Differential Metabolites in Osteoarthritis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Risk of Bias Assessment
2.5. Data Collection
2.6. Data Synthesis
3. Results
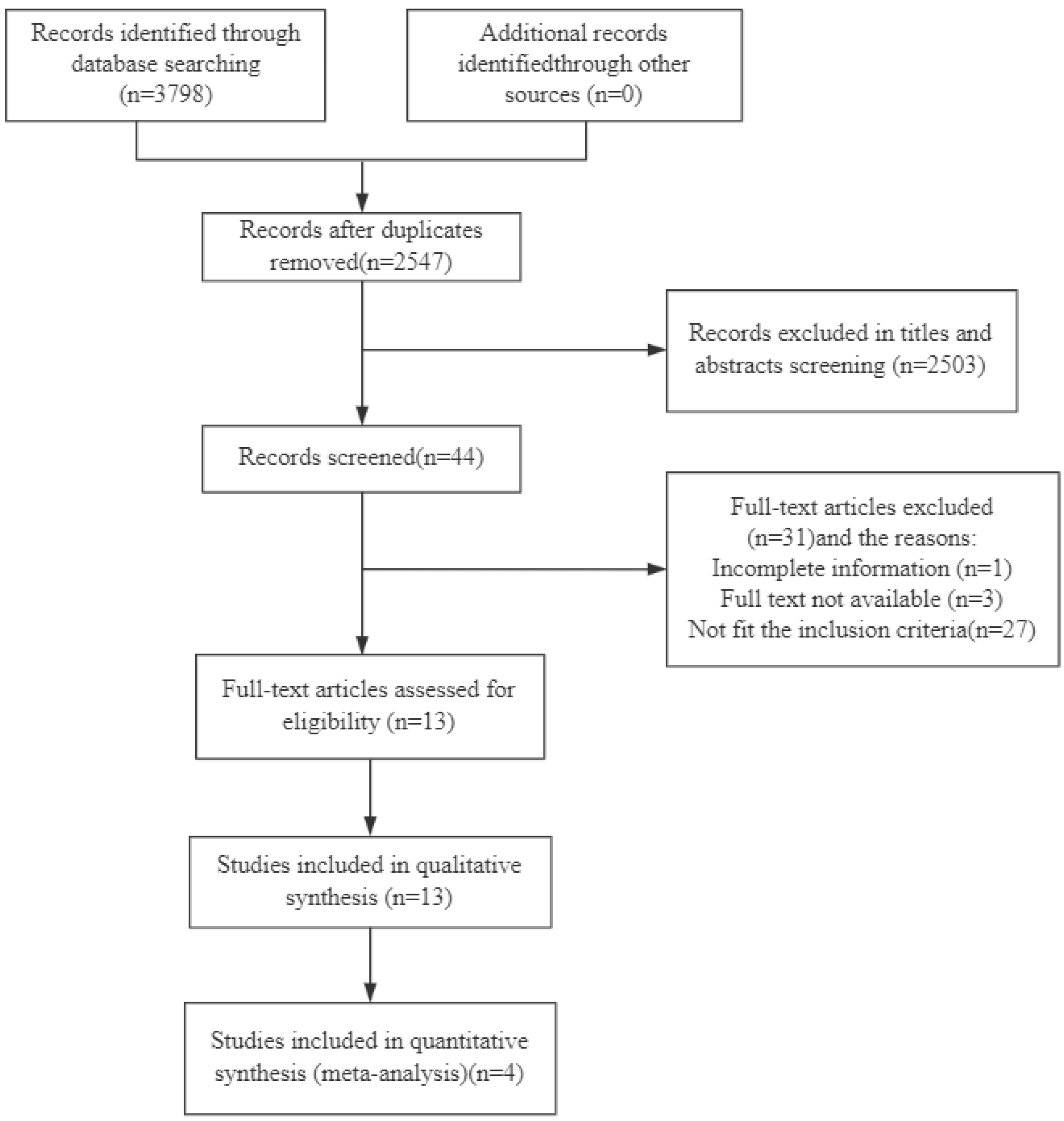
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Risk of Bias of Included Studies
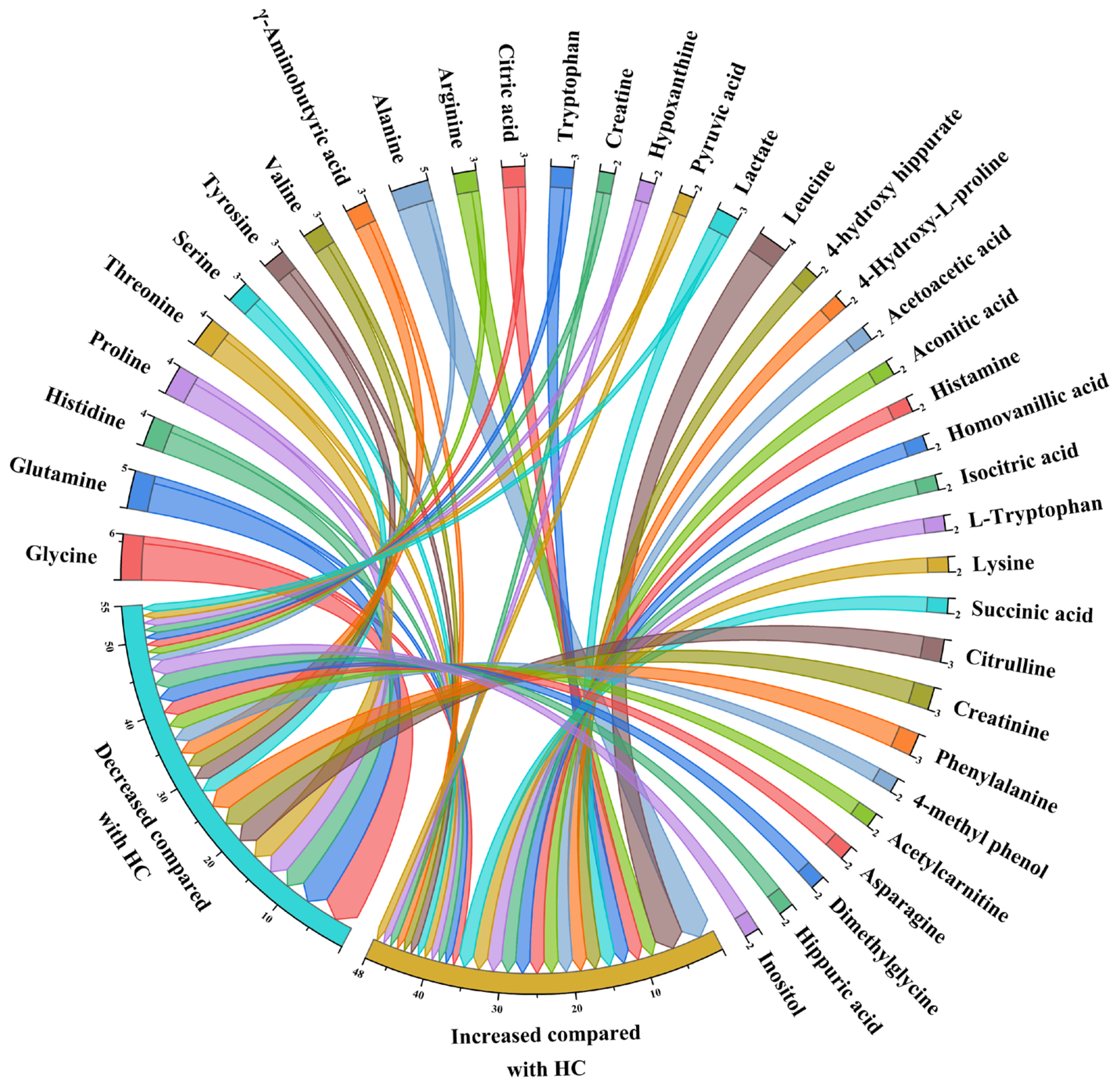
3.4. Qualitative Synthesis
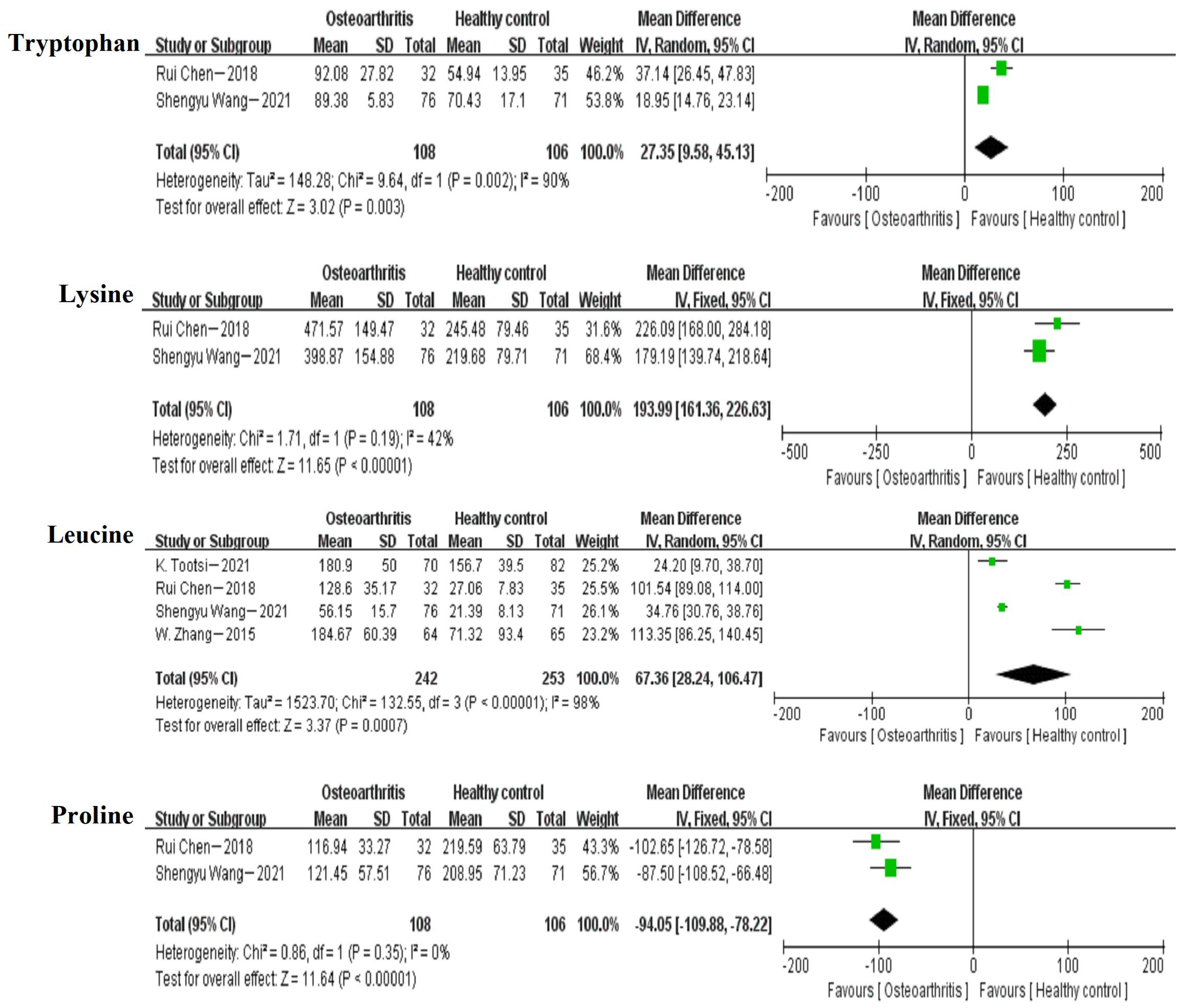
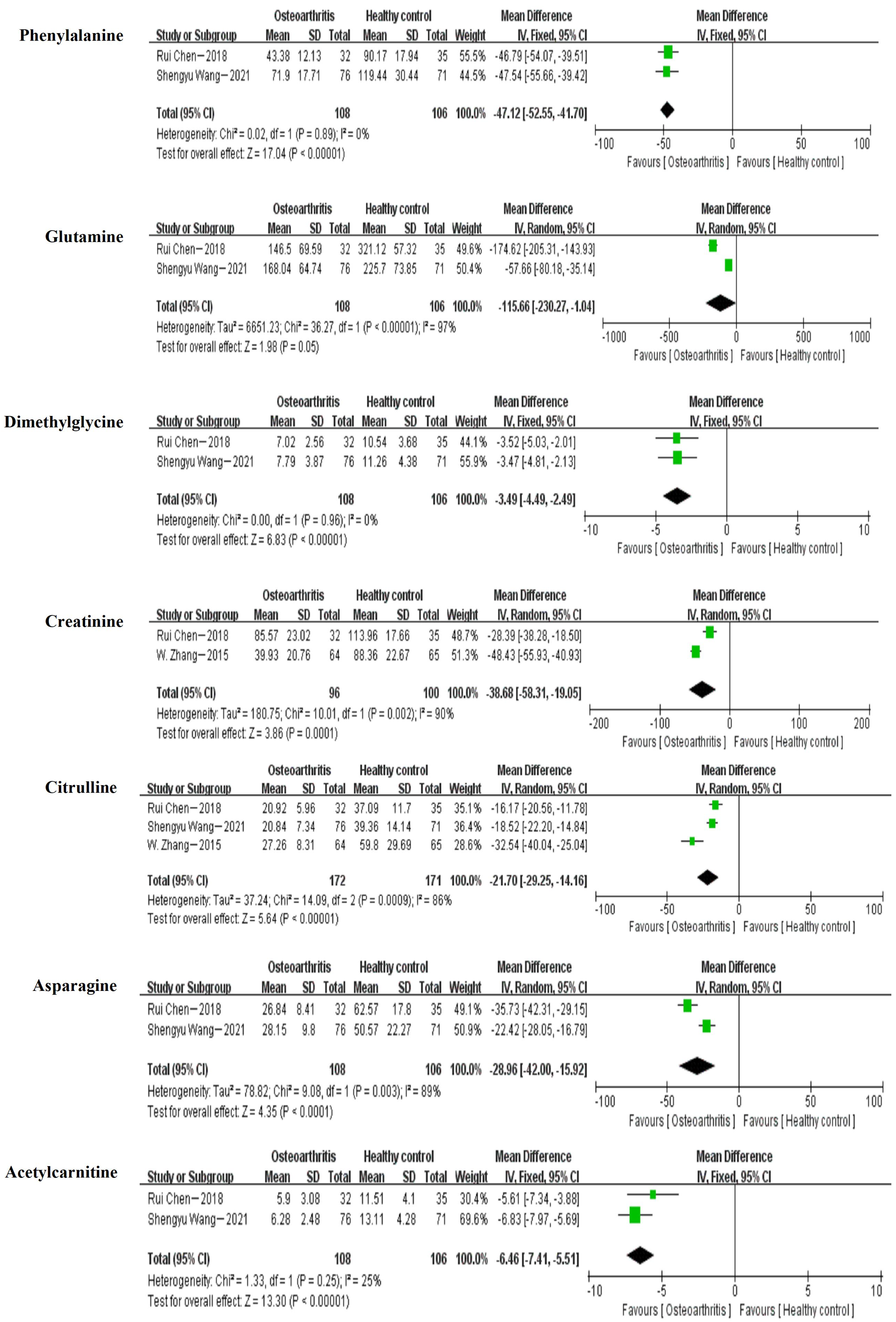
3.5. Meta-Analysis
4. Discussion
5. Limitations and Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Xie, F.; Kovic, B.; Jin, X.; He, X.; Wang, M.; Silvestre, C. Economic and Humanistic Burden of Osteoarthritis: A Systematic Review of Large Sample Studies. Pharmacoeconomics 2016, 34, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Rodrigues, D.; Rodrigues, A.; Martins, T.; Pinto, J.; Amorim, D.; Almeida, A.; Pinto-Ribeiro, F. Correlation between Pain Severity and Levels of Anxiety and Depression in Osteoarthritis Patients: A Systematic Review and Meta-Analysis. Rheumatology 2021, 61, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, L.; Cicuttini, F.; Zhu, Z.; Han, W.; Antony, B.; Wluka, A.E.; Winzenberg, T.; Aitken, D.; Blizzard, L.; et al. Depression in Patients with Knee Osteoarthritis: Risk Factors and Associations with Joint Symptoms. BMC Musculoskelet. Disord. 2021, 22, 40. [Google Scholar] [CrossRef]
- Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30496104/ (accessed on 10 April 2023).
- Sowers, M.R.; Karvonen-Gutierrez, C.A. The Evolving Role of Obesity in Knee Osteoarthritis. Curr. Opin. Rheumatol. 2010, 22, 533–537. [Google Scholar] [CrossRef]
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The Role of Metabolism in the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Dennison, E.M. Osteoarthritis: The Importance of Hormonal Status in Midlife Women. Maturitas 2022, 165, 8–11. [Google Scholar] [CrossRef]
- Young, D.A.; Barter, M.J.; Soul, J. Osteoarthritis Year in Review: Genetics, Genomics, Epigenetics. Osteoarthr. Cartil. 2022, 30, 216–225. [Google Scholar] [CrossRef]
- Kulkarni, P.; Martson, A.; Vidya, R.; Chitnavis, S.; Harsulkar, A. Pathophysiological Landscape of Osteoarthritis. Adv. Clin. Chem. 2021, 100, 37–90. [Google Scholar] [PubMed]
- Pathophysiological Perspective of Osteoarthritis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33207632/ (accessed on 10 April 2023).
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Roos, E.M. Physical Therapy for Patients with Knee and Hip Osteoarthritis: Supervised, Active Treatment Is Current Best Practice. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 112–117. [Google Scholar]
- Cooper, C.; Chapurlat, R.; Al-Daghri, N.; Herrero-Beaumont, G.; Bruyère, O.; Rannou, F.; Roth, R.; Uebelhart, D.; Reginster, J.-Y. Safety of Oral Non-Selective Non-Steroidal Anti-Inflammatory Drugs in Osteoarthritis: What Does the Literature Say? Drugs Aging 2019, 36, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Renkawitz, T.; Voellner, F.; Craiovan, B.; Greimel, F.; Worlicek, M.; Grifka, J.; Benditz, A. Revision Surgery in Total Joint Replacement Is Cost-Intensive. Biomed. Res. Int. 2018, 2018, 8987104. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.H.; Fitzgerald, J. Current Issues in Joint Replacement Surgery. Curr. Opin. Rheumatol. 2006, 18, 163–169. [Google Scholar] [CrossRef]
- Abbott, J.H.; Robertson, M.C.; McKenzie, J.E.; Baxter, G.D.; Theis, J.-C.; Campbell, A.J. MOA Trial team Exercise Therapy, Manual Therapy, or Both, for Osteoarthritis of the Hip or Knee: A Factorial Randomised Controlled Trial Protocol. Trials 2009, 10, 11. [Google Scholar] [CrossRef]
- Sukerkar, P.A.; Doyle, Z. Imaging of Osteoarthritis of the Knee. Radiol. Clin. N. Am. 2022, 60, 605–616. [Google Scholar] [CrossRef]
- Puntmann, V.O. How-to Guide on Biomarkers: Biomarker Definitions, Validation and Applications with Examples from Cardiovascular Disease. Postgrad. Med. J. 2009, 85, 538–545. [Google Scholar] [CrossRef]
- Lamers, R.J.a.N.; van Nesselrooij, J.H.J.; Kraus, V.B.; Jordan, J.M.; Renner, J.B.; Dragomir, A.D.; Luta, G.; van der Greef, J.; DeGroot, J. Identification of an Urinary Metabolite Profile Associated with Osteoarthritis. Osteoarthr. Cartil. 2005, 13, 762–768. [Google Scholar] [CrossRef]
- Li, J.-T.; Zeng, N.; Yan, Z.-P.; Liao, T.; Ni, G.-X. A Review of Applications of Metabolomics in Osteoarthritis. Clin. Rheumatol. 2021, 40, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; He, Z.; Kong, Y.; Liu, Z.; Gong, L. Insight into Osteoarthritis through Integrative Analysis of Metabolomics and Transcriptomics. Clin. Chim. Acta 2020, 510, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.B.; Setton, L.A.; Nettles, D.L. The Role of Metabolomics in Osteoarthritis Research. J. Am. Acad. Orthop. Surg. 2013, 21, 63–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Showiheen, S.A.A.; Sun, A.R.; Wu, X.; Crawford, R.; Xiao, Y.; Wellard, R.M.; Prasadam, I. Application of Metabolomics to Osteoarthritis: From Basic Science to the Clinical Approach. Curr. Rheumatol. Rep. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Wang, X.; Cai, Z. Mass Spectrometry-Based Metabolomics: Targeting the Crosstalk between Gut Microbiota and Brain in Neurodegenerative Disorders. Mass. Spectrom. Rev. 2019, 38, 22–33. [Google Scholar] [CrossRef]
- Rispoli, M.G.; Valentinuzzi, S.; De Luca, G.; Del Boccio, P.; Federici, L.; Di Ioia, M.; Digiovanni, A.; Grasso, E.A.; Pozzilli, V.; Villani, A.; et al. Contribution of Metabolomics to Multiple Sclerosis Diagnosis, Prognosis and Treatment. Int. J. Mol. Sci. 2021, 22, 11112. [Google Scholar] [CrossRef]
- Zhai, G. The Role of Metabolomics in Precision Medicine of Osteoarthritis: How Far Are We? Osteoarthr. Cartil. Open 2021, 3, 100170. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tao, S.; Lin, Y. Target metabolomics of knee osteoarthritis in middle-aged and old people in Harbin. J. Harbin Med. Univ. 2021, 55, 522–527. [Google Scholar]
- Shao, Q.; Zhou, X.; Zhang, Y.; Wu, B.; Wang, S. Metabolomic Study on Serum of Liver-Kidney Deficiency Syndrome of Knee-Joint Osteoarthritis Based on 1H-NMR. Chin. J. Inf. Tradit. Chin. Med. 2017, 24, 27–31. [Google Scholar]
- Zhang, Y. Study on Plasma Metabolomics of Odorized Solitary Parasitic Mixture on Rheumatic and Paralytic Knee Osteoarthritis. Master’s Thesis, Hunan University of Chinese Medicine, Changsha, China, 2020. [Google Scholar]
- Yang, S.; Li, X.; Su, M.; Qiu, Y.; Jia, W.; Chu, L. Correlativity Between Urinary Metabolite Profiles and Chinese Medical Syndromes in Knee Osteoarthritis Patients. J. Shanghai Univ. Tradit. Chin. Med. 2009, 23, 33–37. [Google Scholar]
- Kuang, T.; Zhang, Y.; Shen, F.; Lu, M.; Kuang, G. Metabonomics Study on Knee Osteoarthritis (Liver and Kidney Deficiency Syndrome) Based on GC-MS. Liaoning J. Tradit. Chin. Med. 2021, 48, 10–14. [Google Scholar]
- Abdelrazig, S. Metabolic Signatures of Osteoarthritis in Urine Using Liquid Chromatography-high Resolution Tandem Mass Spectrometry. Metabolomics 2021, 17, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Likhodii, S.; Liu, M.; Aref-Eshghi, E.; Harper, P.E.; Martin, G.; Furey, A.; Green, R.; Randell, E.; et al. Metabolomic Analysis of Human Plasma Reveals That Arginine Is Depleted in Knee Osteoarthritis Patients. Osteoarthr. Cartil. 2016, 24, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Shen, N.; Chen, H.; Ni, S.; Zhang, T.; Hu, M.; Wang, J.; Sun, L.; Yang, X. Global and Targeted Metabolomics of Synovial Fluid Discovers Special Osteoarthritis Metabolites: Metabolomics discovers OA metabolites. J. Orthop. Res. 2017, 35, 1973–1981. [Google Scholar] [CrossRef]
- Pertusa, C.; Mifsut, D.; Morales, J.M.; Tarín, J.J.; Cano, A.; Monleón, D.; García-Pérez, M.Á. Metabolomic Analysis of Severe Osteoarthritis in a Spanish Population of Women Compared to Healthy and Osteoporotic Subjects. Metabolites 2022, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Tootsi, K.; Vilba, K.; Märtson, A.; Kals, J.; Paapstel, K.; Zilmer, M. Metabolomic Signature of Amino Acids and Polyamines in the Serum of Osteoarthritis Patients. Osteoarthr. Cartil. 2021, 29, S372–S373. [Google Scholar] [CrossRef]
- Chen, R.; Han, S.; Liu, X.; Wang, K.; Zhou, Y.; Yang, C.; Zhang, X. Perturbations in Amino Acids and Metabolic Pathways in Osteoarthritis Patients Determined by Targeted Metabolomics Analysis. J. Chromatogr. B 2018, 1085, 54–62. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Zhang, Z.; Yang, F.; Chen, J. Serum Metabolites as Potential Biomarkers for Diagnosis of Knee Osteoarthritis. Dis. Markers 2015, 2015, 684794. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Qiu, Y.; Zhao, T.; Chen, T.; Su, M.; Chu, L.; Lv, A.; Liu, P.; Jia, W. Urinary Metabolomics as a Potentially Novel Diagnostic and Stratification Tool for Knee Osteoarthritis. Metabolomics 2010, 6, 109–118. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Bai, Y.; Gong, X.; Dou, C.; Cao, Z.; Dong, S. Redox Control of Chondrocyte Differentiation and Chondrogenesis. Free Radic. Biol. Med. 2019, 132, 83–89. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- June, R.K.; Liu-Bryan, R.; Long, F.; Griffin, T.M. Emerging Role of Metabolic Signaling in Synovial Joint Remodeling and Osteoarthritis. J. Orthop. Res. 2016, 34, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Terwilliger, E.F.; Kohn, D.; Madry, H. Remodelling of Human Osteoarthritic Cartilage by FGF-2, Alone or Combined with Sox9 via rAAV Gene Transfer. J. Cell Mol. Med. 2009, 13, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The Role of Metabolism in Chondrocyte Dysfunction and the Progression of Osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, W.; Luo, W.; Zeng, C.; Deng, Z.; Ren, W.; Wu, G.; Lei, G. Alterations of Amino Acid Metabolism in Osteoarthritis: Its Implications for Nutrition and Health. Amino Acids 2016, 48, 907–914. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C.-A.A.; Kovacs-Nolan, J.; Mine, Y. Bioactive Dietary Peptides and Amino Acids in Inflammatory Bowel Disease. Amino Acids 2015, 47, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Bartenbach, L.; Karall, T.; Koch, J.; Keller, M.A.; Oberacher, H.; Scholl-Bürgi, S.; Karall, D.; Oemer, G.; Baumgartner, D.; Meinel, K.; et al. Amino Acid and Phospholipid Metabolism as an Indicator of Inflammation and Subtle Cardiomyopathy in Patients with Marfan Syndrome. Metabolites 2021, 11, 805. [Google Scholar] [CrossRef]
- Jian, H.; Xu, Q.; Wang, X.; Liu, Y.; Miao, S.; Li, Y.; Mou, T.; Dong, X.; Zou, X. Amino Acid and Fatty Acid Metabolism Disorders Trigger Oxidative Stress and Inflammatory Response in Excessive Dietary Valine-Induced NAFLD of Laying Hens. Front. Nutr. 2022, 9, 849767. [Google Scholar] [CrossRef]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collée, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 Signaling Leads to Increased Activation of the NLRP3 Inflammasome in Osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef]
- Tang, J.; Liu, T.; Wen, X.; Zhou, Z.; Yan, J.; Gao, J.; Zuo, J. Estrogen-Related Receptors: Novel Potential Regulators of Osteoarthritis Pathogenesis. Mol. Med. 2021, 27, 5. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages Regulate the Progression of Osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ye, J.; Shi, R.; Zhao, B.; Liu, Z.; Lin, W.; Liu, X. Dietary Protein and Amino Acid Restriction: Roles in Metabolic Health and Aging-Related Diseases. Free Radic. Biol. Med. 2022, 178, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Ny, V.; Needham, T.; Ceacero, F. Potential Benefits of Amino Acid Supplementation for Cervid Performance and Nutritional Ecology, with Special Focus on Lysine and Methionine: A Review. Anim. Nutr. 2022, 11, 391–401. [Google Scholar] [CrossRef]
- Sperringer, J.E.; Addington, A.; Hutson, S.M. Branched-Chain Amino Acids and Brain Metabolism. Neurochem. Res. 2017, 42, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Tonomura, H.; Takahashi, K.A.; Mazda, O.; Arai, Y.; Inoue, A.; Terauchi, R.; Shin-Ya, M.; Kishida, T.; Imanishi, J.; Kubo, T. Glutamine Protects Articular Chondrocytes from Heat Stress and NO-Induced Apoptosis with HSP70 Expression. Osteoarthr. Cartil. 2006, 14, 545–553. [Google Scholar] [CrossRef]
- Remst, D.F.G.; Blaney Davidson, E.N.; van der Kraan, P.M. Unravelling Osteoarthritis-Related Synovial Fibrosis: A Step Closer to Solving Joint Stiffness. Rheumatology 2015, 54, 1954–1963. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Z.; Li, Q.; Chen, C.; Luo, Y.; Kang, P. Activated Intestinal Microbiome-Associated Tryptophan Metabolism Upregulates Aryl Hydrocarbon Receptor to Promote Osteoarthritis in a Rat Model. Int. Immunopharmacol. 2023, 118, 110020. [Google Scholar] [CrossRef]
- Stratz, C.; Anakwue, J.; Bhatia, H.; Pitz, S.; Fiebich, B.L. Anti-Inflammatory Effects of 5-HT3 Receptor Antagonists in Interleukin-1beta Stimulated Primary Human Chondrocytes. Int. Immunopharmacol. 2014, 22, 160–166. [Google Scholar] [CrossRef]
- Ohashi, Y.; Uchida, K.; Fukushima, K.; Inoue, G.; Takaso, M. Mechanisms of Peripheral and Central Sensitization in Osteoarthritis Pain. Cureus 2023, 15, e35331. [Google Scholar] [CrossRef]
- Haleem, D.J. Serotonin-1A Receptor Dependent Modulation of Pain and Reward for Improving Therapy of Chronic Pain. Pharmacol. Res. 2018, 134, 212–219. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Li, G. Molecular Insights Into Lysyl Oxidases in Cartilage Regeneration and Rejuvenation. Front. Bioeng. Biotechnol. 2020, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef] [PubMed]
- Blicharski, T.; Tomaszewska, E.; Dobrowolski, P.; Hułas-Stasiak, M.; Muszyński, S. A Metabolite of Leucine (β-Hydroxy-β-Methylbutyrate) given to Sows during Pregnancy Alters Bone Development of Their Newborn Offspring by Hormonal Modulation. PLoS ONE 2017, 12, e0179693. [Google Scholar] [CrossRef]
- Nishizaki, K.; Ikegami, H.; Tanaka, Y.; Imai, R.; Matsumura, H. Effects of Supplementation with a Combination of β-Hydroxy-β-Methyl Butyrate, L-Arginine, and L-Glutamine on Postoperative Recovery of Quadriceps Muscle Strength after Total Knee Arthroplasty. Asia Pac. J. Clin. Nutr. 2015, 24, 412–420. [Google Scholar] [PubMed]
- Andrade, V.S.; Rojas, D.B.; de Andrade, R.B.; Kim, T.D.H.; Vizuete, A.F.; Zanatta, Â.; Wajner, M.; Gonçalves, C.-A.S.; Wannmacher, C.M.D. A Possible Anti-Inflammatory Effect of Proline in the Brain Cortex and Cerebellum of Rats. Mol. Neurobiol. 2018, 55, 4068–4077. [Google Scholar] [CrossRef]
- Roecker, R.; Junges, G.M.; de Lima, D.D.; da Cruz, J.G.P.; Wyse, A.T.S.; Dal Magro, D.D. Proline Alters Antioxidant Enzyme Defenses and Lipoperoxidation in the Erythrocytes and Plasma of Rats: In Vitro and in Vivo Studies. Biol. Trace Elem. Res. 2012, 147, 172–179. [Google Scholar] [CrossRef]
- Li, P.; He, W.; Wu, G. Composition of Amino Acids in Foodstuffs for Humans and Animals. Adv. Exp. Med. Biol. 2021, 1332, 189–210. [Google Scholar] [CrossRef]
- Shrode, R.L.; Cady, N.; Jensen, S.N.; Borcherding, N.; Mangalam, A.K. Isoflavone Consumption Reduces Inflammation through Modulation of Phenylalanine and Lipid Metabolism. Metabolomics 2022, 18, 84. [Google Scholar] [CrossRef]
- Lawson, B.R.; Belkowski, S.M.; Whitesides, J.F.; Davis, P.; Lawson, J.W. Immunomodulation of Murine Collagen-Induced Arthritis by N, N-Dimethylglycine and a Preparation of Perna Canaliculus. BMC Complement. Altern. Med. 2007, 7, 20. [Google Scholar] [CrossRef]
- Mao, Y.; Shi, D.; Li, G.; Jiang, P. Citrulline Depletion by ASS1 Is Required for Proinflammatory Macrophage Activation and Immune Responses. Mol. Cell 2022, 82, 527–541.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Pi, D.; Leng, W.; Wang, X.; Hu, C.-A.A.; Hou, Y.; Xiong, J.; Wang, C.; Qin, Q.; Liu, Y. Asparagine Preserves Intestinal Barrier Function from LPS-Induced Injury and Regulates CRF/CRFR Signaling Pathway. Innate Immun. 2017, 23, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Ravagna, A.; Colombrita, C.; Scapagnini, G.; Guagliano, E.; Calvani, M.; Butterfield, D.A.; Giuffrida Stella, A.M. Acetylcarnitine Induces Heme Oxygenase in Rat Astrocytes and Protects against Oxidative Stress: Involvement of the Transcription Factor Nrf2. J. Neurosci. Res. 2005, 79, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-Y.; Kim, H.W.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Creatinine-Cystatin C Ratio and Mortality in Cancer Patients: A Retrospective Cohort Study. J. Cachexia Sarcopenia Muscle 2022, 13, 2064–2072. [Google Scholar] [CrossRef]
- Park, H.M.; Kim, H.J.; Lee, B.; Kwon, M.; Jung, S.M.; Lee, S.-W.; Park, Y.-B.; Song, J.J. Decreased Muscle Mass Is Independently Associated with Knee Pain in Female Patients with Radiographically Mild Osteoarthritis: A Nationwide Cross-Sectional Study (KNHANES 2010–2011). Clin. Rheumatol. 2018, 37, 1333–1340. [Google Scholar] [CrossRef]
- Mitoma, T.; Maki, J.; Ooba, H.; Eto, E.; Takahashi, K.; Kondo, T.; Ikeda, T.; Sakamoto, Y.; Mitsuhashi, T.; Masuyama, H. Protocol for a Randomised, Placebo-Controlled, Double-Blinded Clinical Trial on the Effect of Oestrogen Replacement on Physical Performance to Muscle Resistance Exercise for Older Women with Osteoarthritis of Knee Joint: The EPOK Trial. BMC Geriatr. 2023, 23, 104. [Google Scholar] [CrossRef]
- Akram, M. Citric Acid Cycle and Role of Its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Johnson, K.; Murphy, A.; Ghosh, S. Invited Review: The Mitochondrion in Osteoarthritis. Mitochondrion 2002, 1, 301–319. [Google Scholar] [CrossRef]
- Role of Cholesterol and Lipid Organization in Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16319881/ (accessed on 8 September 2023).
- Association of Plasma N-6 and n-3 Polyunsaturated Fatty Acids with Synovitis in the Knee: The MOST Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22353693/ (accessed on 8 September 2023).
- Zhou, M.; Guo, Y.; Wang, D.; Shi, D.; Li, W.; Liu, Y.; Yuan, J.; He, M.; Zhang, X.; Guo, H.; et al. The Cross-Sectional and Longitudinal Effect of Hyperlipidemia on Knee Osteoarthritis: Results from the Dongfeng-Tongji Cohort in China. Sci. Rep. 2017, 7, 9739. [Google Scholar] [CrossRef]
- Bao, C.; Zhu, S.; Song, K.; He, C. HK2: A Potential Regulator of Osteoarthritis via Glycolytic and Non-Glycolytic Pathways. Cell Commun. Signal 2022, 20, 132. [Google Scholar] [CrossRef]
- Cao, X.; Cui, Z.; Ding, Z.; Chen, Y.; Wu, S.; Wang, X.; Huang, J. An Osteoarthritis Subtype Characterized by Synovial Lipid Metabolism Disorder and Fibroblast-like Synoviocyte Dysfunction. J. Orthop. Translat. 2022, 33, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Platt, N.; Thorpe, C.; Shakibaei, M. Regulation of 2-Deoxy-D-Glucose Transport, Lactate Metabolism, and MMP-2 Secretion by the Hypoxia Mimetic Cobalt Chloride in Articular Chondrocytes. Ann. N. Y. Acad. Sci. 2006, 1091, 83–93. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Country | Sample | Sample Size (n = M/F) | BMI | Age (OA/HC) | Metabolomics Technique | NOS | ||
|---|---|---|---|---|---|---|---|---|---|
| OA | HC | OA | HC | ||||||
| Clara Pertusa et al. (2022) [46] | Spain | Blood serum | 53 | 23 | 27.66 ± 5.53 | 28.04 ± 4.83 | 76.00 ± 9.49/70.02 ± 7.13 | NMR | 7 |
| Shengyu Wang et al. (2021) [38] | China | Blood serum | 40/36 | 35/36 | NG | NG | 70.5 (50–75)/61.0 (50–75) | UPLC-TQ-MS | 7 |
| Tao Kuang et al. (2021) [42] | China | Urine | 10/15 | 10/15 | NG | NG | 42–70 (M); 40–66 (F)/40–60 (M); 41–68 (F) | GC-MS | 5 |
| Salah Abdelrazig et al. (2021) [43] | UK | Urine | 26/48 | 30/38 | 30.23 | 28.34 | 68 (50–91)/68 (52–88) | LC-HRMS | 8 |
| K. Tootsi et al. (2021) [47] | Estonia | Blood serum | 36/34 | 38/44 | 27.8 ± 3.2 | 26.0 ± 3.5 | 62 ±7/61 ± 8 | LC- and FIA-MS (AbsoluteIDQ™ p180) | 8 |
| Yi Zhang (2020) [40] | China | Blood serum | 14/16 | 15/15 | NG | NG | 63.45 ± 8.53/67.86 ± 7.45 | GC-MS | 7 |
| Rui Chen et al. (2018) [48] | China | Blood serum | 3 15/17 | 17/18 | 25.3 ± 2.3 | 24.3 ± 3.0 | 56.4 ± 3.6/54.8 ± 4.5 | UPLC-TQ-MS | 7 |
| Qin Shao et al. (2017) [39] | China | Blood serum | 31/69 | 7/13 | 23.4 ± 5.8 | 22.6 ± 2.7 | 62.7 ± 8.6/61.9 ± 4.1 | 1H-NMR | 7 |
| Kaidi Zheng et al. (2016) [45] | China | knee synovial fluid | 49 | 21 | NG | NG | NG | GC-TOF/MS; LC/MS | 7 |
| Qingmeng Zhang et al. (2015) [49] | China | Blood serum | 20/20 | 10/10 | 28.2 ± 3.4 | 24.2 ± 2.3 | 58.1 ± 6.93/56.3 ± 7. 9 | UPLC-MS | 7 |
| W. Zhang et al. (2016) [44] | Canada | Blood serum | 64 | 45 | 33.9 ± 7.3 | 30.09 ± 6.7 | 65 ± 7/46 ± 8 | UPLC | 6 |
| Xin Li et al. (2010) [50] | China | Urine | 7/30 | 11/26 | 25.1 ± 3.2 | 23.8 ± 1.9 | 56.8 ± 7.2/56.3 ± 7.9 | GC–MS | 8 |
| Songbin Yang et al. (2009) [41] | China | Urine | 7/30 | 11/26 | 25.13 ± 3.25 | 24.06 ± 2.11 | 56.8 ± 7.1/56.0 ± 7.4 | GC-MS | 7 |
| Trend | Differential Small Molecule Metabolites Name | |||
|---|---|---|---|---|
| Blood Samples | Urine Samples | Knee Synovial Fluid | ||
| Upward | Leucine, 4-Hydroxy-L-proline, L-Tryptophan, Lysine, Succinic acid, 2-Ketoisopropionic acid, 3-Carboxy-4-methyl-2-oxopentanoate, Acetic acid, Acetone, ADMA, fatty acidsC16: 0, fatty acidsC18: 0, fatty acidsC18: 2, fatty acidsC18: 4, fatty acidsC20: 3, fatty acidsC20: 4, fatty acidsC22: 5, fatty acidsC22: 6, fatty acid, Galactose, Glucose, Glycerol, Homocysteine, Isoleucine, Lipid, LysoPC a C16:0, LysoPC a C18:0, Methionine, Methyl-hippuric acid, N-Acetylgalactosamine, Pyruvate, Ribotide, Spermidine | 4-hydroxy hippurate, Acetoacetic acid, Aconitic acid, Histamine, Homovanillic acid, Isocitric acid, Succinic acid, 2,3-Diaminopropionic acid, 2-Keto-glutaramic acid, 3-Nitrotyrosine, 4-Methyleneproline, 4-Methylproline, Acetylphosphate, D-glucose, Fumarate, Mannitol, Phosphoric acid, Prolyl-Glutamate, Ribose, S-Lactoylglutathione, Suberic acid, Urate | 1,5-Anhydroglucitol, 8-Aminocaprylic acid, Gluconic, lactone, Tyramine | |
| Downward | Citrulline, Creatinine, Phenylalanine, Acetylcarnitine, Asparagine, Dimethylglycine, Inositol, 2-aminobutyrate, 4-aminobutyrate, 4-Oxoproline, Acetate, Aminomalonic acid, Dimethylamine, fatty acidsC18: 1, fatty acidsC20: 2, glutamic acid, L-Glycine, L-Histidine, LysoPC(18:0), LysoPC a C28:1, N(CH3)3, Ornithine, Hydroxyproline, PC aa C36:6, PC ae C36:2, PC ae C38:0, Phosphocholine, Propionyl-L-carnitine, Pyridoxine, SM (OH) C14:1, SM (OH) C22:1, SM (OH) C22:2, SM (OH) C24:1, SM C16:0, SM C16:1, SM C24:0, Sphingomyelin (d18:1/16:0), Taurine, α-Aminobutyric acid, α-glucose, β-glucose | Creatinine, 4-methyl phenol, Hippuric acid, Inositol, 2-Hydroxyhippuric acid, 3-Methoxyphenylacetic acid, 3-Methylcrotonylglycine, 3-Oxoalanine, 4-Hydroxybutyric acid, Aminoadipic acid, Cytosine, Homocysteine sulphinic acid, Hydroxykynurenine, L-Homoserine, N-Acetyl-L-glutamate 5-semialdehyde, N-Phenylacetyl-L-glutamine, Pipecolic acid, Tartaric acid | ||
| Inconsistent | Upward | Glycine, Histidine, Serine, Tyrosine, Valine, γ-Aminobutyric acid, Alanine, Arginine, Tryptophan, Creatine, Hypoxanthine, Pyruvic acid, Lactate | Proline, Citric acid | Glutamine, Threonine |
| Downward | Glycine, Glutamine, Histidine, Proline, Threonine, Serine, Tyrosine, Valine, γ-Aminobutyric acid, Alanine, Arginine, Creatine, Hypoxanthine, Pyruvic acid, Lactate | Glycine, Glutamine, Histidine, Threonine, Citric acid, Tryptophan | ||
| Metabolite Name | Studies for Synthesis | MD (μmol/L) | 95%CI |
|---|---|---|---|
| Tryptophan | 2 (1, 2) | 27.35 | [9.58,45.13] |
| Lysine | 2 (1, 2) | 193.99 | [161.36,226.63] |
| Leucine | 4 (1, 2, 3, 4) | 67.36 | [28.24,106.47] |
| Proline | 2 (1, 2) | −94.05 | [−109.88, −78.22] |
| Phenylalanine | 2 (1, 2) | −47.12 | [−52.55, −41.70] |
| Glutamine | 2 (1, 2) | −115.66 | [−230.27, −1.04] |
| Dimethylglycine | 2 (1, 2) | −3.49 | [−4.49, −2.49] |
| Creatinine | 2 (1, 3) | −38.68 | [−58.31, −19.05] |
| Citrulline | 3 (1, 2, 3) | −21.70 | [−29.25, −14.16] |
| Asparagine | 2 (1, 2) | −28.96 | [−28.05, −16.79] |
| Acetylcarnitine | 2 (1, 2) | −6.46 | [−7.41, −5.51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.; Han, X.; Wang, Y.; Shi, J.; Zhang, Y.; Zhao, H.; Zhang, L.; Jiang, M.; Liu, M. Differential Metabolites in Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4191. https://doi.org/10.3390/nu15194191
Liao Z, Han X, Wang Y, Shi J, Zhang Y, Zhao H, Zhang L, Jiang M, Liu M. Differential Metabolites in Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(19):4191. https://doi.org/10.3390/nu15194191
Chicago/Turabian StyleLiao, Zeqi, Xu Han, Yuhe Wang, Jingru Shi, Yuanyue Zhang, Hongyan Zhao, Lei Zhang, Miao Jiang, and Meijie Liu. 2023. "Differential Metabolites in Osteoarthritis: A Systematic Review and Meta-Analysis" Nutrients 15, no. 19: 4191. https://doi.org/10.3390/nu15194191
APA StyleLiao, Z., Han, X., Wang, Y., Shi, J., Zhang, Y., Zhao, H., Zhang, L., Jiang, M., & Liu, M. (2023). Differential Metabolites in Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients, 15(19), 4191. https://doi.org/10.3390/nu15194191






