The Minimum Methionine Requirement for Adults Aged ≥60 Years Is the Same in Males and Females
Abstract
1. Introduction
2. Materials and Methods
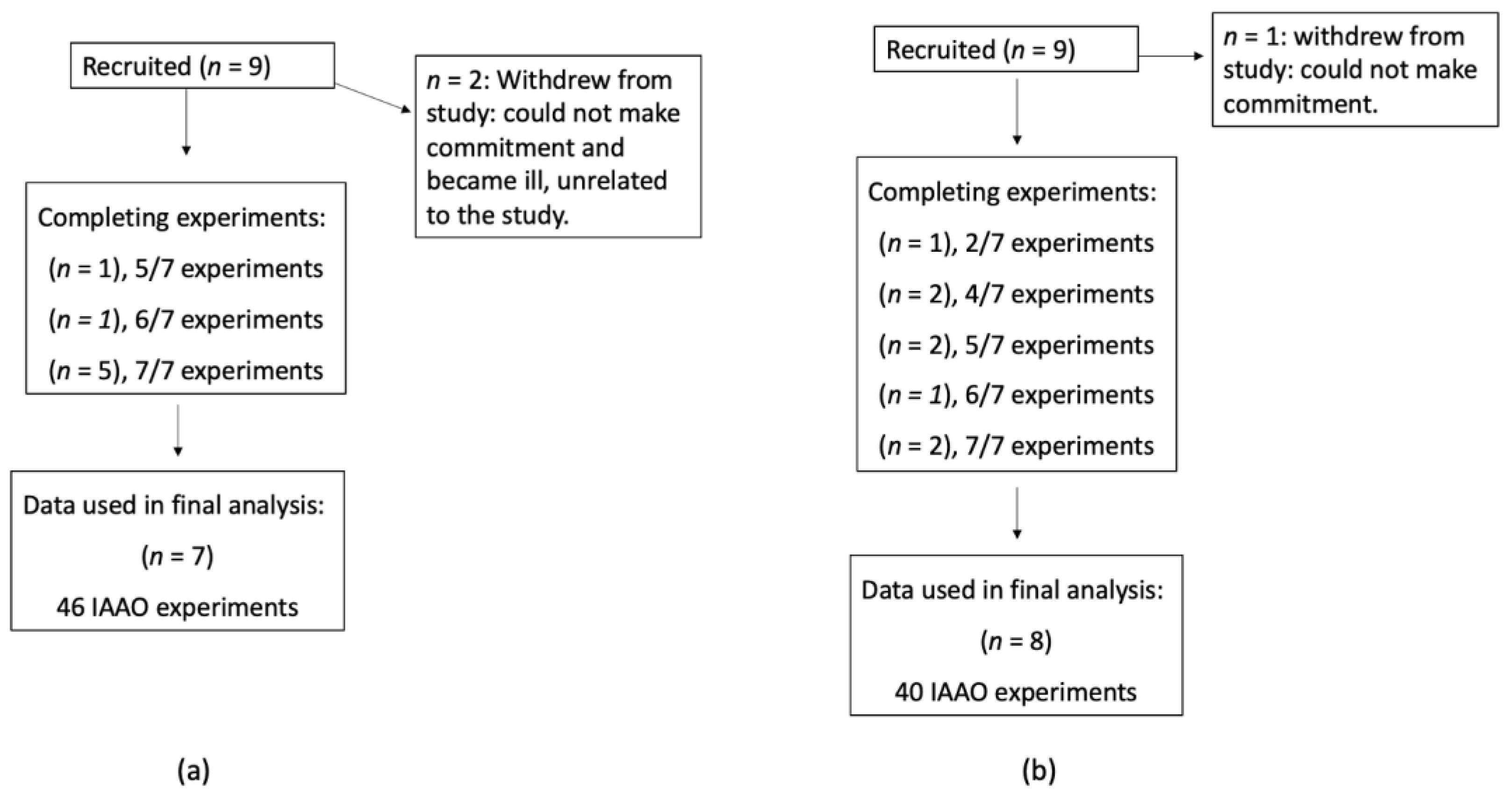
2.1. Subjects
2.2. Experimental Design
2.3. Study Diets
2.4. Tracer Protocol
2.5. Sample Collection and Analysis
2.6. Estimation of Isotope Kinetics
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
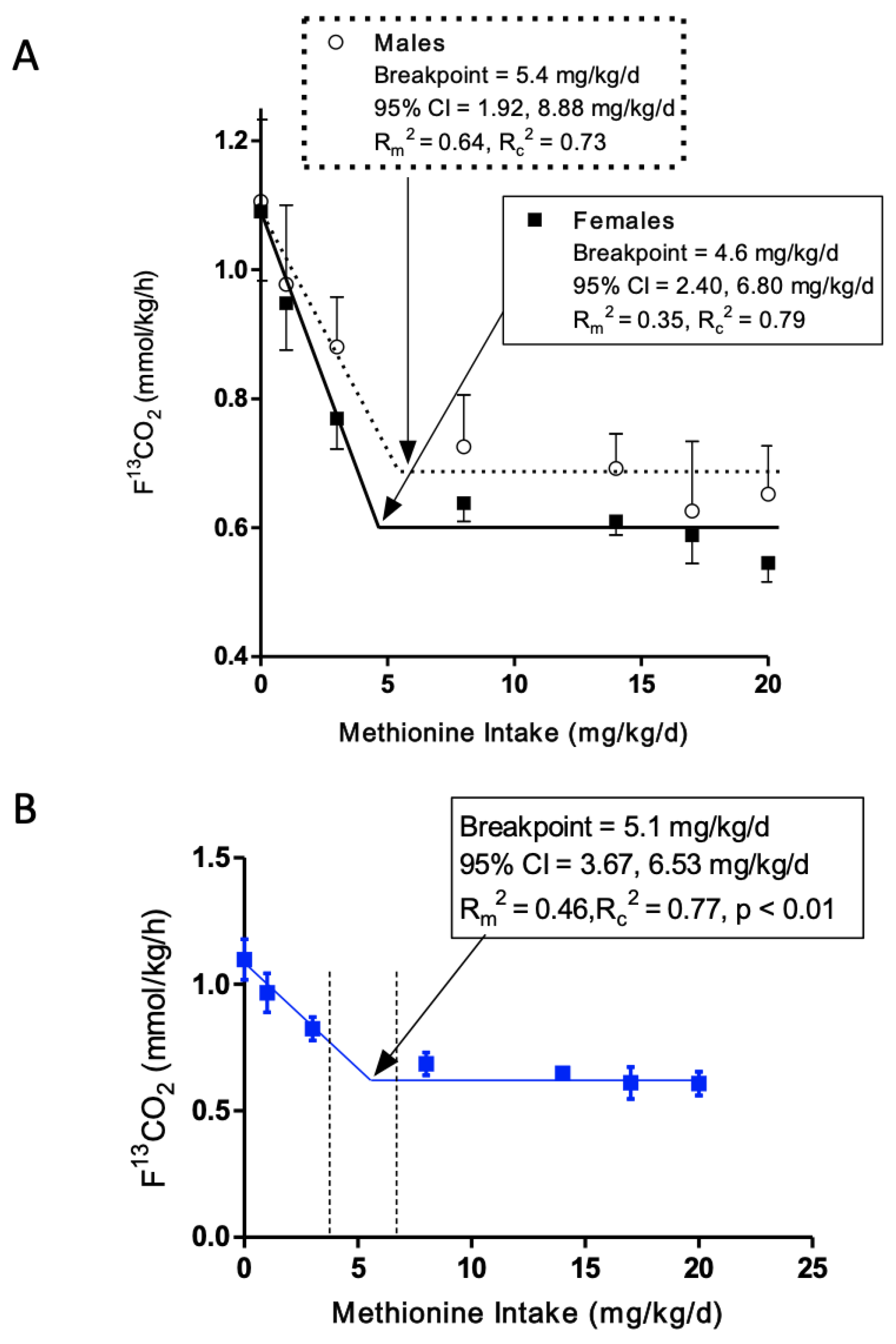
3.2. L-[1-13C]Phenylalanine Oxidation
3.3. Minimum Methionine Requirement Based on FFM
3.4. Comparison of Current Minimum Methionine Requirement Estimate of Older Adults to Young Adults
3.5. Effect of Methionine Intake and Sex on Secondary Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; ISBN 030965646X. [Google Scholar]
- Paoletti, A.; Pencharz, P.B.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. The Dietary Requirement for Total Sulphur Amino Acids in Adults ≥60 Years Appears Higher in Males than in Females. Am. J. Clin. Nutr. 2023, 118, 538–548. [Google Scholar] [CrossRef]
- Health Canada. Canada’s Dietary Guidelines for Health Professionals and Policy Makers; Health Canada: Ottawa, ON, Canada, 2019. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Sepehr, E. Protein Digestibility and Quality in Products Containing Antinutritional Factors Are Adversely Affected by Old Age in Rats. J. Nutr. 2003, 133, 220–225. [Google Scholar] [CrossRef]
- World Health Organization & Food and Agriculture Organization of the United Nations & United Nations University. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation, Rome 17–24 October 2001; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Womack, M.; Rose, C. The Partial Replacement of Dietary Methionine by Cysteine for Purposes of Growth. J. Biol. Chem. 1941, 141, 375–379. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J.; Harris, B.J. Methionine Metabolism in Mammals. The Methionine-Sparing Effect of Cystine. J. Biol. Chem. 1988, 263, 11750–11754. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Martin, J.J.; Harris, B.J. Effect of Dietary Cystine on Methionine Metabolism in Rat Liver. J. Nutr. 1986, 116, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.S.; Steel, D.L.; Jones, E.M.; Baumann, C.A. Nitrogen Balances of Women Maintained on Various Levels of Methionine and Cystine. J. Nutr. 1958, 64, 99–111. [Google Scholar] [CrossRef]
- Rose, W.C.; Wixom, R.L. The Amino Acid Requirements of Man. XIII. The Sparing Effect of Cystine on the Methionine Requirement. J. Biol. Chem. 1955, 216, 753–773. [Google Scholar]
- Clark, H.E.; Howe, J.M.; Shannon, B.M.; Carlson, K.; Kolski, S.M. Requirements of Adult Human Subjects for Methionine and Cystine. Am. J. Clin. Nutr. 1970, 23, 731–738. [Google Scholar] [CrossRef]
- Di Buono, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Dietary Cysteine Reduces the Methionine Requirement in Men. Am. J. Clin. Nutr. 2001, 74, 761–766. [Google Scholar] [CrossRef]
- Di Buono, M.; Wykes, L.J.; Cole, D.E.C.; Ball, R.O.; Pencharz, P.B. Regulation of Sulfur Amino Acid Metabolism in Men in Response to Changes in Sulfur Amino Acid Intakes. J. Nutr. 2003, 133, 733–739. [Google Scholar] [CrossRef][Green Version]
- Fukagawa, N.K.; Yu, Y.M.; Young, V.R. Methionine and Cysteine Kinetics at Different Intakes of Methionine and Cystine in Elderly Men and Women. Am. J. Clin. Nutr. 1998, 68, 380–388. [Google Scholar] [CrossRef]
- Mercier, S.; Breuillé, D.; Buffière, C.; Gimonet, J.; Papet, I.; Mirand, P.P.; Obled, C. Methionine Kinetics Are Altered in the Elderly Both in the Basal State and after Vaccination. Am. J. Clin. Nutr. 2006, 83, 291–298. [Google Scholar] [CrossRef][Green Version]
- WHO. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO: Rome, Italy, 1985; Volume 724. [Google Scholar]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J. Protein Content and Amino Acid Composition of Commercially Available Plant—Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Rafii, M.; Chapman, K.; Owens, J.; Elango, R.; Campbell, W.W.; Ball, R.O.; Pencharz, P.B.; Courtney-Martin, G. Dietary Protein Requirement of Female Adults >65 Years Determined by the Indicator Amino Acid Oxidation Technique Is Higher than Current Recommendations. J. Nutr. 2015, 145, 18–24. [Google Scholar] [CrossRef]
- Szwiega, S.; Pencharz, P.B.; Rafii, M.; Lebarron, M.; Chang, J.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. Dietary Leucine Requirement of Older Men and Women Is Higher than Current Recommendations. Am. J. Clin. Nutr. 2020, 113, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Gao, X.; Chinchilli, V.M.; Sinha, R.; Muscat, J.; Winkels, R.M.; Richie, J.P. Association of Sulfur Amino Acid Consumption with Cardiometabolic Risk Factors: Cross-Sectional Findings from NHANES III. EClinicalMedicine 2020, 19, 100248. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.J.; Wagner, D.A.; Burke, J.F.; Young, V.R. Quantitative Study in Vivo of Methionine Cycle in Humans Using [Methyl-2H3]- and [1-13C]Methionine. Am. J. Physiol. -Endocrinol. Metab. 1988, 255, E322–E331. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Silbershatz, H.; Rosenberg, I.H.; Selhub, J.; D’Agostino, R.B.; Wolf, P.A.; Jacques, P.F.; Wilson, P.W.F. Nonfasting Plasma Total Homocysteine Levels and All-Cause and Cardiovascular Disease Mortality in Elderly Framingham Men and Women. Arch. Intern. Med. 1999, 159, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.O.; Courtney-Martin, G.; Pencharz, P.B. The in Vivo Sparing of Methionine by Cysteine in Sulfur Amino Acid Requirements in Animal Models and Adult Humans. J. Nutr. 2006, 136, 1682S–1693S. [Google Scholar] [CrossRef]
- Zello, G.A.; Pencharz, P.B.; Ball, R.O. The Design and Validation of a Diet for Studies of Amino Acid Metabolism in Adult Humans. Nutr. Res. 1990, 10, 1353–1365. [Google Scholar] [CrossRef]
- Kriengsinyos, W.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Oral and Intravenous Tracer Protocols of the Indicator Amino Acid Oxidation Method Provide the Same Estimate of the Lysine Requirement in Healthy Men. J. Nutr. 2002, 132, 2251–2257. [Google Scholar] [CrossRef]
- Martin, K.E.; Pencharz, P.B.; Rafii, M.; Ball, R.O.; Szwiega, S.; Elango, R.; Courtney-Martin, G. The Phenylalanine Requirement of Elderly Men and Women Measured by Direct 13C Carbon Oxidation Method Is Similar to That of Young Adults. J. Nutr. 2019, 149, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- FAO. Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Shiman, R.; Gray, D.W. Formation and Fate of Tyrosine. Intracellular Partitioning of Newly Synthesized Tyrosine in Mammalian Liver. J. Biol. Chem. 1998, 273, 34760–34769. [Google Scholar] [CrossRef] [PubMed]
- Di, A.; Meo, M.; Yazdanpanah, V.; Higgins, M.; Nichols, M.; Bohn, A.; Tan, S.; Zainab, L.; Sepiashvili, L.; Adeli, K. Analytical Development, Validation, and Pediatric CALIPER Reference Interval Establishment for a LC-MS/MS High Sensitivity Estradiol Method in Serum without Derivatization. In Proceedings of the AACC Annual Scientific Meeting & Clinical Lab Expo Taking Place, Chicago, IL, USA, 24–28 July 2022. [Google Scholar]
- Swaroop, A. A Micromethod for the Determination of Urinary Inorganic Sulfates. Clin. Chim. Acta 1973, 32646, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Gelius, S.S. An Improved Barium-Rhodizonate Method for Determination of Sulfate Ion in Biological Fluids. Anal. Biochem. 2020, 598, 113703. [Google Scholar] [CrossRef]
- Matthews, D.E.; Motil, K.J.; Rohrbaugh, D.K.; Burke, J.F.; Young, V.R.; Bier, D.M. Measurement of Leucine Metabolism in Man from a Primed, Continuous Infusion of L-[1-3C]Leucine. Am. J. Physiol. 1980, 238, E473–E479. [Google Scholar] [CrossRef]
- Hoerr, R.A.; Yu, Y.M.; Wagner, D.A.; Burke, J.F.; Young, V.R. Recovery of 13C in Breath from NaH13CO3 Infused by Gut and Vein: Effect of Feeding. Am. J. Physiol. Endocrinol. Metab. 1989, 257, E426–E438. [Google Scholar] [CrossRef]
- Hayamizu, K.; Kato, M.; Hattori, S. Determining Amino Acid Requirements from Repeated Observations on Indicator Amino Acid Oxidation Method by Mixed-Effect Change-Point Regression Models. J. Clin. Biochem. Nutr. 2011, 48, 154–160. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R2 from Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Staggs, V. Parametric Bootstrap Interval Approach to Inference for; University of Kansas: Lawrence, KS, USA, 2009. [Google Scholar]
- Di Buono, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Total Sulfur Amino Acid Requirement in Young Men as Determined by Indicator Amino Acid Oxidation with L-[1-13c]Phenylalanine. Am. J. Clin. Nutr. 2001, 74, 756–760. [Google Scholar] [CrossRef]
- Matthews, D.R.; Rudenski, A.S.; Burnett, M.A.; Darling, P.; Turner, R.C. The Half-Life of Endogenous Insulin and C-Peptide in Man Assessed by Somatostatin Suppression. Clin. Endocrinol. (Oxf). 1985, 23, 71–79. [Google Scholar] [CrossRef]
- Fukagawa, N.K.; Galbraith, R.A. Advancing Age and Other Factors Influencing the Balance between Amino Acid Requirements and Toxicity. J. Nutr. 2004, 134, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.M.; Mulkey, Z.; Lado-Abeal, J.; Yarbrough, S. Diagnosing and Managing Low Serum Testosterone. Baylor Univ. Med. Cent. Proc. 2014, 27, 321–324. [Google Scholar] [CrossRef]
- Verdonk, S.J.E.; Vesper, H.W.; Martens, F.; Sluss, P.M.; Hillebrand, J.J.; Heijboer, A.C. Estradiol Reference Intervals in Women during the Menstrual Cycle, Postmenopausal Women and Men Using an LC-MS/MS Method. Clin. Chim. Acta 2019, 495, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F.; Grimble, G.K. Immunonutrition: Role of Sulfur Amino Acids, Related Amino Acids, and Polyamines. Nutrition 1998, 14, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Mazor, K.M.; Lee, J.I.; Roman, H.B.; Hirschberger, L.L.; Stipanuk, M.H. Upregulation of Capacity for Glutathione Synthesis in Response to Amino Acid Deprivation: Regulation of Glutamate-Cysteine Ligase Subunits. Amino Acids 2014, 46, 1285–1296. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient Synthesis of Glutathione Underlies Oxidative Stress in Aging and Can Be Corrected by Dietary Cysteine and Glycine Supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Reid, M.; Jahoor, F. Methods for Measuring Glutathione Concentration and Rate of Synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 385–390. [Google Scholar] [CrossRef]
- Liu, R.M.; Choi, J. Age-Associated Decline in γ-Glutamylcysteine Synthetase Gene Expression in Rats. Free Radic. Biol. Med. 2000, 28, 566–574. [Google Scholar] [CrossRef]
- Liu, R.M. Down-Regulation of γ-Glutamylcysteine Synthetase Regulatory Subunit Gene Expression in Rat Brain Tissue during Aging. J. Neurosci. Res. 2002, 68, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Liu, R.M. Gender Difference in Glutathione Metabolism during Aging in Mice. Exp. Gerontol. 2003, 38, 507–517. [Google Scholar] [CrossRef]
- Bray, G.A.; Jablonski, K.A.; Fujimoto, W.Y.; Barrett-Connor, E.; Haffner, S.; Hanson, R.L.; Hill, J.O.; Hubbard, V.; Kriska, A.; Stamm, E.; et al. Relation of Central Adiposity and Body Mass Index to the Development of Diabetes in the Diabetes Prevention Program. Am. J. Clin. Nutr. 2008, 87, 1212–1218. [Google Scholar] [CrossRef]
- Carey, V.J.; Walters, E.E.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Rosner, B.A.; Speizer, F.E.; Manson, J.E. Body Fat Distribution and Risk of Non-Insulin-Dependent Diabetes Mellitus in Women: The Nurses’ Health Study. Am. J. Epidemiol. 1997, 145, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, R. Obesity—A Perspective Based on the Biochemical Interrelationship of Lipids and Carbohydrates. Med. Hypotheses 2007, 68, 1159–1171. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Gámez-Nava, J.I.; Díaz-De La Cruz, E.N.; Cardona-Muñoz, E.G.; Becerra-Alvarado, I.N.; Aceves-Aceves, J.A.; Sánchez-Rodríguez, E.N.; Miranda-Díaz, A.G. The Effect of Visceral Abdominal Fat Volume on Oxidative Stress and Proinflammatory Cytokines in Subjects with Normal Weight, Overweight and Obesity. Diabetes, Metab. Syndr. Obes. Targets Ther. 2020, 13, 1077–1087. [Google Scholar] [CrossRef]
- Anoop, S.; Jebasingh, K.F.; Philip, D.M.; Kurian, M.E.; Mohan, V.; Finney, G.; Thomas, N. The Waist-Height Ratio Is a Potential Anthropometric Index of Insulin Resistance: Observations Based on Oral Glucose Tolerance Test in Lean, Normo-Glycaemic, Asian Indian Males from Southern India. Clin. Epidemiol. Glob. Health 2021, 11, 100762. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Xiang, J.; Dai, H.; Li, M.; Wang, T.; Wang, S.; Lin, H.; Lu, J.; Xu, Y.; et al. Associations of Body Shapes with Insulin Resistance and Cardiometabolic Risk in Middle-Aged and Elderly Chinese. Nutr. Metab. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender Differences in Insulin Resistance, Body Composition, and Energy Balance. Gend Med. 2009, 6, 60–75. [Google Scholar] [CrossRef]
- Couillard, C.; Bergeron, N.; Prud’homme, D.; Bergeron, J.; Tremblay, A.; Bouchard, C.; Mauriège, P.; Després, J.-P. Gender Difference in Postprandial Lipemia. Importance of Visceral Adipose Tissue Accumulation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2448–2455. [Google Scholar] [CrossRef]
- Cai, J.; Huang, Z.Z.; Lu, S. Differential Regulation of γ-Glutamylcysteine Synthetase Heavy and Light Subunit Gene Expression. Biochem. J. 1997, 326, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Li, Y.L.; Shao, C.H.; Bidasee, K.R.; Rozanski, G.J. Insulin Regulation of Glutathione and Contractile Phenotype in Diabetic Rat Ventricular Myocytes. Am. J. Physiol. -Hear. Circ. Physiol. 2007, 292, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.; Weber, D.; Kochlik, B.; Stuetz, W.; Toussaint, O.; Debacq-Chainiaux, F.; Dollé, M.E.T.; Jansen, E.H.J.M.; Gonos, E.S.; Sikora, E.; et al. Gender- and Age-Dependencies of Oxidative Stress, as Detected Based on the Steady State Concentrations of Different Biomarkers in the MARK-AGE Study. Redox Biol. 2019, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wonisch, W.; Falk, A.; Sundl, I.; Winklhofer-Roob, B.M.; Lindschinger, M. Oxidative Stress Increases Continuously with BMI and Age with Unfavourable Profiles in Males. Aging Male 2012, 15, 159–165. [Google Scholar] [CrossRef]
- Segovia-Siapco, G.; Sabaté, J. Health and Sustainability Outcomes of Vegetarian Dietary Patterns: A Revisit of the EPIC-Oxford and the Adventist Health Study-2 Cohorts. Eur. J. Clin. Nutr. 2018, 72, 60–70. [Google Scholar] [CrossRef]
- Health Canada. Summary of Health Canada’s Assessment of a Health Claim about Barley Products and Blood Cholesterol Lowering; Health Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]

| Value 1 | ||
|---|---|---|
| Baseline Characteristics | Females (n = 8) | Males (n = 7) |
| Age (years) | 70.6 ± 2.05 | 68.3 ± 2.09 |
| Weight (kg) | 60.6 a ± 3.33 | 85.5 b ± 5.74 |
| Height (cm) | 159 a ± 2.56 | 177 b ± 2.10 |
| BMI (kg/m2) | 23.9 ± 1.23 | 27.3 ± 1.67 |
| FFM-SF 2 (kg) | 41.8 a ± 2.22 | 58.0 b ± 2.64 |
| FFM-BIA 3 (kg) | 43.0 ± 1.67 | 62.1 ± 2.59 |
| Fat -SF 2 (%) | 29.3 ± 2.38 | 31.3 ± 2.73 |
| Fat-BIA 3 (%) | 28.9 ± 4.10 | 26.3 ± 3.00 |
| WHR | 0.85 a ± 0.03 | 0.97 b ± 0.04 |
| REE 4 (kcal/d) | 1229 a ± 48.1 | 1698 b ± 81.5 |
| Blood Hb A1C (%) | 5.44 ± 0.08 | 5.39 ± 0.12 |
| Fasting Blood Glucose (mmol/L) | 5.25 ± 0.19 | 5.37 ± 0.20 |
| Fasting Insulin (pmol/L) | 42.0 ± 8.55 | 45.6 ± 7.78 |
| HOMA-IR 5 | 1.49 ± 0.29 | 1.63 ± 0.35 |
| Fasting Blood Urea (mmol/L) | 5.03 a ± 0.38 | 6.33 b ± 0.45 |
| Fasting Blood Creatinine (µmol/L) | 65.5 a ± 3.5 | 78.6 b ± 4.6 |
| C-reactive Protein (mg/L) | 1.43 ± 0.56 | 1.07 ± 0.15 |
| Vitamin B12 (pmol/L) | 477 ± 117 | 291 ± 49.7 |
| Vitamin B6 (nmol/L) | 155 ± 80.8 | 60.1 ± 25.6 |
| Red Blood Cell Folate (nmol/L) | 963 ± 80.2 | 885 ± 113 |
| Testosterone (nmol/L) | 1.07 a ± 0.21 | 20.0 b ± 3.18 |
| Estradiol (pmol/L) | 20.0 a ± 3.42 | 70.8 b ± 10.8 |
| GSH (mmol/L) | 2.79 ± 0.28 | 2.87 ± 0.36 |
| Value 1 | ||||
|---|---|---|---|---|
| Nutrient | Females (n = 7) | % RDA | Males (n = 6) | % RDA |
| Calories (kcal/d) | 1558 ± 149 | - | 2464 ± 135 | - |
| Calories (kcal/kg/d) | 25.6 ± 0.92 | - | 28.5 ± 1.56 | - |
| Protein (g) | 58.2 ± 4.53 | - | 111 ± 10.4 | - |
| Protein (g/kg/d) | 0.99 ± 0.10 | 124 | 1.37 ± 0.27 | 171 |
| Total sulphur amino acid (mg/kg/d) | 19.1 ± 2.43 | 100 | 28.6 ± 8.15 | 151 |
| Total Fat 2 (g) | 66.4 ± 4.85 | 110 | 90.2 ± 4.90 | 94 |
| Saturated fat 2 (g) | 19.5 ± 1.63 | 113 | 23.6 ± 3.24 | 86 |
| MUFA (g) | 26.0 ± 2.56 | - | 33.3 ± 3.22 | - |
| PUFA (g) | 14.7 ± 1.63 | - | 19.7 ± 2.40 | - |
| Trans fat (g) | 0.98 ± 0.41 | - | 0.63 ± 0.16 | - |
| Selenium (µg) | 77.1 a ± 18.3 | 140 | 146 b ± 16.3 | 266 |
| Vitamin C (mg) | 110 ± 13.2 | 147 | 185 ± 39.6 | 206 |
| Vitamin E (mg) | 9.07 ± 1.22 | 61 | 14.6 ± 2.81 | 97 |
| Folate (µg) | 324 ± 27.9 | 81 | 492 ± 89.5 | 123 |
| Vitamin B6 (µg) | 3.44 ± 2.10 | 229 | 2.81 ± 0.35 | 165 |
| Vitamin B12 (µg) | 3.35 ± 0.63 | 140 | 7.35 ± 1.90 | 306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoletti, A.; Pencharz, P.B.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. The Minimum Methionine Requirement for Adults Aged ≥60 Years Is the Same in Males and Females. Nutrients 2023, 15, 4112. https://doi.org/10.3390/nu15194112
Paoletti A, Pencharz PB, Ball RO, Kong D, Xu L, Elango R, Courtney-Martin G. The Minimum Methionine Requirement for Adults Aged ≥60 Years Is the Same in Males and Females. Nutrients. 2023; 15(19):4112. https://doi.org/10.3390/nu15194112
Chicago/Turabian StylePaoletti, Alyssa, Paul B. Pencharz, Ronald O. Ball, Dehan Kong, Libai Xu, Rajavel Elango, and Glenda Courtney-Martin. 2023. "The Minimum Methionine Requirement for Adults Aged ≥60 Years Is the Same in Males and Females" Nutrients 15, no. 19: 4112. https://doi.org/10.3390/nu15194112
APA StylePaoletti, A., Pencharz, P. B., Ball, R. O., Kong, D., Xu, L., Elango, R., & Courtney-Martin, G. (2023). The Minimum Methionine Requirement for Adults Aged ≥60 Years Is the Same in Males and Females. Nutrients, 15(19), 4112. https://doi.org/10.3390/nu15194112





