Redefining Nutritional Requirements in End-Stage Liver Disease: Towards a Personalized Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Nutritional Assessment
2.3. Energy Expenditure
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Difference between Measured Resting Energy Expenditure and Estimation of Energy Requirements
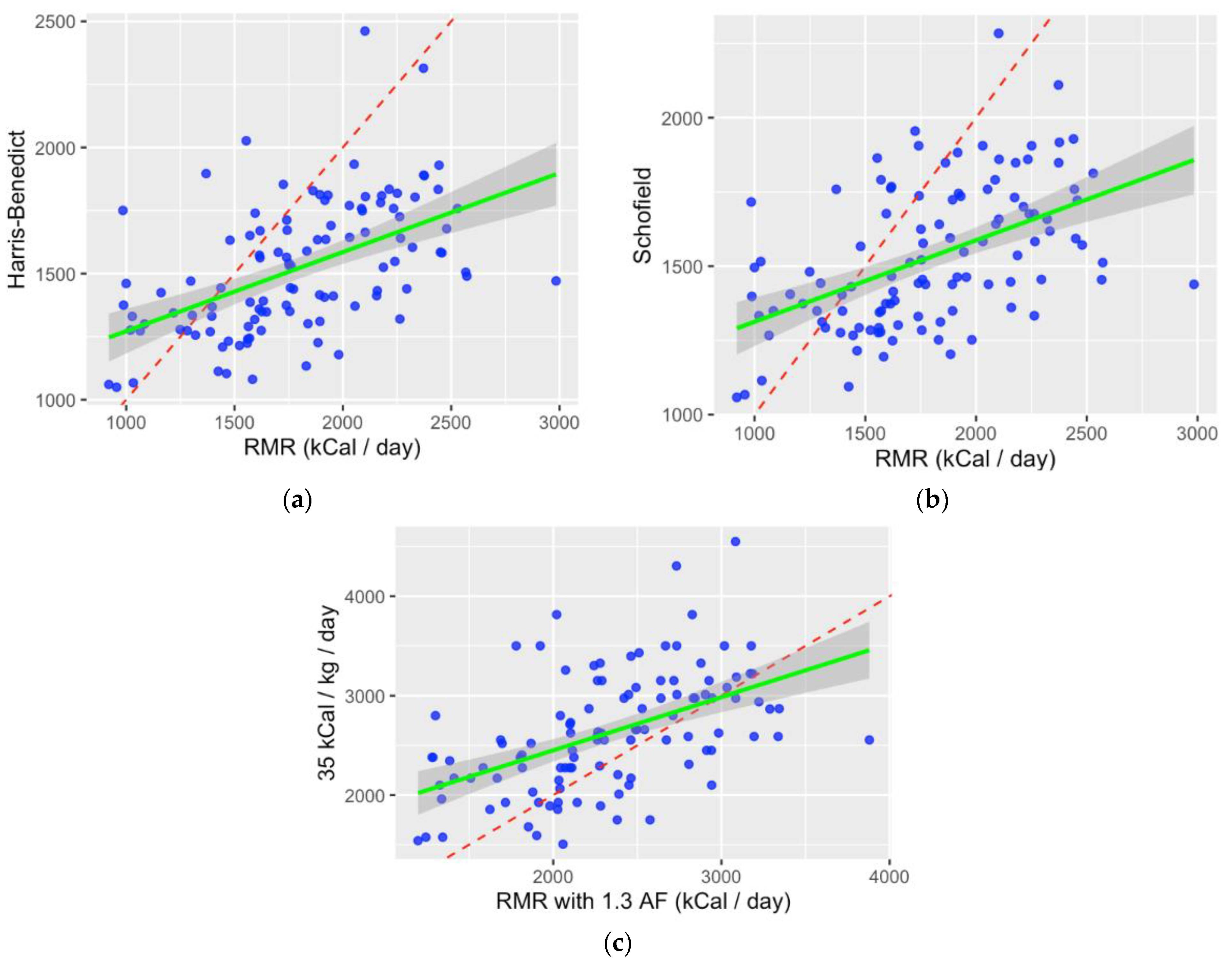
3.3. Correlation between Measured and Predicted Energy Expenditure
3.4. Parameters Associated with Hypermetabolism
3.5. Development of a New Prediction Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, B.; Goh, S.K.; Parker, F.; Romero, S.; Sinclair, M.; Gow, P.; Ma, R.; Angus, P.; Jones, R.; Luke, J.; et al. Malnutrition and low muscle strength are independent predictors of clinical outcomes and healthcare costs after liver transplant. Clin. Nutr. ESPEN 2022, 48, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Giusto, M.; Gentili, F.; Novelli, G.; Ferretti, G.; Riggio, O.; Corradini, S.G.; Siciliano, M.; Farcomeni, A.; Attili, A.F.; et al. Nutritional status: Its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010, 30, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kalafateli, M.; Mantzoukis, K.; Choi Yau, Y.; Mohammad, A.O.; Arora, S.; Rodrigues, S.; de Vos, M.; Papadimitriou, K.; Thorburn, D.; O’Beirne, J.; et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J. Cachexia Sarcopenia Muscle 2017, 8, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.; Sharpe, M.D.; Lowndes, R.; Ghent, C.N. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation 1994, 57, 469–472. [Google Scholar] [CrossRef]
- Vidot, H.; Bowen, D.G.; Carey, S.; McCaughan, G.W.; Allman-Farinelli, M.; Shackel, N.A. Aggressive nutrition intervention reduces ascites and frequency of paracentesis in malnourished patients with cirrhosis and ascites. JGH Open 2017, 1, 92–97. [Google Scholar] [CrossRef]
- Le Cornu, K.A.; McKierman, F.J.; Kaadia, S.A.; Neuberger, J.M. A prospective randomised study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation 2000, 69, 1364–1369. [Google Scholar] [CrossRef]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef]
- Cabre, E.; Gonzalez-Huix, F.; Abad-Lacruz, A.; Esteve, M.; Acero, D.; Fernandez-Banares, F.; Xiol, X.; Gassull, M.A. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology 1990, 98, 715–720. [Google Scholar] [CrossRef]
- Antar, R.; Wong, P.; Ghali, P. A Meta-Analysis of Nutritional Supplementation for Management of Hospitalized Alcoholic Hepatitis. Can. J. Gastroenterol. 2012, 26, 945707. [Google Scholar] [CrossRef]
- Fialla, A.D.; Israelsen, M.; Hamberg, O.; Krag, A.; Gluud, L.L. Nutritional therapy in cirrhosis or alcoholic hepatitis: A systematic review and meta-analysis. Liver Int. 2015, 35, 2072–2078. [Google Scholar] [CrossRef]
- Koretz, R.L.; Avenell, A.; Lipman, T.O. Nutritional support for liver disease. Cochrane Database Syst. Rev. 2012, 5, CD008344. [Google Scholar] [CrossRef] [PubMed]
- Ney, M.; Vandermeer, B.; van Zanten, S.J.V.; Ma, M.M.; Gramlich, L.; Tandon, P. Meta-analysis: Oral or enteral nutrition supplementation in cirrhosis. Aliment. Pharmacol. Ther. 2013, 37, 672–679. [Google Scholar] [CrossRef]
- Chapman, B.; Testro, A.; Gow, P.; Whitcher, B.; Sinclair, M. Determining Energy Requirements in Cirrhosis: An Update on the Role of Indirect Calorimetry. Curr. Hepatol. Rep. 2021, 20, 85–95. [Google Scholar] [CrossRef]
- EASL. Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Basal Metabolism in Man; Carnegie Institute: Washington, DC, USA, 1919. [Google Scholar]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. 1), 5–41. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Sonnenday, C.; Tapper, E.B.; Tandon, P.; Duarte-Rojo, A.; Dunn, M.A.; Tsien, C.; Kallwitz, E.R.; Ng, V.; et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019, 70, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Feng, S.; Covinsky, K.E.; Hayssen, H.; Zhou, L.Q.; Yeh, B.M.; Lai, J.C. A Comparison of Muscle Function, Mass, and Quality in Liver Transplant Candidates: Results From the Functional Assessment in Liver Transplantation Study. Transplantation 2016, 100, 1692–1698. [Google Scholar] [CrossRef]
- Lai, J.C.; Dodge, J.L.; Kappus, M.R.; Dunn, M.A.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; Rahimi, R.S.; McCulloch, C.E.; Haugen, C.E.; et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J. Hepatol. 2020, 73, 575–581. [Google Scholar] [CrossRef]
- Lai, J.C.; Dodge, J.L.; Sen, S.; Covinsky, K.; Feng, S. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016, 63, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Frias, C.; Conchillo, M.; Payeras, M.; Inarrairaegui, M.; Davola, D.; Fruhbeck, G.; Salvador, J.; Rodriguez, M.; Richter, J.A.; Mugueta, C.; et al. Factors related to increased resting energy expenditure in men with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Santos, L.F.; Silva, T.R.; Anastacio, L.R.; Lima, A.S.; Correia, M.I. Hyper- and hypometabolism are not related to nutritional status of patients on the waiting list for liver transplantation. Clin. Nutr. 2014, 33, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Schock, L.; Lam, L.; Tandon, P.; Taylor, L.; Raman, M. Indirect Calorimetry Performance Using a Handheld Device Compared to the Metabolic Cart in Outpatients with Cirrhosis. Nutrients 2019, 11, 1030. [Google Scholar] [CrossRef]
- Bot, D.; Droop, A.; Tushuizen, M.E.; Van Hoek, B. For dietary advice in end-stage liver cirrhosis resting metabolic rate should be measured, not estimated. Hepatoma Res. 2020, 2020, 45. [Google Scholar] [CrossRef]
- Meng, Q.H.; Hou, W.; Yu, H.W.; Lu, J.; Li, J.; Wang, J.H.; Zhang, F.Y.; Zhang, J.; Yao, Q.W.; Wu, J.; et al. Resting energy expenditure and substrate metabolism in patients with acute-on-chronic hepatitis B liver failure. J. Clin. Gastroenterol. 2011, 45, 456–461. [Google Scholar] [CrossRef]
- Teramoto, A.; Yamanaka-Okumura, H.; Urano, E.; Nakamura-Kutsuzawa, T.; Sugihara, K.; Katayama, T.; Miyake, H.; Imura, S.; Utsunomiya, T.; Shimada, M.; et al. Comparison of measured and predicted energy expenditure in patients with liver cirrhosis. Asia Pac. J. Clin. Nutr. 2014, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hasson, R.E.; Howe, C.A.; Jones, B.L.; Freedson, P.S. Accuracy of four resting metabolic rate prediction equations: Effects of sex, body mass index, age, and race/ethnicity. J. Sci. Med. Sport 2011, 14, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.W.; Krag, A.; Nordgaard-Lassen, I.; Frandsen, E.; Tofteng, F.; Mortensen, C.; Becker, U. Effect of paracentesis on metabolic activity in patients with advanced cirrhosis and ascites. Scand. J. Gastroenterol. 2016, 51, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Vandermeer, B.; Raman, M.; Gramlich, L.; Den Heyer, V.; Belland, D.; Ma, M.; Tandon, P. Are Predictive Energy Expenditure Equations Accurate in Cirrhosis? Nutrients 2019, 11, 334. [Google Scholar] [CrossRef]
- Mathur, S.; Peng, S.; Gane, E.J.; McCall, J.L.; Plank, L.D. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition 2007, 23, 398–403. [Google Scholar] [CrossRef]
- Kato, M.; Miwa, Y.; Tajika, M.; Hiraoka, T.; Muto, Y.; Moriwaki, H. Preferential use of branched-chain amino acids as an energy substrate in patients with liver cirrhosis. Intern. Med. 1998, 37, 429–434. [Google Scholar] [CrossRef]
- Mansour, A.; Motamed, S.; Hekmatdoost, A.; Karimi, S.; Mohajeri-Tehrani, M.R.; Abdollahi, M.; Jelodar, R.; Sajjadi-Jazi, S.M. Factors related to hypermetabolism in individuals with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Sci. Rep. 2023, 13, 3669. [Google Scholar] [CrossRef]
- Kalaitzakis, E.; Bosaeus, I.; Ohman, L.; Bjornsson, E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: Correlations with energy intake and resting energy expenditure. Am. J. Clin. Nutr. 2007, 85, 808–815. [Google Scholar] [CrossRef]
- Lindqvist, C.; Nordstedt, P.; Nowak, G.; Slinde, F.; Majeed, A.; Bottai, M.; Wahlin, S. Energy expenditure early after liver transplantation: Better measured than predicted. Nutrition 2020, 79–80, 110817. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 110) |
|---|---|
| Male, n (%) | 70 (64) |
| Age (years), median (IQR) | 59 (50–64) |
| [Age range] | (21–71) |
| Aetiology: | |
| Alcohol | 36 (33) |
| NASH | 28 (25) |
| HCC | 12 (11) |
| Cholestatic disease | 12 (11) |
| HCV | 9 (8) |
| Other | 13 (12) |
| MELD, median (IQR) | 19 (13–25) |
| MELD-Na, median (IQR) | 21 (14–28) |
| Child–Pugh Score, median (IQR) | 9 (8–10) |
| Child–Pugh Category, n (%) | |
| A | 13 (12) |
| B | 56 (51) |
| C | 41 (37) |
| Ascites, n (%) | 65 (60) |
| History hepatic encephalopathy, n (%) | 52 (47) |
| Weight (kg), median (IQR) | 74 (62–86) |
| BMI (kg/m2), median (IQR) | 25.2 (22.2–28.4) |
| Nutritional status, n (%) | |
| SGA-A (well nourished) | 16 (15) |
| SGA-B (mild-moderately malnourished) | 53 (48) |
| SGA-C (severely malnourished) | 41 (37) |
| HGS (kg), median (IQR) | |
| Male | 26.3 (22.6–31.6) |
| Female | 17.1 (14.4–22.4) |
| MUAC (cm), median (IQR) | |
| Male | 29 (25.5–31.7) |
| Female | 26.7 (23.7–32.1) |
| TSF (mm), median (IQR) | |
| Male | 11.6 (8.4–15) |
| Female | 11.0 (7.9–16.9) |
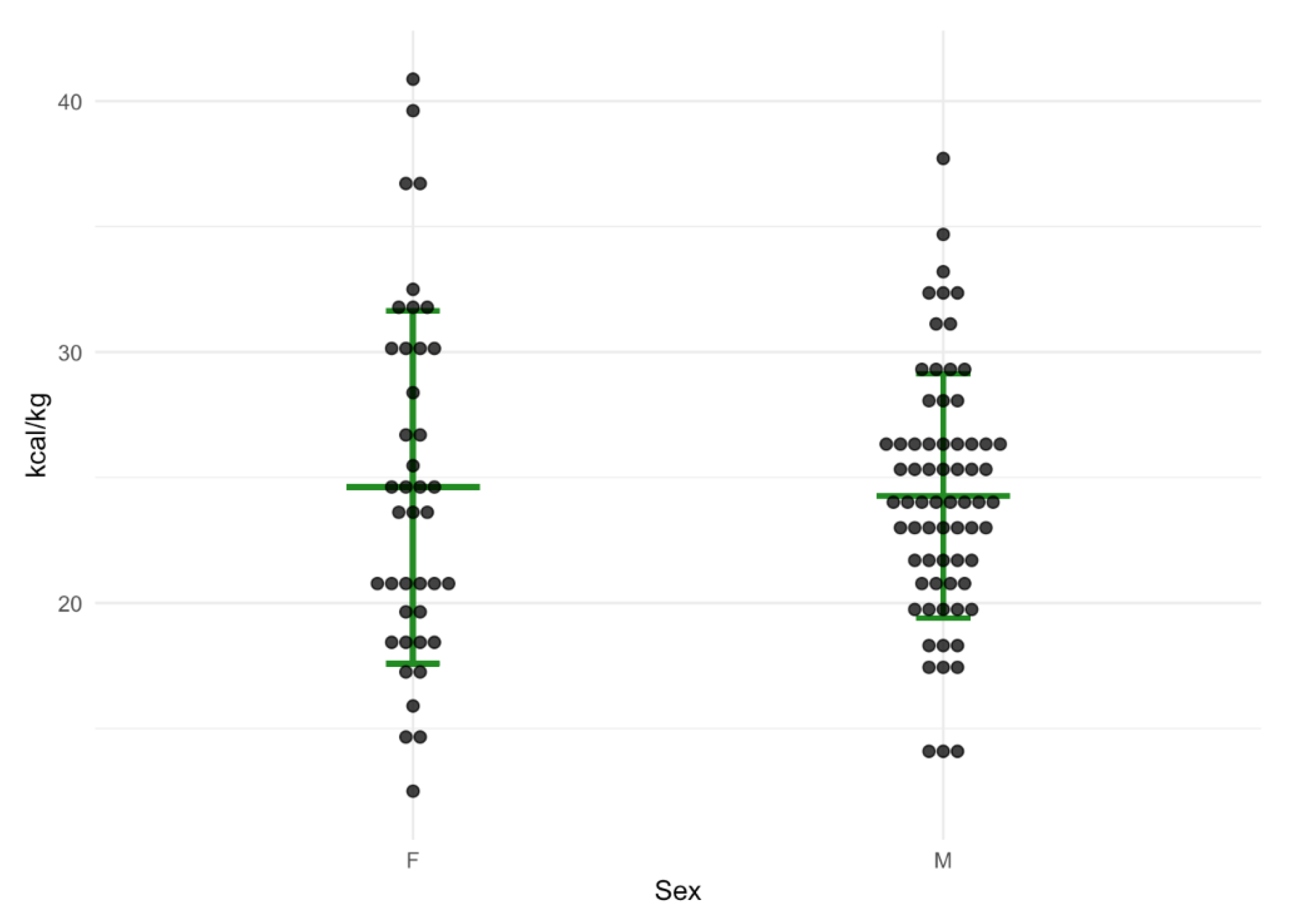
| Method | Total (n = 110) Median | IQR | Female (n = 41) Median | IQR | Male (n = 69) Median | IQR |
|---|---|---|---|---|---|---|
| REE IC | 1756 | 1531–2104 | 1583 | 1305–1755 | 1926 | 1616–2229 |
| REE H-B | 1480 | 1322–1722 | 1318 | 1256–1424 | 1638 | 1435–1800 |
| REE Schofield | 1474 | 1349–1723 | 1344 | 1276–1414 | 1641 | 1454–1766 |
| REE IC×1.3 | 2283 | 1990–2735 | 2058 | 1697–2282 | 2504 | 2100–2898 |
| REE EASL | 2590 | 2178–3010 | 2275 | 1925–2555 | 2800 | 2468–3150 |
| Method | Mean Adjusted Difference | p |
|---|---|---|
| IC with Harris–Benedict | 271.03 | <0.001 |
| IC with Schofield | 260.81 | <0.001 |
| IC×1.3 with EASL | −297.92 | <0.001 |
| Normometabolic (n = 57) | Hypermetabolic (n = 53) | p | |
|---|---|---|---|
| Male, n (%) | 37 (65) | 33 (62) | NS |
| MELD, median (IQR) | 17 (12–23) | 20 (16–28) | 0.003 |
| MELD-Na, median (IQR) | 20 (13–23) | 24 (16–31) | 0.002 |
| Refractory Ascites, n (%) | 30 (52) | 34 (64) | 0.04 |
| Hepatic Encephalopathy, n (%) | 27 (47) | 25 (47) | 0.46 |
| Weight (kg), median (IQR) | 75 (65–90) | 74 (60–85) | 0.14 |
| Well-nourished, n (%) | 13 (23) | 3 (6) | |
| Moderately malnourished, n (%) | 29 (51) | 24 (45) | |
| Severely malnourished, n (%) | 15 (26) | 26 (49) | 0.01 |
| HGS (kg), median (IQR) | |||
| Male | 26.7 (22.7–33.6) | 25.9 (22.5–30.3) | 0.18 |
| Female | 16.95 (14.3–22.7) | 17.1 (14.8–20.5) | 0.47 |
| MUAC (cm), median (IQR) | |||
| Male | 29.2 (26.5–32.7) | 28.3 (25–30.7) | 0.15 |
| Female | 28 (26.5–32.5) | 23.7 (21.4–26.9) | 0.03 |
| TSF (mm), median (IQR) | |||
| Male | 12.2 (8.6–17.7) | 10.4 (8.2–13.7) | 0.08 |
| Female | 13 (10.8–19.1) | 8.0 (6.4–12.8) | 0.02 |
| Characteristic | n | OR | 95% CI | p |
|---|---|---|---|---|
| Diuretics | 110 | 2.81 | 1.14, 7.49 | 0.03 |
| MELD | 110 | 1.08 | 1.02, 1.14 | 0.008 |
| MELD-Na | 110 | 1.07 | 1.02, 1.13 | 0.005 |
| Child–Pugh score | 110 | 1.25 | 1.02, 1.54 | 0.032 |
| Nutritional Status: | ||||
| SGA A | 13 | - | - | |
| SGA B | 56 | 3.59 | 1.01, 17.0 | 0.067 |
| SGA C | 41 | 7.51 | 2.04, 36.8 | 0.005 |
| Modifier | Change to Estimate |
|---|---|
| Child–Pugh Category | |
| B | +281.3 |
| C | +390.4 |
| MELD Score | |
| 16–20 | +108.5 |
| 21–30 | +250.1 |
| >31 | +292.7 |
| If age >65 years | +73.9 |
| If SGA-C | +15.3 × dry weight − 956.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapman, B.; Wong, D.; Whitcher, B.; Sinclair, M.; Gow, P.; Majumdar, A.; Testro, A. Redefining Nutritional Requirements in End-Stage Liver Disease: Towards a Personalized Approach. Nutrients 2023, 15, 4770. https://doi.org/10.3390/nu15224770
Chapman B, Wong D, Whitcher B, Sinclair M, Gow P, Majumdar A, Testro A. Redefining Nutritional Requirements in End-Stage Liver Disease: Towards a Personalized Approach. Nutrients. 2023; 15(22):4770. https://doi.org/10.3390/nu15224770
Chicago/Turabian StyleChapman, Brooke, Darren Wong, Bethany Whitcher, Marie Sinclair, Paul Gow, Avik Majumdar, and Adam Testro. 2023. "Redefining Nutritional Requirements in End-Stage Liver Disease: Towards a Personalized Approach" Nutrients 15, no. 22: 4770. https://doi.org/10.3390/nu15224770
APA StyleChapman, B., Wong, D., Whitcher, B., Sinclair, M., Gow, P., Majumdar, A., & Testro, A. (2023). Redefining Nutritional Requirements in End-Stage Liver Disease: Towards a Personalized Approach. Nutrients, 15(22), 4770. https://doi.org/10.3390/nu15224770







