J. Immunol. 1994, 152, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J. Immunological relationship between the mother and the fetus. Int. Rev. Immunol. 2002, 21, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Breckler, L.A.; Hale, J.; Taylor, A.; Dunstan, J.A.; Thornton, C.A.; Prescott, S.L. Pregnancy IFN-gamma responses to foetal alloantigens are altered by maternal allergy and gravidity status. Allergy 2008, 63, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Vieira, P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004, 10, 801–805. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Bhutta, Z.A. Maternal nutrition and birth outcomes: Effect of balanced protein-energy supplementation. Paediatr. Perinat. Epidemiol. 2012, 26, 178–190. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Ricciardi, L.; Furci, F.; Gangemi, S. Nickel sensitization influence microbiota in allergic and non-allergic disorders: What’s up? J. Biol. Regul. Homeost. Agents. 2021, 35, 757–760. [Google Scholar] [PubMed]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell Infect. Microbiol. 2012, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018, 50, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Gili, T.; Conte, F.; Del Chierico, F.; Conta, G.; Miccheli, A.; Botticelli, A.; Pacci, P.; Caldarelli, G.; Noti, M.; et al. Network analysis of gut microbiome and metabolome to discover microbiota-linked biomarkers in patients affected by non-small cell lung cancer. Int. J. Mol. Sci. 2020, 21, 8730. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Ramírez, P.; Cadena-Ullauri, S.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Ruiz-Pozo, V.A.; Zambrano, A.K. Role of the gut microbiota in hematgueologic cancer. Front. Microbiol. 2023, 14, 1185787. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; FrutosRde, L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Gaboriau-Routhiau, V.; Rakotobe, S.; Lecuyer, E.; Mulder, I.; Lan, A.; Bridonneau, C.; Rochet, V.; Pisi, A.; De Paepe, M.; Brandi, G.; et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009, 31, 677–689. [Google Scholar] [CrossRef]
- Tsilimigras, M.C.; Fodor, A.; Jobin, C. Carcinogenesis and therapeutics: The microbiota perspective. Nat. Microbiol. 2017, 2, 17008. [Google Scholar] [CrossRef]
- Song, Y.; Gyarmati, P. Microbiota changes in a pediatric acute lymphocytic leukemia mouse model. Microbiology 2020, 9, 982. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Yooseph, S.; Harkins, D.M.; Moncera, K.J.; Zabokrtsky, K.B.; Torralba, M.G.; Tovchigrechko, A.; Highlander, S.K.; Pieper, R.; Sender, L.; et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent acute lymphoblastic leukemia (ALL) at the time of disease diagnosis. BMC Genom. 2016, 17, 635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, C.; Zhang, A. Gut microbiota in acute leukemia: Current evidence and future directions. Front. Microbiol. 2022, 13, 1045497. [Google Scholar] [CrossRef]
- Strick, R.; Strissel, P.L.; Borgers, S.; Smith, S.L.; Rowley, J.D. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc. Natl. Acad. Sci. USA 2000, 97, 4790–4795. [Google Scholar] [CrossRef]
- Vicente-Dueñas, C.; Janssen, S.; Oldenburg, M.; Auer, F.; González-Herrero, I.; Casado-García, A.; Isidro-Hernandez, M.; Raboso-Gallego, J.; Westhoff, P.; Pandyra, A.A.; et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 2020, 136, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Garrett, W.S. Microbes, Microbiota, and Colon Cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the microbiomeinfluence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.C.; Jeha, S.; et al. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Rajasuriar, R.; Lim, Y.A.L.; Woo, Y.L.; Loke, P.; Ariffin, H. Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy. BMC Cancer 2020, 20, 151. [Google Scholar] [CrossRef]
- De Pietri, S.; Ingham, A.C.; Frandsen, T.L.; Rathe, M.; Krych, L.; Castro Mejia, J.L.; Nielsen, D.S.; Nersting, J.; Wehner, P.S.; Schmiegelow, K.; et al. Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: The impact of the gut microbiota. Int. J. Cancer 2020, 147, 1953–1962. [Google Scholar] [CrossRef]
- Nearing, J.T.; Connors, J.; Whitehouse, S.; Van Limbergen, J.; Macdonald, T.; Kulkarni, K.; Langille, M.G.I. Infectious complications are associated with alterations in the gut microbiome in pediatric patients with acute lymphoblastic leukemia. Front. Cell. Infect. Microbiol. 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Galloway-Pena, J.R.; Smith, D.P.; Sahasrabhojane, P.; Wadsworth, W.D.; Fellman, B.M.; Ajami, N.J.; Shpall, E.J.; Daver, N.; Guindani, M.; Petrosino, J.F.; et al. Characterization of oral and gut microbiome temporal variability in hospitalized cancer patients. Genome Med. 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Lucafo, M.; Franzin, M.; Lagatolla, C.; Franca, R.; Bramuzzo, M.; Stocco, G.; Decorti, G. Emerging insights on the interaction between anticancer and immunosuppressant drugs and intestinal microbiota in pediatric patients. Clin. Transl. Sci. 2020, 13, 238–259. [Google Scholar] [CrossRef]
- Zhou, B.; Xia, X.; Wang, P.; Chen, S.; Yu, C.; Huang, R.; Zhang, R.; Wang, Y.; Lu, L.; Yuan, F.; et al. Induction and amelioration of methotrexate-induced gastrointestinal toxicity are related to immune response and gut microbiota. EBioMedicine 2018, 33, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, P.P.; et al. Enterococcus hirae and Barnesiellaintestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2663. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and Microbiota: IsThere a Link with Allergies? Int. J. Mol. Sci. 2021, 22, 4288. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Abe, E.; Miyaura, C.; Shiina, Y.; Suda, T. 1α, 25-dihydroxyvitamin D3 induces differentiation of human promyelocyticleukemia cells (HL-60) into monocyte- macrophages, but not into granulocytes. Biochem. Biophys. Res. Commun. 1983, 117, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Kizaki, M.; Yamato, K.; Kawai, Y.; Kamata-Matsushita, M.; Ueno, H.; Ohguchi, M.; Nishihara, T.; Koeffler, H.P.; Ikeda, Y. 1, 25-dihydroxyvitamin D3 induces differentiation of a retinoic acid–resistant acute promyelocyticleukemia cell line (UF-1) associated with expression of p21 WAF1/CIP1 and p27 KIP1. Blood 1999, 93, 2225–2233. [Google Scholar] [CrossRef]
- Abe, E.; Miyaura, C.; Sakagami, H.; Takeda, M.; Konno, K.; Yamazaki, T.; Yoshiki, S.; Suda, T. Differentiation of mouse myeloid leukemia cells induced by 1α, 25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1981, 78, 4990–4994. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Studzinski, G.P. Retinoblastoma protein and CCAAT/enhancer-binding protein β are required for 1, 25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004, 64, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Motomura, S.; Kanamori, H.; Maruta, A.; Kodama, F.; Ohkubo, T. The effect of 1-hydroxyvitamin D3 for prolongation of leukemic transformation-free survival in myelodysplastic syndromes. Am. J. Hematol. 1991, 38, 67–68. [Google Scholar] [CrossRef]
- Siitonen, T.; Timonen, T.; Juvonen, E.; Terävä, V.; Kutila, A.; Honkanen, T.; Mikkola, M.; Hallman, H.; Kauppila, M.; Nyländen, P.; et al. Valproic acid combined with 13-cis retinoic acid and 1, 25-dihydroxyvitamin D3 in the treatment of patients with myelodysplastic syndromes. Haematologica 2007, 92, 1119–1122. [Google Scholar] [CrossRef]
- Gemelli, C.; Orlandi, C.; Zanocco Marani, T.; Martello, A.; Vignudelli, T.; Ferrari, F.; Montanari, M.; Parenti, S.; Testa, A.; Grande, A.; et al. The vitamin D3/Hox-A10 pathway supports MafB function during the monocyte differentiation of human CD34+ hemopoietic progenitors. J. Immunol. 2008, 181, 5660–5672. [Google Scholar] [CrossRef]
- Callens, C.; Coulon, S.; Naudin, J.; Radford-Weiss, I.; Boissel, N.; Raffoux, E.; Wang, P.H.; Agarwal, S.; Tamouza, H.; Paubelle, E.; et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J. Exp. Med. 2010, 207, 731–750. [Google Scholar] [CrossRef]
- Harrison, J.S.; Bershadskiy, A. Clinical experience using vitamin D and analogs in the treatment of myelodysplasia and acute myeloid leukemia: A review of the literature. Leuk. Res. Treat. 2012, 2012, 125814. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Figueroa, J.; Barron-Calvillo, E.; Garcia-Parra, C.; Galindo-Delgado, P.; Contreras-Ochoa, C.; Lagunas-Martinez, A.; Campos-Romero, F.H.; Silva-Estrada, J.A.; Limon-Rojas, A.E. Probiotic supplementation decreases chemotherapy-induced gastrointestinal side effects in patients with acute leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Vindigni, S.M.; Surawicz, C.M. Fecalmicrobiota transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Tkach, S.; Dorofeyev, A.; Kuzenko, I.; Boyko, N.; Falalyeyeva, T.; Boccuto, L.; Scarpellini, E.; Kobyliak, N.; Abenavoli, L. Current status and future therapeutic options for fecalmicrobiota. Medicina 2022, 58, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecalmicrobiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Li, T.; Wei, H.; et al. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamazaki, S. Probiotics and safety. Am. J. Clin. Nutr. 2001, 73, 46–70. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Musolino, C.; Tonacci, A.; Pioggia, G.; Gangemi, S. Interactions between the MicroRNAs and Microbiota in Cancer Development: Roles and Therapeutic Opportunities. Cancers 2020, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Furci, F.; Allegra, A.; Tonacci, A.; Isola, S.; Senna, G.; Pioggia, G.; Gangemi, S. Air Pollution and microRNAs: The Role of Association in Airway Inflammation. Life 2023, 13, 1375. [Google Scholar] [CrossRef]
- De Martinis, M.; Ginaldi, L.; Allegra, A.; Sirufo, M.M.; Pioggia, G.; Tonacci, A.; Gangemi, S. The Osteoporosis/MicrobiotaLinkage: The Role of miRNA. Int. J. Mol. Sci. 2020, 21, 8887. [Google Scholar] [CrossRef]
- Hermetet, F.; Mshaik, R.; Simonet, J.; Callier, P.; Delva, L.; Quéré, R. High-fat diet intensifies MLL-AF9-induced acute myeloid leukaemia through activation of the FLT3 signalling in mouse primitive hematopoietic cells. Sci. Rep. 2020, 10, 16187. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Shen, N.; Pang, J.X.; Zhang, Y.W.; Rao, E.Y.; Bode, A.M.; Al-Kali, A.; Zhang, D.E.; Litzow, M.R.; Li, B.; et al. Fatty acid-binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia 2017, 31, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Oum, R.; Gbito, K.Y.; Garcia-Manero, G.; Strom, S.S. Dietary intake of vegetables, fruits, and meats/beans as potential risk factors of acute myeloid leukemia: A Texas case-control study. Nutr. Cancer. 2013, 65, 1132–1140. [Google Scholar] [CrossRef]
- Lanoue, L.; Green, K.K.; Kwik-Uribe, C.; Keen, C.L. Dietary factors and the risk for acute infant leukemia: Evaluating the effects of cocoa-derived flavanols on DNA topoisomerase activity. ExpBiol. Med. 2010, 235, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Park, Y.; Mayne, S.T.; Wang, R.; Sinha, R.; Hollenbeck, A.R.; Schatzkin, A.; Cross, A.J. Diet, lifestyle, and acute myeloid leukemia in the NIH-AARP cohort. Am. J. Epidemiol. 2010, 171, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A. Maternal diet and infant leukaemia: A role for DNA topoisomerase II inhibitors? Int. J. Cancer Suppl. 1998, 11, 26–28. [Google Scholar] [CrossRef]
- Li, Y.; Moysich, K.B.; Baer, M.R.; Weiss, J.R.; Brasure, J.; Graham, S.; McCann, S.E. Intake of selected food groups and beverages and adult acute myeloid leukaemia. Leuk. Res. 2006, 30, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M.; Davies, S.M.; Jacobs, D.R.; Folsom, A.R.; Potter, J.D. Diet and risk of leukaemia in the Iowa Women’s Health Study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 777–781. [Google Scholar]
- Kwiatkowski, A. Dietary and other environmental risk factors in acute leukaemias: A case-control study of 119 patients. Eur. J. Cancer Prev. 1993, 2, 139–146. [Google Scholar] [CrossRef]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can. we prevent childhood Leukaemia? Leukemia 2021, 35, 1258–1264. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).