Partially Hydrolyzed Guar Gum Intake Supports the Gut Microbiota and Attenuates Inflammation during Influenza H1N1 Virus Infection in Mice
Abstract
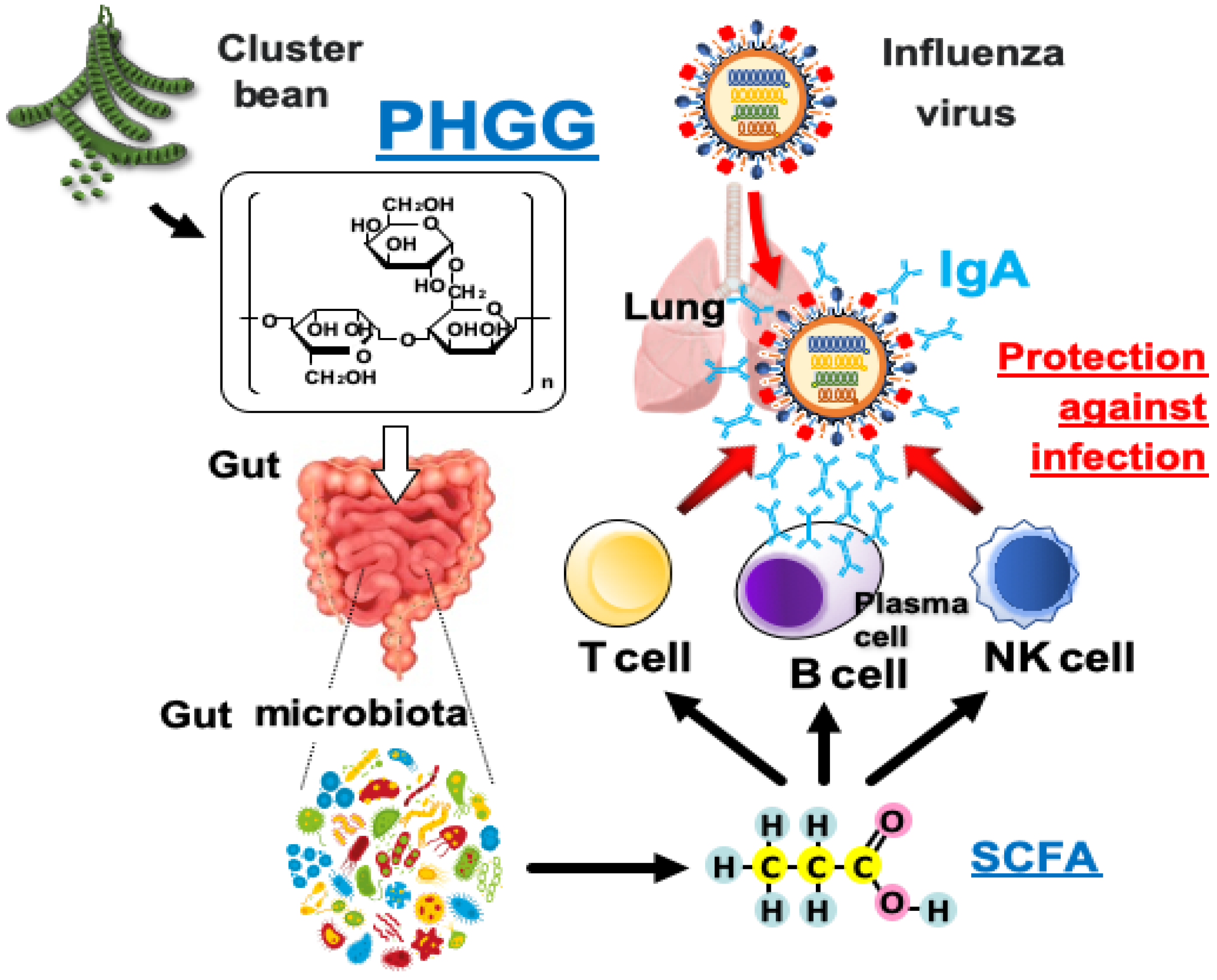
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. H1N1 Virus Preparation
2.3. Histology
2.4. Gene Expression Analysis
2.5. Flow Cytometry
2.6. Measurement of IgA and Cytokine Levels
2.7. Determination of Microbiota via 16S rRNA Gene Sequencing
2.8. Measurements of SCFAs in Cecum with GC-MS
2.9. Statistical Analysis
3. Results
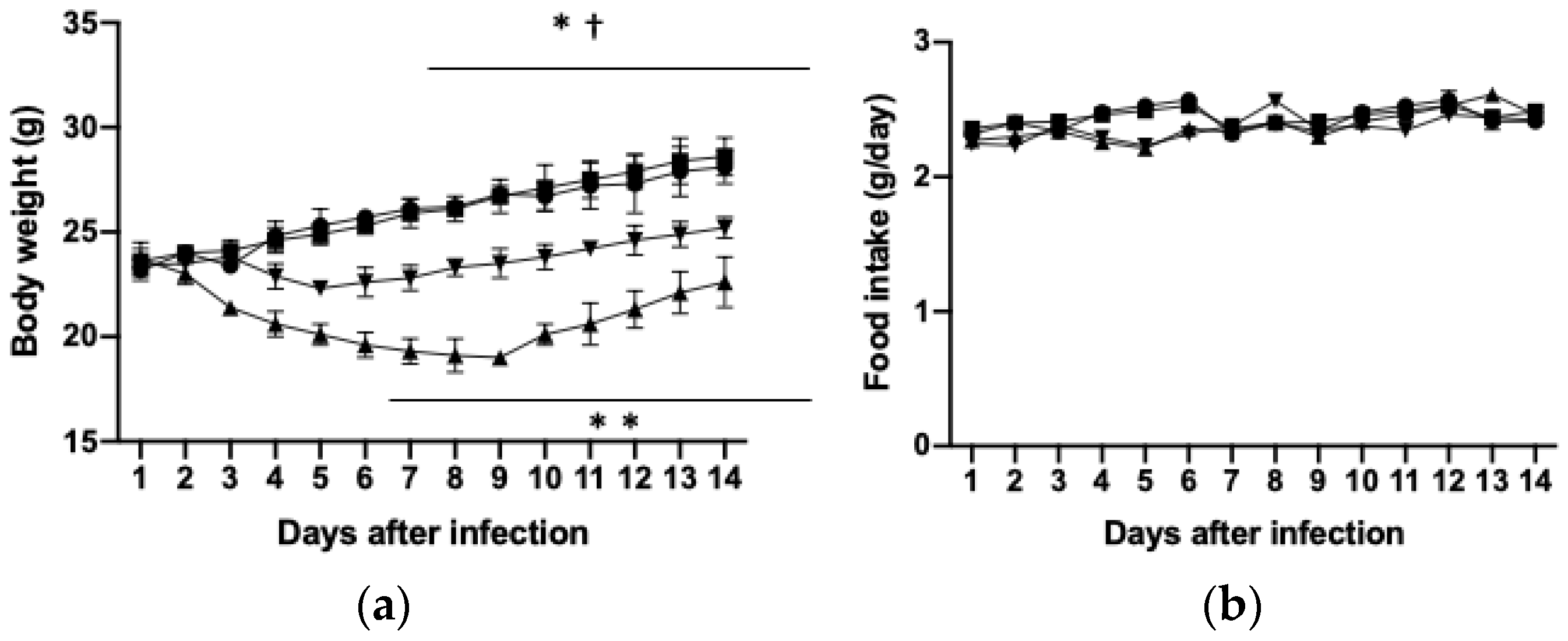
3.1. Effects of PHGG on Body and Tissue Weights and Food Intake
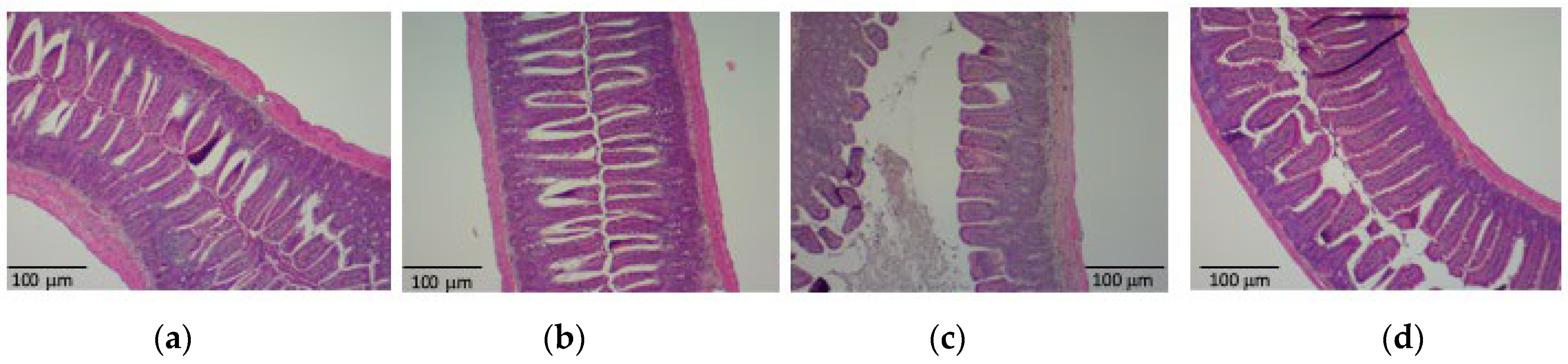
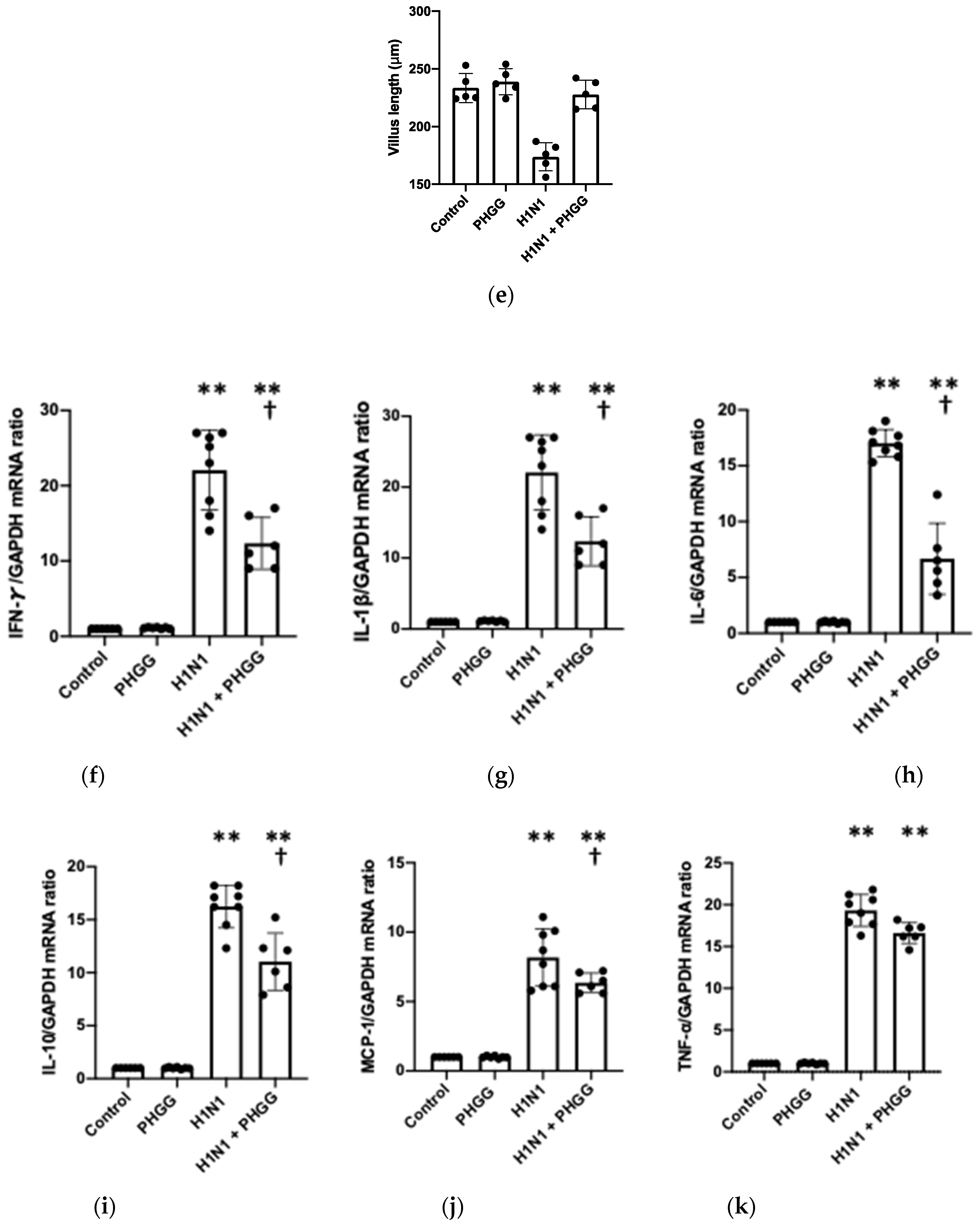
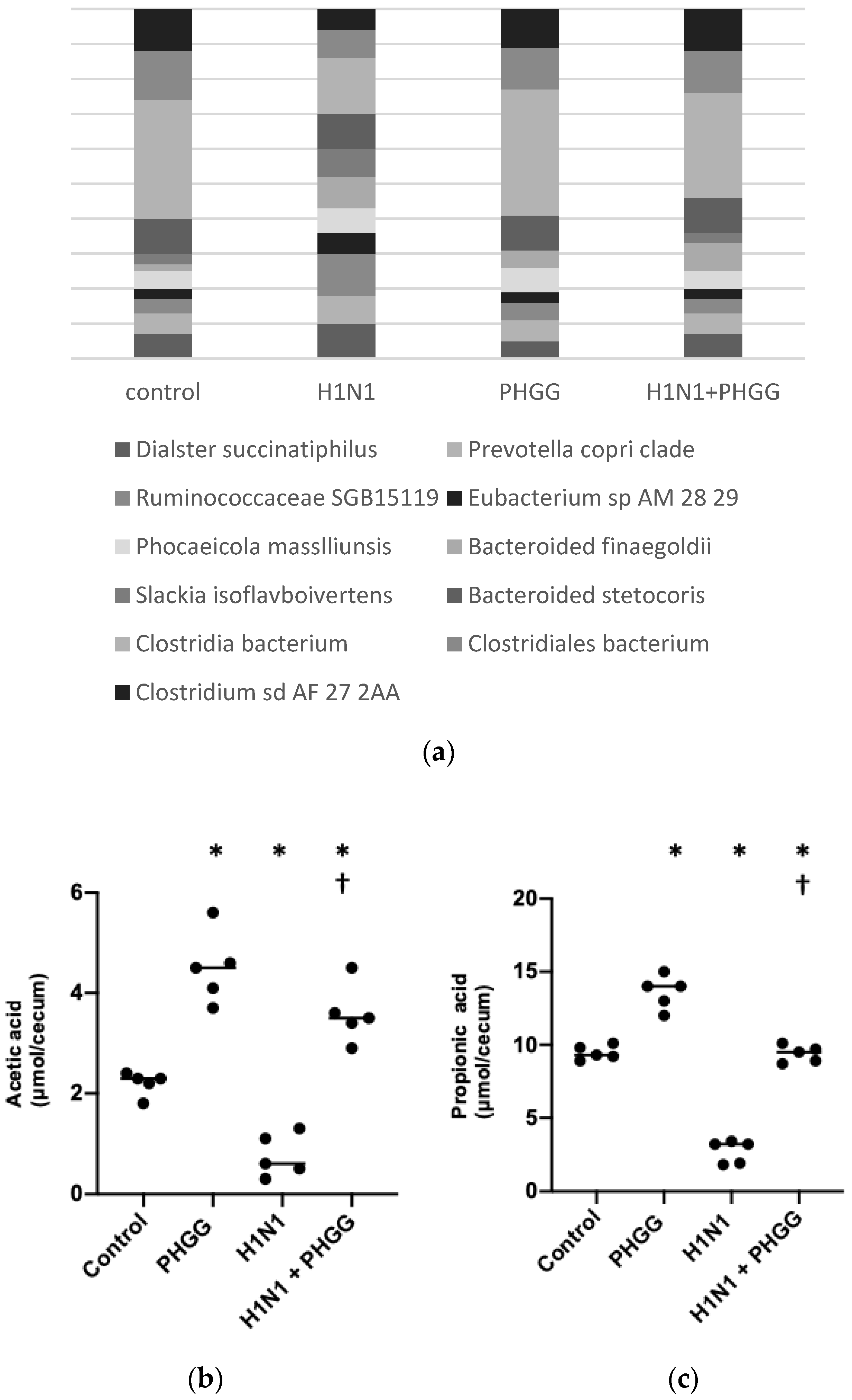
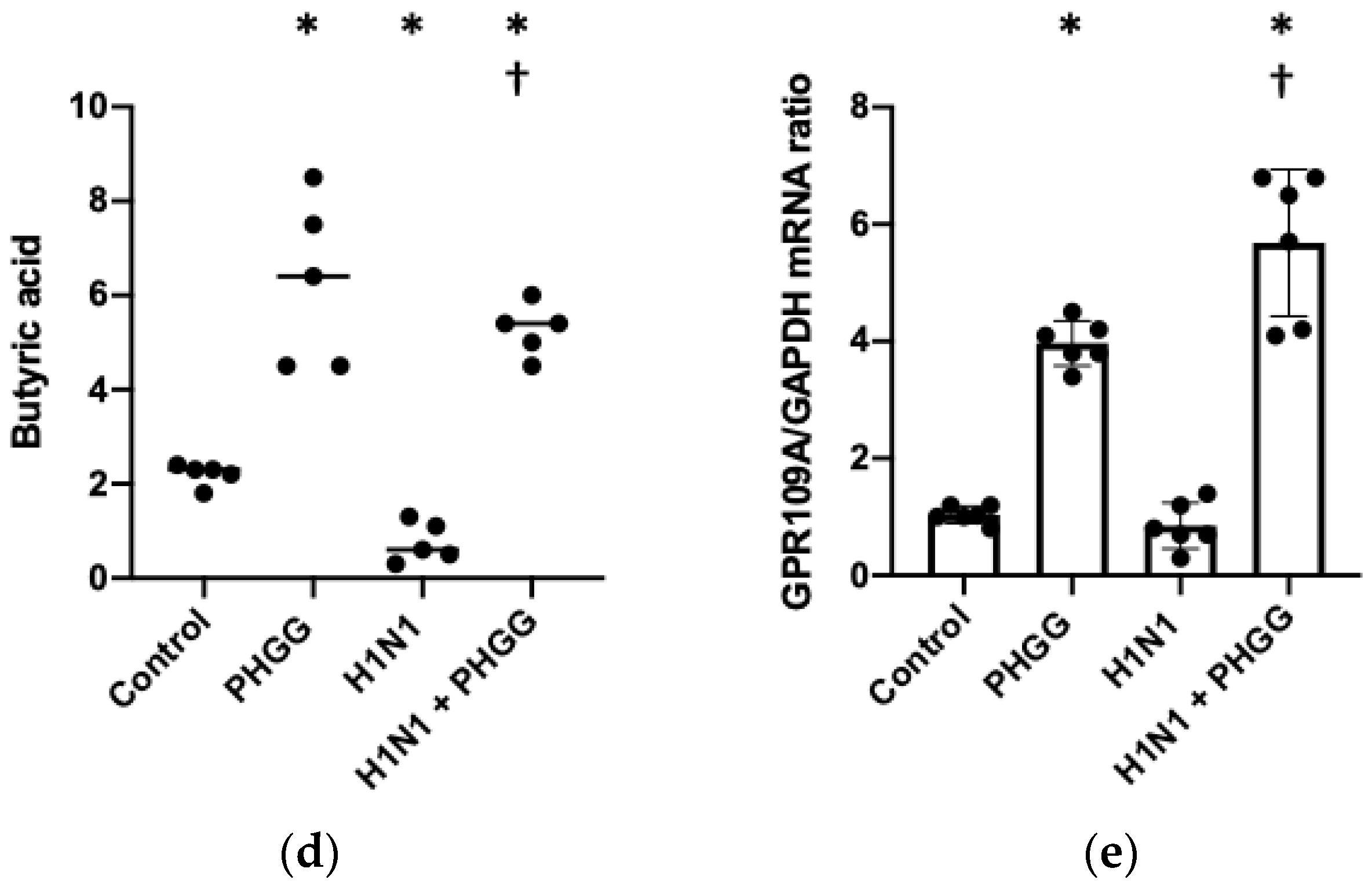
3.2. PHGG Protected the Gut Environment
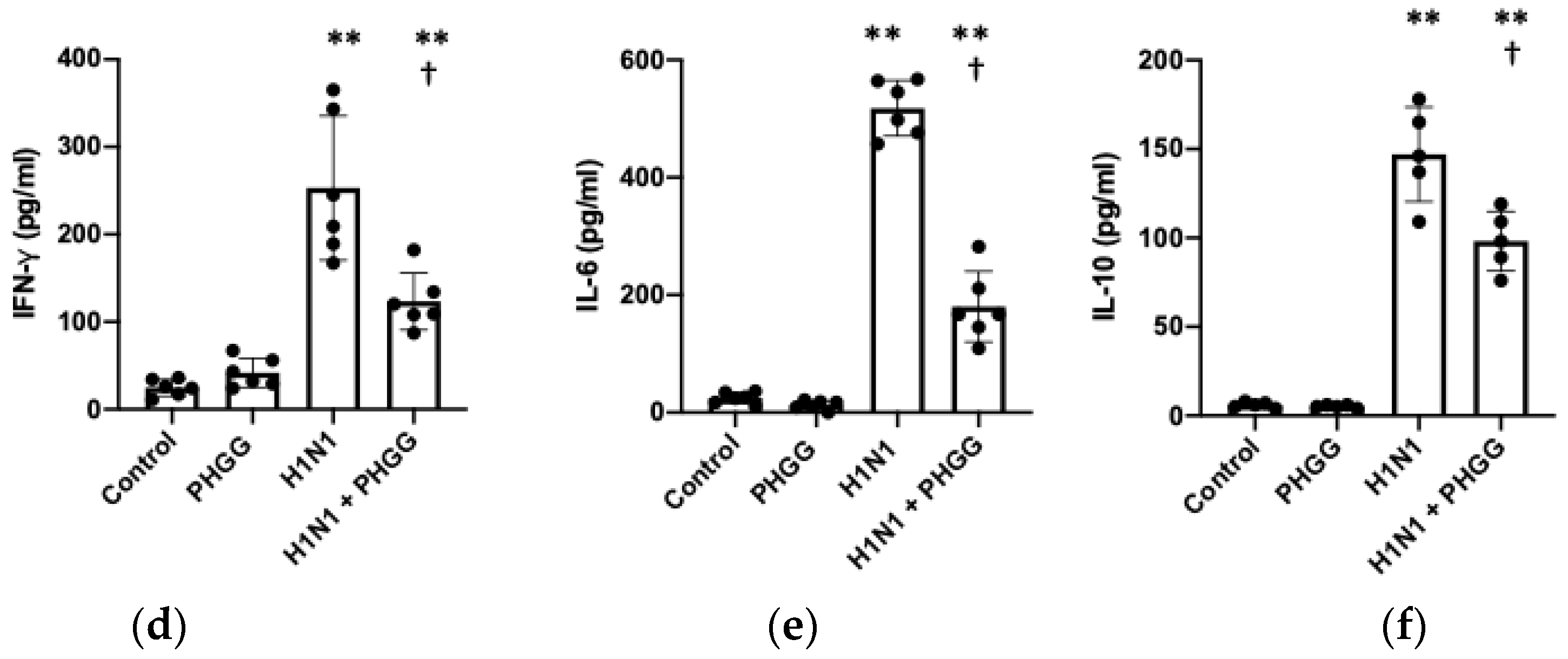
3.3. PHGG Inhibited Increases in IgA Production and Serum Cytokine Levels Induced by H1N1 Infection
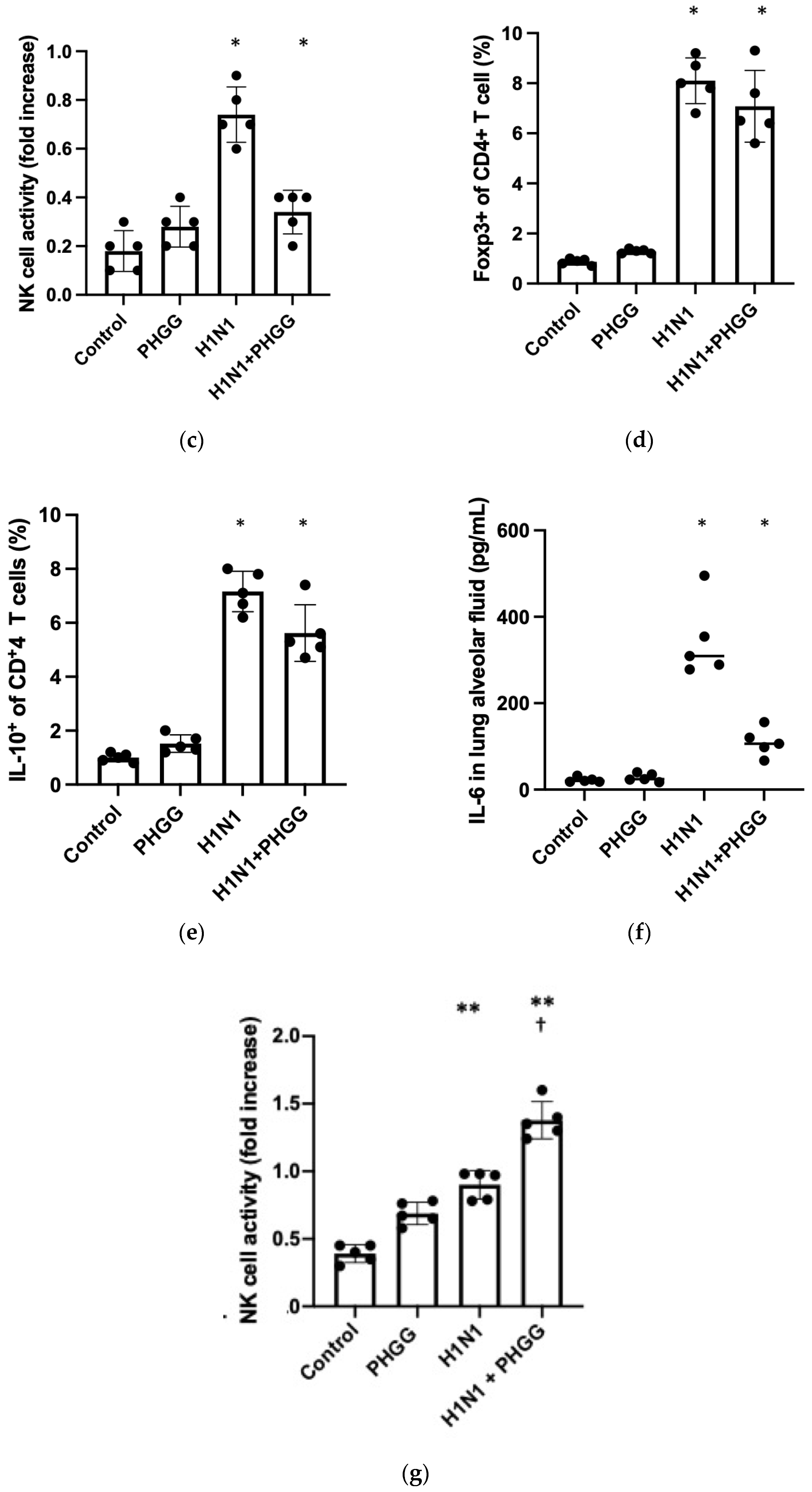
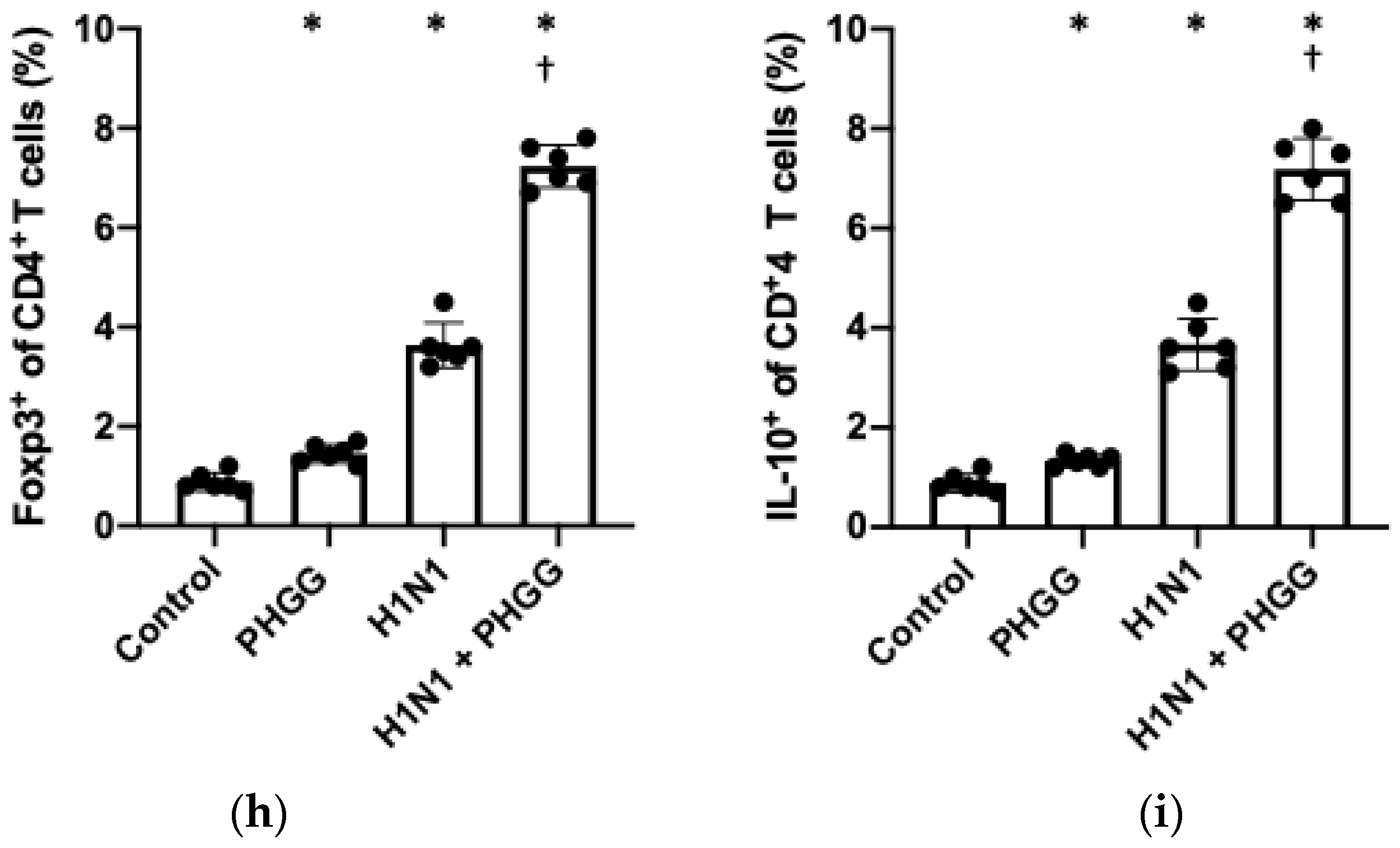
3.4. Effects of PHGG on Lung Cytokine Gene Expression and Splenocyte Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Johnson, I.R.; Schley, P.D. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 2002, 71, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, B.R.; Blackburn, G.L.; Scrimshaw, N.S.; Flatt, J.P. Cellular immunity in semistarved states in hospitalized adults. Am. J. Clin. Nutr. 1975, 28, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Usmanova, G.; Mokdad, A.H. Results of the Global Youth Tobacco Survey and implementation of the WHO Framework Convention on Tobacco Control in the WHO Eastern Mediterranean Region (EMR) countries. J. Epidemiol. Glob. Health 2013, 3, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef]
- Seo, K.; Seo, J.; Yeun, J.; Choi, H.; Kim, Y.I.; Chang, S.Y. The role of mucosal barriers in human gut health. Arch. Pharm. Res. 2021, 44, 325–341. [Google Scholar] [CrossRef]
- Shi, H.Y.; Zhu, X.; Li, W.L.; Mak, J.W.Y.; Wong, S.H.; Zhu, S.T.; Guo, S.L.; Chan, F.K.L.; Zhang, S.T.; Ng, S.C. Modulation of gut microbiota protects against viral respiratory tract infections: A systematic review of animal and clinical studies. Eur. J. Nutr. 2021, 60, 4151–4174. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, Q.; Luo, M.; Xiong, L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 729346. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Silvestre, M.P.; Vasques, M.; Araujo, J.R.; Morais, J.; Duarte, M.I.; Pestana, D.; Faria, A.; Pereira-Leal, J.B.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Womble, J.T.; Gunsch, C.K.; Ingram, J.L. The Gut/Lung Microbiome Axis in Obesity, Asthma, and Bariatric Surgery: A Literature Review. Obesity 2021, 29, 636–644. [Google Scholar] [CrossRef]
- Abdulla, M.H.; Agarwal, D.; Singh, J.K.; Traiki, T.B.; Pandey, M.K.; Ahmad, R.; Srivastava, S.K. Association of the microbiome with colorectal cancer development (Review). Int. J. Oncol. 2021, 58, 17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Li, H.X.; Xu, R.C.; Miao, W.T.; Dai, M.Y.; Ding, S.T.; Liu, H.D. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 2021, 69, 101347. [Google Scholar] [CrossRef]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef]
- Alam, N.H.; Ashraf, H.; Sarker, S.A.; Olesen, M.; Troup, J.; Salam, M.A.; Gyr, N.; Meier, R. Efficacy of partially hydrolyzed guar gum-added oral rehydration solution in the treatment of severe cholera in adults. Digestion 2008, 78, 24–29. [Google Scholar] [CrossRef]
- Furnari, M.; Parodi, A.; Gemignani, L.; Giannini, E.G.; Marenco, S.; Savarino, E.; Assandri, L.; Fazio, V.; Bonfanti, D.; Inferrera, S.; et al. Clinical trial: The combination of rifaximin with partially hydrolysed guar gum is more effective than rifaximin alone in eradicating small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2010, 32, 1000–1006. [Google Scholar] [CrossRef]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the metabolic syndrome profile by soluble fibre-guar gum-in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, Z.; Naito, Y.; Takagi, T.; Mizushima, K.; Tokunaga, M.; Ishihara, N.; Juneja, L.R.; Yoshikawa, T. Partially hydrolyzed guar gum affects the expression of genes involved in host defense functions and cholesterol absorption in colonic mucosa of db/db male mice. J. Clin. Biochem. Nutr. 2012, 51, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.V.; Suzuki, T. Dietary Fermentable Fiber Reduces Intestinal Barrier Defects and Inflammation in Colitic Mice. J. Nutr. 2016, 146, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Sumitani, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Fujisawa, T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef. Microbes 2015, 6, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota-Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Kozawa, M. The effect of partially hydrolyzed guar gum on preventing influenza infection. Clin. Nutr. ESPEN 2021, 42, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Toapanta, F.R.; Ross, T.M. Impaired immune responses in the lungs of aged mice following influenza infection. Respir. Res. 2009, 10, 112. [Google Scholar] [CrossRef]
- Johann, S.; Blumel, G.; Lipp, M.; Forster, R. A versatile flow cytometry-based assay for the determination of short- and long-term natural killer cell activity. J. Immunol. Methods 1995, 185, 209–216. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Han, D.; Liu, J.; Liu, H.; Jiang, Z. Partially Hydrolyzed Guar Gum Attenuates d-Galactose-Induced Oxidative Stress and Restores Gut Microbiota in Rats. Int. J. Mol. Sci. 2019, 20, 4861. [Google Scholar] [CrossRef]
- Pylkas, A.M.; Juneja, L.R.; Slavin, J.L. Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J. Med. Food 2005, 8, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c− Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.e1008. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Ichinohe, T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. USA 2019, 116, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R., Jr.; et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Mazanec, M.B.; Coudret, C.L.; Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 1995, 69, 1339–1343. [Google Scholar] [CrossRef]
- Tezuka, H.; Abe, Y.; Asano, J.; Sato, T.; Liu, J.; Iwata, M.; Ohteki, T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 2011, 34, 247–257. [Google Scholar] [CrossRef]
- Gao, P.; Adachi, T.; Okai, S.; Morita, N.; Kitamura, D.; Shinkura, R. Integrin CD11b provides a new marker of pre-germinal center IgA + B cells in murine Peyer’s patches. Int. Immunol. 2021, 34, 249–262. [Google Scholar] [CrossRef]
- Du, S.W.; Jacobs, H.M.; Arkatkar, T.; Rawlings, D.J.; Jackson, S.W. Integrated B Cell, Toll-like, and BAFF Receptor Signals Promote Autoantibody Production by Transitional B Cells. J. Immunol. 2018, 201, 3258–3268. [Google Scholar] [CrossRef]
- Fukunaga, T.; Sasaki, M.; Araki, Y.; Okamoto, T.; Yasuoka, T.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Effects of the soluble fibre pectin on intestinal cell proliferation, fecal short chain fatty acid production and microbial population. Digestion 2003, 67, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Blauwiekel, R.; Bunn, J.Y.; Jetton, T.L.; Frankel, W.L.; Holst, J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007, 137, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shimizu, T.; Fujita, H.; Imai, Y.; Drucker, D.J.; Seino, Y.; Yamada, Y. GLP-1 Receptor Signaling Differentially Modifies the Outcomes of Sterile vs Viral Pulmonary Inflammation in Male Mice. Endocrinology 2020, 161, bqaa201. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef] [PubMed]
- Mindt, B.C.; Fritz, J.H.; Duerr, C.U. Group 2 Innate Lymphoid Cells in Pulmonary Immunity and Tissue Homeostasis. Front. Immunol. 2018, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Miyazawa, K.; Kubota, A.; Yoda, K.; Hiramatsu, M. Orally administered heat-killed Lactobacillus gasseri TMC0356 can upregulate cell-mediated immunity in senescence-accelerated mice. FEMS Microbiol. Lett. 2012, 326, 125–130. [Google Scholar] [CrossRef]
- Park, M.K.; Ngo, V.; Kwon, Y.M.; Lee, Y.T.; Yoo, S.; Cho, Y.H.; Hong, S.M.; Hwang, H.S.; Ko, E.J.; Jung, Y.J.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]
- Groeger, D.; Schiavi, E.; Grant, R.; Kurnik-Lucka, M.; Michalovich, D.; Williamson, R.; Beinke, S.; Kiely, B.; Akdis, C.A.; Hessel, E.M.; et al. Intranasal Bifidobacterium longum protects against viral-induced lung inflammation and injury in a murine model of lethal influenza infection. EBioMedicine 2020, 60, 102981. [Google Scholar] [CrossRef]


| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward | TGTCCGTCGTGGATCTGAC |
| Reverse | CCTGCTTCACCACCTTCTTG | |
| IFN-γ | Forward | TACTGCCACGGCACAGTCATTGAAA |
| Reverse | GCTCTGCAGGATTTTCATGTCACCA | |
| IL-6 | Forward | CCTCTCTGCAAGAGACTTCCATCCA |
| Reverse | AGCCTCCGACTTGTGAAGTGGT | |
| TNF-α | Forward | CAGGCGGTGCCTATGTCTC |
| Reverse | CGATCACCCCGAAGTTCAAGTAG | |
| MCP-1 | Forward | CTGTTCACAGTTGCCGGCTG |
| Reverse | AGCTTCTTTGGGACACCTGCT | |
| IL-10 | Forward | TTTGAATTCCCTGGGTGAGAA |
| Reverse | GCTCCACTGCCTTGCTCTTATT | |
| IL-1-β | Forward | TGTTCTTTGAAGTTGACGGACCCC |
| Reverse | TGATGTGCTGCTGCGAGATTTGA | |
| IP-10 | Forward | TGAGGGCCATAGGGAAGCTTGAAAT |
| Reverse | TCCGGATTCAGACATCTCTGCTCAT |
| Control | PHGG | H1N1 | H1N1 + PHGG | |
|---|---|---|---|---|
| Body weight (g) | 28.1 ± 0.8 | 28.6 ± 0.9 | 22.6 ± 1.2 | 25.2 ± 0.5 |
| Liver (mg) | 1232 ± 45.6 | 1256 ± 38.6 | 1431 ± 56.4 | 1278 ± 47.5 |
| Soleus (mg) | 22.3 ± 1.6 | 22.4 ± 1.8 | 18.4 ± 1.9 | 20.5 ± 2.5 |
| EDL (mg) | 10.2 ± 0.7 | 10.1 ± 0.6 | 8.7 ± 0.9 | 9.8 ± 0.8 |
| Gastrocnemius (mg) | 154.2 ± 10.3 | 153.8 ± 9.5 | 121.4 ± 11.1 | 140.6 ± 10.5 |
| Visceral fat (mg) | 45.8 ± 6.7 | 28.9 ± 5.5 | 21.2 ± 9.7 | 22.7 ± 8.7 |
| Subcutaneous fat (mg) | 87.3 ± 10.2 | 70.3 ± 8.9 | 30.7 ± 12.9 | 54.2 ± 8.6 |
| Spleen (mg) | 89.3 ± 2.3 | 85.8 ± 1.8 | 98.1 ± 1.4 | 90.2 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Kamiya, S.; Narasaki, S.; Sumii, A.; Tsutsumi, Y.M.; Machida, K.; Hara, K.; Izumi-Mishima, Y.; Tsutsumi, R.; Sakaue, H. Partially Hydrolyzed Guar Gum Intake Supports the Gut Microbiota and Attenuates Inflammation during Influenza H1N1 Virus Infection in Mice. Nutrients 2023, 15, 4252. https://doi.org/10.3390/nu15194252
Kato T, Kamiya S, Narasaki S, Sumii A, Tsutsumi YM, Machida K, Hara K, Izumi-Mishima Y, Tsutsumi R, Sakaue H. Partially Hydrolyzed Guar Gum Intake Supports the Gut Microbiota and Attenuates Inflammation during Influenza H1N1 Virus Infection in Mice. Nutrients. 2023; 15(19):4252. https://doi.org/10.3390/nu15194252
Chicago/Turabian StyleKato, Takahiro, Satoshi Kamiya, Soshi Narasaki, Ayako Sumii, Yasuo M. Tsutsumi, Kyoka Machida, Kanako Hara, Yuna Izumi-Mishima, Rie Tsutsumi, and Hiroshi Sakaue. 2023. "Partially Hydrolyzed Guar Gum Intake Supports the Gut Microbiota and Attenuates Inflammation during Influenza H1N1 Virus Infection in Mice" Nutrients 15, no. 19: 4252. https://doi.org/10.3390/nu15194252
APA StyleKato, T., Kamiya, S., Narasaki, S., Sumii, A., Tsutsumi, Y. M., Machida, K., Hara, K., Izumi-Mishima, Y., Tsutsumi, R., & Sakaue, H. (2023). Partially Hydrolyzed Guar Gum Intake Supports the Gut Microbiota and Attenuates Inflammation during Influenza H1N1 Virus Infection in Mice. Nutrients, 15(19), 4252. https://doi.org/10.3390/nu15194252







