Use of Branched-Chain Amino Acids as a Potential Treatment for Improving Nutrition-Related Outcomes in Advanced Chronic Liver Disease
Abstract
:1. Introduction
2. The Role of Nutritional Status in the Prognosis of Advanced Chronic Liver Disease
2.1. The Multifactorial Pathogenesis of Malnutrition in ACLD and Its Consequences on Health Outcomes
2.2. The Importance of Addressing Secondary Sarcopenia in ACLD
2.3. Clinical Significance of Frailty in Decompensated and Compensated ACLD
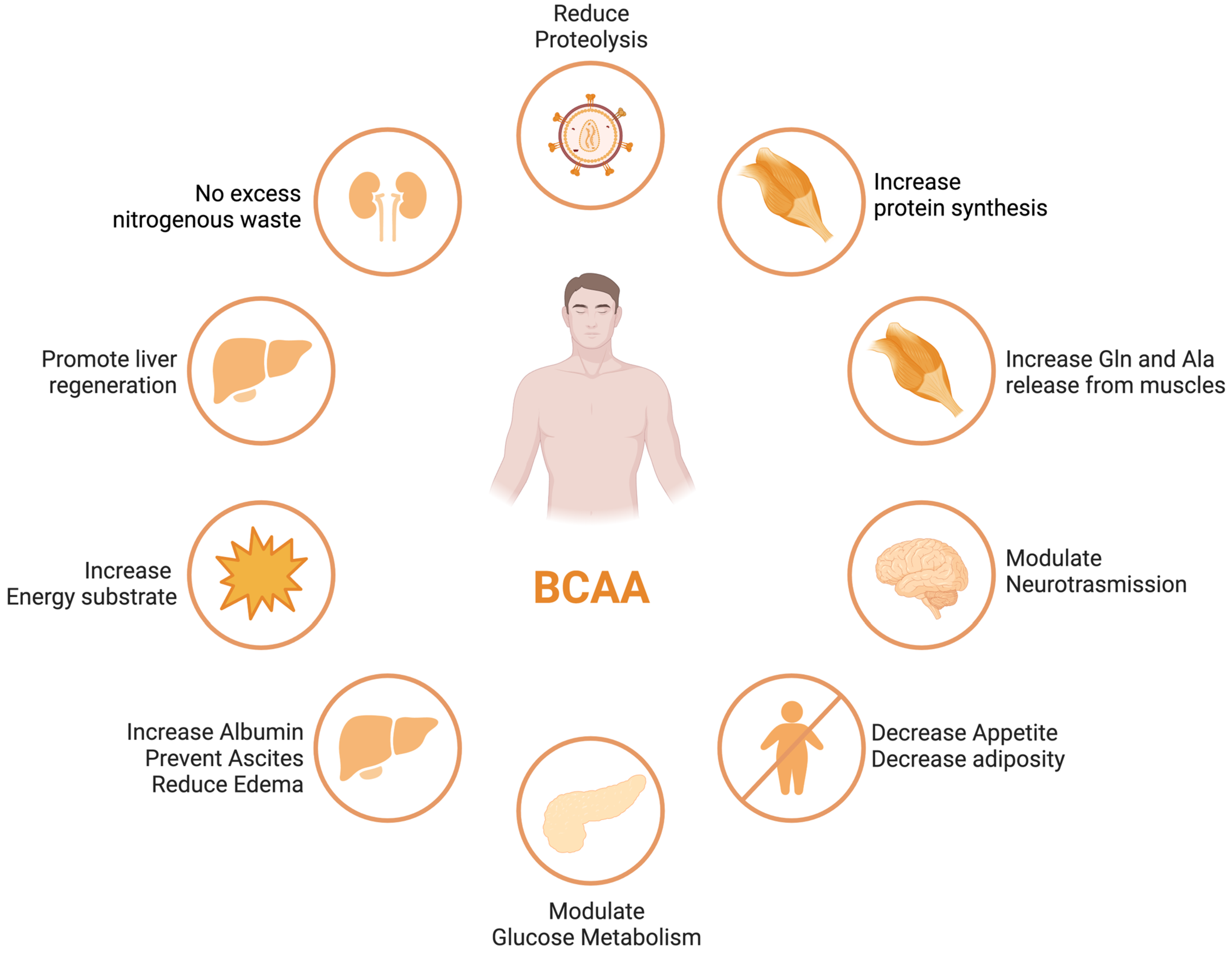
3. Branched-Chain Amino Acids (BCAAs) in Liver Disease
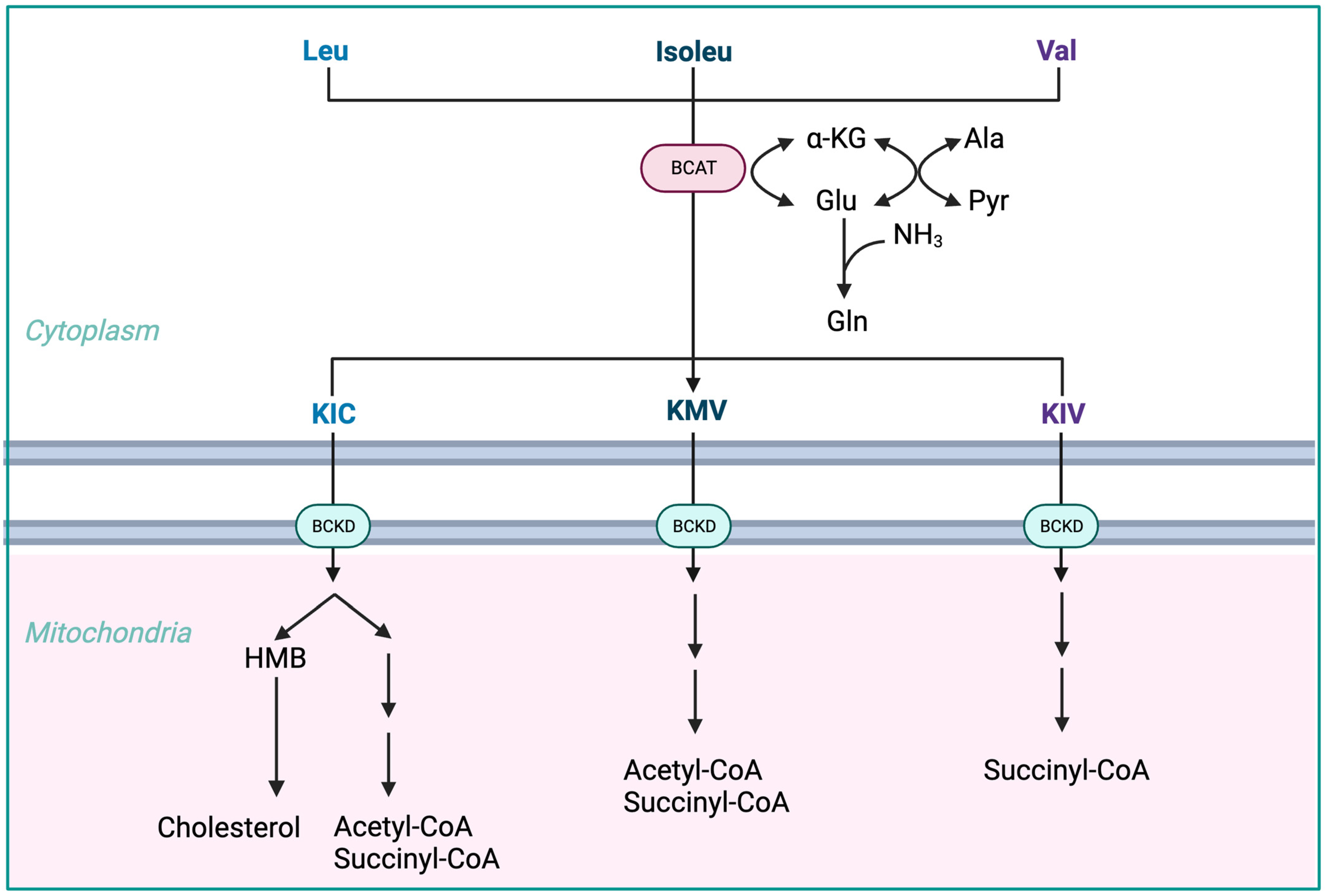
3.1. Low Fischer’s Ratio and BCAAs Metabolism in ACLD
- The initial stage of BCAA breakdown occurs outside the liver due to low BCAT activity, allowing BCAAs to quickly accumulate in the bloodstream, providing a unique advantage to BCAA-based nutritional formulas;
- Skeletal muscle plays a vital role in BCAA breakdown, facilitated by branched-chain amino acid aminotransferase (BCAT). This leads to the production of branched-chain keto acids (BCKAs), glutamate, alanine, and glutamine, which are released into the bloodstream;
- BCKD, an enzyme complex located in the inner mitochondrial membrane, irreversibly decarboxylates BCKAs into branched-chain acyl-CoA esters;
- The activity of BCKD is regulated through phosphorylation and dephosphorylation processes mediated by specific kinases and phosphatases;
- The liver exhibits the highest BCKD activity, while muscles, adipose tissue, and the brain have relatively lower activity levels;
- Muscles, comprising a significant proportion of total body weight, are major contributors to overall BCAA utilization, alongside the liver;
- Factors like cytokines, hormones, nutrients, and metabolites affect BCKD activity, with endotoxin or TNF-α administration inducing increased BCKD activity in muscles;
- BCAAs follow diverse metabolic pathways post-BCKD reaction, with KIC being ketogenic, KIV being glucogenic, and KMV being both glycogenic and ketogenic;
- The catabolism of KIC results in the synthesis of β-hydroxy-β-methylbutyrate (HMB) through the action of KIC dioxygenase.
3.2. Effects of BCAA Supplementation on Outcomes in ACLD
3.2.1. Hepatic Encephalopathy
3.2.2. Overall Survival
3.2.3. Event-Free Survival
3.2.4. Nutritional Status
3.2.5. Quality of Life
3.2.6. Liver Disease Severity and Hepatocellular Carcinoma
4. BCAA Supplementation and Body Composition: Evidence for Optimal Dose, Timing, and Formulations
5. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Fukui, A.; Kawabe, N.; Hashimoto, S.; Murao, M.; Nakano, T.; Shimazaki, H.; Kan, T.; Nakaoka, K.; Ohki, M.; Takagawa, Y.; et al. Additional BCAA-enriched nutrient mixture improves the nutritional condition in cirrhotic patients with hypoalbuminemia despite treatment with regular BCAA granules: A pilot study. Turk. J. Gastroenterol. 2015, 26, 328–335. [Google Scholar] [CrossRef]
- Ruiz-Margáin, A.; Macías-Rodríguez, R.; Ríos-Torres, S.; Román-Calleja, B.; Méndez-Guerrero, O.; Rodríguez-Córdova, P.; Torre, A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev. Gastroenterol. Mex. 2018, 83, 9–15. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [CrossRef]
- Ascione, A.; Fontanella, L.; Imparato, M.; Rinaldi, L.; De Luca, M. Mortality from cirrhosis and hepatocellular carcinoma in Western Europe over the last 40 years. Liver Int. 2017, 37, 1193–1201. [Google Scholar] [CrossRef]
- Guglielmi, F.; Panella, C.; Buda, A.; Budillon, G.; Caregaro, L.; Clerici, C.; Conte, D.; Federico, A.; Gasbarrini, G.; Guglielmi, A.; et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism: Multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE). Dig. Liver Dis. 2005, 37, 681–688. [Google Scholar] [CrossRef]
- Traub, J.; Reiss, L.; Aliwa, B.; Stadlbauer, V. Malnutrition in Patients with Liver Cirrhosis. Nutrients 2021, 13, 540. [Google Scholar] [CrossRef]
- Chin, S.; Shepherd, R.W.; Thomas, B.J.; Cleghorn, G.J.; Patrick, M.K.; Wilcox, J.; Ong, T.H.; Lynch, S.V.; Strong, R. Nutritional support in children with end-stage liver disease: A randomized crossover trial of a branched-chain amino acid supplement. Am. J. Clin. Nutr. 1992, 56, 158–163. [Google Scholar] [CrossRef]
- Carvalho, L.; Parise, E.R. Evaluation of nutritional status of nonhospitalized patients with liver cirrhosis. Arq. de Gastroenterol. 2006, 43, 269–274. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- van Dijk, A.M.; Coppens, B.J.; van Beers, M.A.; Slot, A.S.B.; Verstraete, C.J.; de Bruijne, J.; Vleggaar, F.P.; van Erpecum, K.J. Nutritional status in patients with hepatocellular carcinoma: Potential relevance for clinical outcome. Eur. J. Intern. Med. 2022, 104, 80–88. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Merli, M.; Giusto, M.; Gentili, F.; Novelli, G.; Ferretti, G.; Riggio, O.; Corradini, S.G.; Siciliano, M.; Farcomeni, A.; Attili, A.F.; et al. Nutritional status: Its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2009, 30, 208–214. [Google Scholar] [CrossRef]
- Beaudart, C.; Reginster, J.; Petermans, J.; Gillain, S.; Quabron, A.; Locquet, M.; Slomian, J.; Buckinx, F.; Bruyère, O. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp. Gerontol. 2015, 69, 103–110. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Okubo, T.; Atsukawa, M.; Tsubota, A.; Yoshida, Y.; Arai, T.; Iwashita, A.; Itokawa, N.; Kondo, C.; Iwakiri, K. Relationship between serum vitamin D level and sarcopenia in chronic liver disease. Hepatol. Res. 2020, 50, 588–597. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Yang, C.; Chen, M.; Shi, Q.; Zhou, C.; Huang, S.; Chen, Y.; Wang, Y.; Li, T.; et al. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology 2022, 303, 711–719. [Google Scholar] [CrossRef]
- Xiong, B.; Yang, C.; Zhou, C.; Wu, X.; Huang, S. TIPS placement as the first-line therapy to prevent variceal rebleeding in patients with cirrhosis and sarcopenia. Eur. J. Radiol. 2022, 158, 110630. [Google Scholar] [CrossRef]
- Toshikuni, N.; Arisawa, T.; Tsutsumi, M. Nutrition and exercise in the management of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7286–7297. [Google Scholar] [CrossRef]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef]
- Sugimoto, T.; Arai, H.; Sakurai, T. An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr. Gerontol. Int. 2021, 22, 99–109. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients with Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef]
- Tandon, P.; Montano-Loza, A.J.; Lai, J.C.; Dasarathy, S.; Merli, M. Sarcopenia and frailty in decompensated cirrhosis. J. Hepatol. 2021, 75, S147–S162. [Google Scholar] [CrossRef]
- Cron, D.C.; Friedman, J.F.; Winder, G.S.; Thelen, A.E.; Derck, J.E.; Fakhoury, J.W.; Gerebics, A.D.; Englesbe, M.J.; Sonnenday, C.J. Depression and Frailty in Patients with End-Stage Liver Disease Referred for Transplant Evaluation. Am. J. Transplant. 2016, 16, 1805–1811. [Google Scholar] [CrossRef]
- Tandon, P.; Tangri, N.; Thomas, L.; Zenith, L.; Shaikh, T.; Carbonneau, M.; Ma, M.; Bailey, R.J.; Jayakumar, S.; Burak, K.W.; et al. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients with Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Am. J. Gastroenterol. 2016, 111, 1759–1767. [Google Scholar] [CrossRef]
- Lai, J.C.; Rahimi, R.S.; Verna, E.C.; Kappus, M.R.; Dunn, M.A.; McAdams-DeMarco, M.; Haugen, C.E.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; et al. Frailty Associated with Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019, 156, 1675–1682. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Dunn, M.A.; Josbeno, D.A.; Tevar, A.D.; Rachakonda, V.; Ganesh, S.R.; Schmotzer, A.R.; Kallenborn, E.A.; Behari, J.; Landsittel, D.P.; DiMartini, A.F.; et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am. J. Gastroenterol. 2016, 111, 1768–1775. [Google Scholar] [CrossRef]
- Siramolpiwat, S.; Kiattikunrat, K.; Soontararatpong, R.; Pornthisarn, B.; Vilaichone, R.-K.; Chonprasertsuk, S.; Bhanthumkomol, P.; Nunanun, P.; Issariyakulkarn, N. Frailty as tested by the Liver Frailty Index is associated with decompensation and unplanned hospitalization in patients with compensated cirrhosis. Scand. J. Gastroenterol. 2021, 56, 1210–1219. [Google Scholar] [CrossRef]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Dunn, M.A.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; McCulloch, C.E.; Haugen, C.E.; et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J. Hepatol. 2020, 73, 575–581. [Google Scholar] [CrossRef]
- Les, I.; Doval, E.; García-Martínez, R.; Planas, M.; Cárdenas, G.; Gómez, P.; Flavià, M.; Jacas, C.; Mínguez, B.; Vergara, M.; et al. Effects of Branched-Chain Amino Acids Supplementation in Patients With Cirrhosis and a Previous Episode of Hepatic Encephalopathy: A Randomized Study. Am. J. Gastroenterol. 2011, 106, 1081–1088. [Google Scholar] [CrossRef]
- Dejong, C.H.C.; van de Poll, M.C.G.; Soeters, P.B.; Jalan, R.; Damink, S.W.M.O. Aromatic Amino Acid Metabolism during Liver Failure. J. Nutr. 2007, 137, 1579S–1585S. [Google Scholar] [CrossRef]
- Kakazu, E.; Kanno, N.; Ueno, Y.; Shimosegawa, T. Extracellular Branched-Chain Amino Acids, Especially Valine, Regulate Maturation and Function of Monocyte-Derived Dendritic Cells. J. Immunol. 2007, 179, 7137–7146. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Branched-Chain Amino Acids: Enzyme and Substrate Regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [CrossRef]
- Marchesini, G.; Dioguardi, F.; Bianchi, G.; Zoli, M.; Bellati, G.; Roffi, L.; Martines, D.; Abbiati, R. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy: A randomized double-blind casein-controlled trial. J. Hepatol. 1990, 11, 92–101. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kiguchi, D.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Tomida, H.; et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1416–1423. [Google Scholar] [CrossRef]
- Konstantis, G.; Pourzitaki, C.; Chourdakis, M.; Kitsikidou, E.; Germanidis, G. Efficacy of branched chain amino acids supplementation in liver cirrhosis: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1171–1190. [Google Scholar] [CrossRef]
- Holecek, M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition 2010, 26, 482–490. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef]
- Trillos-Almanza, M.C.; Wessel, H.; Martínez-Aguilar, M.; van den Berg, E.H.; Douwes, R.M.; Moshage, H.; Connelly, M.A.; Bakker, S.J.L.; de Meijer, V.E.; Dullaart, R.P.F.; et al. Branched Chain Amino Acids Are Associated with Physical Performance in Patients with End-Stage Liver Disease. Biomolecules 2023, 13, 824. [Google Scholar] [CrossRef]
- Urata, Y.; Okita, K.; Korenaga, K.; Uchida, K.; Yamasaki, T.; Sakaida, I. The effect of supplementation with branched-chain amino acids in patients with liver cirrhosis. Hepatol. Res. 2007, 37, 510–516. [Google Scholar] [CrossRef]
- Fukushima, H.; Miwa, Y.; Shiraki, M.; Gomi, I.; Toda, K.; Kuriyama, S.; Nakamura, H.; Wakahara, T.; Era, S.; Moriwaki, H. Oral branched-chain amino acid supplementation improves the oxidized/reduced albumin ratio in patients with liver cirrhosis. Hepatol. Res. 2007, 37, 765–770. [Google Scholar] [CrossRef]
- Aoyama, K.; Tsuchiya, M.; Mori, K.; Kubo, Y.; Shiraishi, K.; Sakaguchi, E.; Yamashita, S.; Sakaida, I. Effect of a late evening snack on outpatients with liver cirrhosis. Hepatol. Res. 2007, 37, 608–614. [Google Scholar] [CrossRef]
- Tsien, C.; Davuluri, G.; Singh, D.; Allawy, A.; Have, G.A.T.; Thapaliya, S.; Schulze, J.M.; Barnes, D.; McCullough, A.J.; Engelen, M.P.; et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015, 61, 2018–2029. [Google Scholar] [CrossRef]
- Pelletier, V.; Marks, L.; Wagner, D.A.; Hoerr, R.A.; Young, V.R. Branched-chain amino acid interactions with reference to amino acid requirements in adult men: Leucine metabolism at different valine and isoleucine intakes. Am. J. Clin. Nutr. 1991, 54, 402–407. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A.; Pagliaro, L.; Marubini, E.; The Liver Study Group of “V. Cervello” Hospital. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig. Dis. Sci. 1986, 31, 468–475. [Google Scholar] [CrossRef]
- Marchesini, G.; Marzocchi, R.; Noia, M.; Bianchi, G. Branched-Chain Amino Acid Supplementation in Patients with Liver Diseases. J. Nutr. 2005, 135, 1596S–1601S. [Google Scholar] [CrossRef]
- Naylor, C.; O’Rourke, K.; Detsky, A.S.; Baker, J.P. Parenteral nutrition with branched-chain amino acids in hepatic encephalopathy. Gastroenterology 1989, 97, 1033–1042. [Google Scholar] [CrossRef]
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017, 2017, CD001939. [Google Scholar] [CrossRef]
- Cabre, E.; Gonzalez-Huix, F.; Abad-Lacruz, A.; Esteve, M.; Acero, D.; Fernandez-Bañares, F.; Xiol, X.; Gassull, M. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. Gastroenterology 1990, 98, 715–720. [Google Scholar] [CrossRef]
- Tai, M.-L.S.; Razlan, H.; Goh, K.-L.; Taib, S.H.M.; Huzaini, A.H.M.; Rampal, S.; Mahadeva, S. Short term nasogastric versus oral feeding in hospitalised patients with advanced cirrhosis: A randomised trial. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2011, 6, e242–e247. [Google Scholar] [CrossRef]
- Tejavath, A.S.; Mathur, A.; Nathiya, D.; Singh, P.; Raj, P.; Suman, S.; Mundada, P.R.; Atif, S.; Rai, R.R.; Tomar, B.S. Impact of Branched Chain Amino Acid on Muscle Mass, Muscle Strength, Physical Performance, Combined Survival, and Maintenance of Liver Function Changes in Laboratory and Prognostic Markers on Sarcopenic Patients with Liver Cirrhosis (BCAAS Study): A Randomized Clinical Trial. Front. Nutr. 2021, 8, 715795. [Google Scholar] [CrossRef]
- Hernández-Conde, M.; Llop, E.; Gómez-Pimpollo, L.; Carrillo, C.F.; Rodríguez, L.; Brule, E.V.D.; Perelló, C.; López-Gómez, M.; Abad, J.; Martínez-Porras, J.L.; et al. Adding Branched-Chain Amino Acids to an Enhanced Standard-of-Care Treatment Improves Muscle Mass of Cirrhotic Patients with Sarcopenia: A Placebo-Controlled Trial. Am. J. Gastroenterol. 2021, 116, 2241–2249. [Google Scholar] [CrossRef]
- Kawamura, E.; Habu, D.; Morikawa, H.; Enomoto, M.; Kawabe, J.; Tamori, A.; Sakaguchi, H.; Saeki, S.; Kawada, N.; Shiomi, S. A randomized pilot trial of oral branched-chain amino acids in early cirrhosis: Validation using prognostic markers for pre-liver transplant status. Liver Transplant. 2009, 15, 790–797. [Google Scholar] [CrossRef]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of Oral Branched-Chain Amino Acid Granules on Event-Free Survival in Patients with Liver Cirrhosis. Clin. Gastroenterol. Hepatol. 2005, 3, 705–713. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ikeda, K.; Arase, Y.; Suzuki, Y.; Suzuki, F.; Akuta, N.; Hosaka, T.; Murashima, N.; Saitoh, S.; Someya, T.; et al. Inhibitory effect of branched-chain amino acid granules on progression of compensated liver cirrhosis due to hepatitis C virus. J. Gastroenterol. 2008, 43, 63–70. [Google Scholar] [CrossRef]
- Gil Park, J.; Tak, W.Y.; Park, S.Y.; Kweon, Y.O.; Chung, W.J.; Jang, B.K.; Bae, S.H.; Lee, H.J.; Jang, J.Y.; Suk, K.T.; et al. Effects of Branched-Chain Amino Acid (BCAA) Supplementation on the Progression of Advanced Liver Disease: A Korean Nationwide, Multicenter, Prospective, Observational, Cohort Study. Nutrients 2020, 12, 1429. [Google Scholar] [CrossRef]
- Kawamura, N.; Nakajima, H.; Takashi, S.-I. Administration of granulated BCAA and quality of life. Hepatol. Res. 2004, 30, 42–45. [Google Scholar] [CrossRef]
- Nakaya, Y.; Okita, K.; Suzuki, K.; Moriwaki, H.; Kato, A.; Miwa, Y.; Shiraishi, K.; Okuda, H.; Onji, M.; Kanazawa, H.; et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 2007, 23, 113–120. [Google Scholar] [CrossRef]
- van Dijk, A.M.; Slot, A.S.B.; Portincasa, P.; Siegerink, S.N.; Chargi, N.; Verstraete, C.J.R.; de Bruijne, J.; Vleggaar, F.P.; van Erpecum, K.J. Systematic review with meta-analysis: Branched-chain amino acid supplementation in liver disease. Eur. J. Clin. Investig. 2022, 53, e13909. [Google Scholar] [CrossRef]
- Dioguardi, F.S.; Brigatti, M.; Dell’Oca, M.; Ferrario, E.; Abbiati, R. Effects of chronic oral branched-chain amino acid supplementation in a subpopulation of cirrhotics. Clin. Physiol. Biochem. 1990, 8, 101–107. [Google Scholar]
- Kitajima, Y.; Takahashi, H.; Akiyama, T.; Murayama, K.; Iwane, S.; Kuwashiro, T.; Tanaka, K.; Kawazoe, S.; Ono, N.; Eguchi, T.; et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J. Gastroenterol. 2017, 53, 427–437. [Google Scholar] [CrossRef]
- Nishida, Y.; Ide, Y.; Okada, M.; Otsuka, T.; Eguchi, Y.; Ozaki, I.; Tanaka, K.; Mizuta, T. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol. Res. 2016, 47, E193–E200. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Habu, D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol. Res. 2004, 30, 36–41. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Yu, W.-C.; Fan, S.-T.; Wong, J. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: A randomized trial. Aliment. Pharmacol. Ther. 2004, 19, 779–788. [Google Scholar] [CrossRef]
- Saito, M.; Yano, Y.; Minami, A.; Hirano, H.; Momose, K.; Sugimoto, M.; Yoshida, M.; Azuma, T. Branched-chain Amino Acid Granules Improve the Non-protein Respiratory Quotient after Radiofrequency Ablation. Intern. Med. 2014, 53, 1469–1475. [Google Scholar] [CrossRef]
- Yatsuhashi, H.; Ohnishi, Y.; Nakayama, S.; Iwase, H.; Nakamura, T.; Imawari, M. Anti-hypoalbuminemic effect of branched-chain amino acid granules in patients with liver cirrhosis is independent of dietary energy and protein intake. Hepatol. Res. 2011, 41, 1027–1035. [Google Scholar] [CrossRef]
- Harima, Y.; Yamasaki, T.; Hamabe, S.; Saeki, I.; Okita, K.; Terai, S.; Sakaida, I. Effect of a late evening snack using branched-chain amino acid-enriched nutrients in patients undergoing hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol. Res. 2010, 40, 574–584. [Google Scholar] [CrossRef]
- Hidaka, H.; Nakazawa, T.; Kutsukake, S.; Yamazaki, Y.; Aoki, I.; Nakano, S.; Asaba, N.; Minamino, T.; Takada, J.; Tanaka, Y.; et al. The efficacy of nocturnal administration of branched-chain amino acid granules to improve quality of life in patients with cirrhosis. J. Gastroenterol. 2012, 48, 269–276. [Google Scholar] [CrossRef]
- Iwasa, M.; Sugimoto, R.; Ishihara, T.; Sekoguchi-Fujikawa, N.; Yoshikawa, K.; Mifuji-Moroka, R.; Tanaka, H.; Kobayashi, Y.; Hasegawa, H.; Takei, Y. Usefulness of Levocarnitine and/or Branched-Chain Amino Acids during Invasive Treatment for Hepatocellular Carcinoma. J. Nutr. Sci. Vitaminol. 2015, 61, 433–440. [Google Scholar] [CrossRef]
- Lee, I.J.; Seong, J.; Bae, J.I.; You, S.H.; Rhee, Y.; Lee, J.H. Effect of Oral Supplementation with Branched-chain Amino Acid (BCAA) during Radiotherapy in Patients with Hepatocellular Carcinoma: A Double-Blind Randomized Study. Cancer Res. Treat. 2011, 43, 24–31. [Google Scholar] [CrossRef]
- Morihara, D.; Iwata, K.; Hanano, T.; Kunimoto, H.; Kuno, S.; Fukunaga, A.; Yotsumoto, K.; Takata, K.; Tanaka, T.; Sakurai, K.; et al. Late-evening snack with branched-chain amino acids improves liver function after radiofrequency ablation for hepatocellular carcinoma. Hepatol. Res. 2012, 42, 658–667. [Google Scholar] [CrossRef]
- Park, J.G.; Tak, W.Y.; Park, S.Y.; Kweon, Y.O.; Jang, S.Y.; Lee, Y.R.; Bae, S.H.; Jang, J.Y.; Kim, D.Y.; Lee, J.S.; et al. Effects of branched-chain amino acids (BCAAs) on the progression of advanced liver disease: A Korean nationwide, multicenter, retrospective, observational, cohort study. Medicine 2017, 96, e6580. [Google Scholar] [CrossRef]
- Yoshida, T.; Muto, Y.; Moriwaki, H.; Yamato, M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol. Jpn. 1989, 24, 692–698. [Google Scholar] [CrossRef]
- Plauth, M.; Egberts, E.-H.; Hamster, W.; Török, M.; Müller, P.H.; Brand, O.; Fürst, P.; Dölle, W. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids: A double-blind placebo-controlled crossover study. J. Hepatol. 1993, 17, 308–314. [Google Scholar] [CrossRef]
- Ooi, P.; Gilmour, S.; Yap, J.; Mager, D. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin. Nutr. ESPEN 2018, 28, 41–51. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosimo, S.; Bertoli, S.; Saffioti, F. Use of Branched-Chain Amino Acids as a Potential Treatment for Improving Nutrition-Related Outcomes in Advanced Chronic Liver Disease. Nutrients 2023, 15, 4190. https://doi.org/10.3390/nu15194190
Colosimo S, Bertoli S, Saffioti F. Use of Branched-Chain Amino Acids as a Potential Treatment for Improving Nutrition-Related Outcomes in Advanced Chronic Liver Disease. Nutrients. 2023; 15(19):4190. https://doi.org/10.3390/nu15194190
Chicago/Turabian StyleColosimo, Santo, Simona Bertoli, and Francesca Saffioti. 2023. "Use of Branched-Chain Amino Acids as a Potential Treatment for Improving Nutrition-Related Outcomes in Advanced Chronic Liver Disease" Nutrients 15, no. 19: 4190. https://doi.org/10.3390/nu15194190




