The Supplementation of Sechium edule var. nigrum spinosum (Chayote) Promotes Nrf2-Mediated Antioxidant Protection in Older Adults with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Intervention
2.3. Anthropometric and Clinical Measurements
2.4. Biochemical Analysis
2.4.1. Samples
2.4.2. SOD Enzyme Activity
2.4.3. GPx Enzyme Activity
2.4.4. Catalase Activity
2.4.5. Total Oxidant Status (TOS)
2.4.6. Total Antioxidant Status (TAS)
2.4.7. Inflammatory Cytokines
2.4.8. Lymphocyte Isolation and RNA Extraction
2.4.9. Gene Expression Analysis
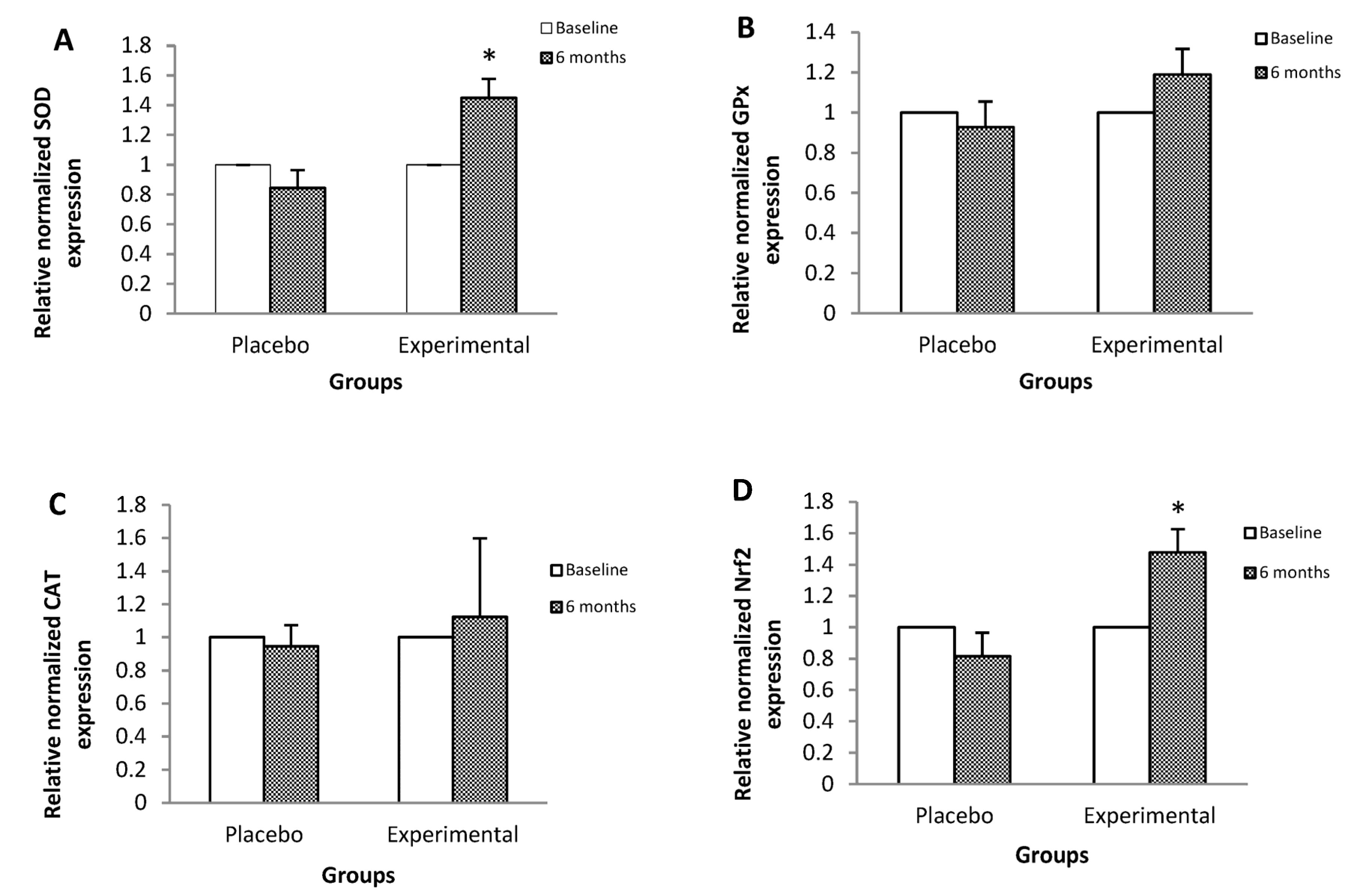
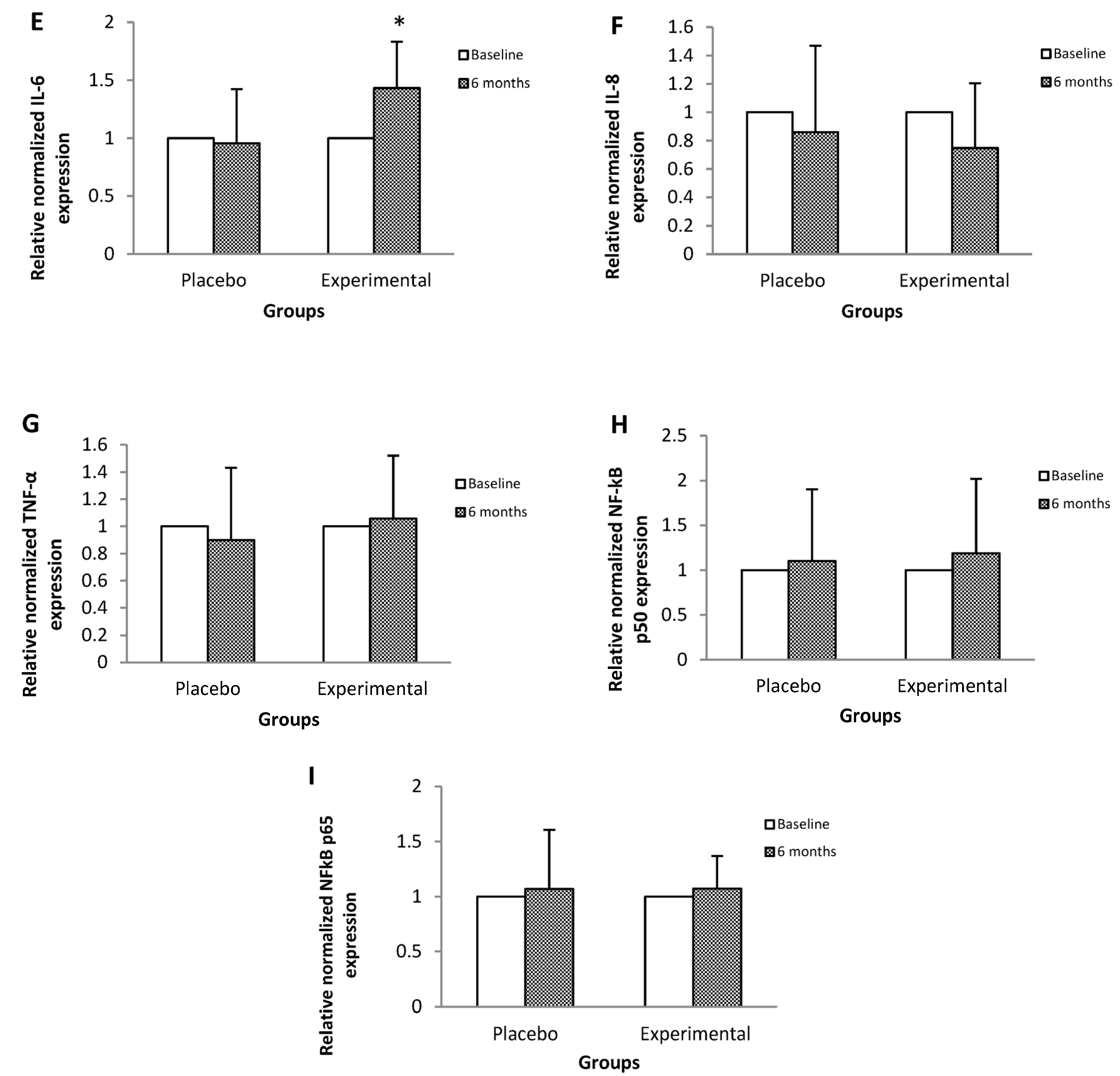
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ageing and Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 15 May 2023).
- Mendoza-Núñez, V.M.; Martínez-Maldonado, M.L.; Vivaldo-Martínez, M. What is the onset age of human aging and old age? Int. J. Gerontol. 2016, 10, 56. [Google Scholar] [CrossRef]
- Caterson, I.D.; Hubbard, V.; Bray, G.A.; Grunstein, R.; Hansen, B.C.; Hong, Y.; Labarthe, D.; Seidell, J.C.; Smith, S.C.; American Heart Association. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: Worldwide comorbidities of obesity. Circulation 2004, 110, e476–e483. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Costa, C. Oxidative Stress, Inflammation and Angiogenesis in the Metabolic Syndrome; Springer: Amsterdam, The Netherlands, 2009; pp. 85–121. [Google Scholar]
- Gouveia, É.R.; Gouveia, B.R.; Marques, A.; Peralta, M.; França, C.; Lima, A.; Campos, A.; Jurema, J.; Kliegel, M.; Ihle, A. Predictors of metabolic syndrome in adults and older adults from Amazonas, Brazil. Int. J. Environ. Res. Public. Health 2021, 18, 1303. [Google Scholar] [CrossRef] [PubMed]
- Boleti, A.P.d.A.; Almeida, J.A.; Migliolo, L. Impact of the metabolic syndrome on the evolution of neurodegenerative diseases. Neural Regen. Res. 2021, 16, 688–689. [Google Scholar]
- Matsumori, A. Nuclear factor-κB is a prime candidate for the diagnosis and control of inflammatory cardiovascular disease. Eur. Cardiol. Rev. 2023, 18, e40. [Google Scholar] [CrossRef]
- Shen, C.; Liu, C.; Qiu, A. Metabolism-related brain morphology accelerates aging and predicts neurodegenerative diseases and stroke: A UK Biobank study. Transl. Psychiatry 2023, 13, 233. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez, P.S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña, G.E.; Guzman, E.; Kostara, C.E. The triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, P.F. PPARα: An emerging target of metabolic syndrome, neurodegenerative and cardiovascular diseases. Front. Endocrinol. 2022, 13, 1074911. [Google Scholar] [CrossRef]
- Motamedi, S.; Karimi, I.; Jafari, F. The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone. Metab. Brain Dis. 2017, 32, 651–665. [Google Scholar] [CrossRef]
- Márquez-Sandoval, F.; Macedo-Ojeda, G.; Viramontes-Hörner, D.; Fernández, B.J.D.; Salas, S.J.; Vizmanos, B. The prevalence of metabolic syndrome in Latin America: A systematic review. Public. Health Nutr. 2011, 14, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H.; Montani, J.P. Angiogenesis; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, S.; Ajmera, P.; Mathur, S. Oxidative stress in metabolic syndrome. Indian J. Clin. Biochem. 2005, 20, 145–149. [Google Scholar] [CrossRef]
- Fernandez-Garcia, J.C.; Cardona, F.J.; Tinahones, F. Inflammation, oxidative stress and metabolic syndrome: Dietary modulation. Curr. Vasc. Pharmacol. 2013, 11, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Pruchniak, M.P.; Aražna, M.; Demkow, U. Biochemistry of oxidative stress. Adv. Exp. Med. Biol. 2016, 878, 9–19. [Google Scholar]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial hypertension—Oxidative stress and inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Avendaño, A.C.H.; Cadena, I.J.; Arévalo, G.M.L.; Campos, R.E.; Cisneros, S.V.M.; Aguirre, M.J.F. Las Variedades del Chayote Mexicano, Recurso Ancestral con Ppotencial de Comercialización; Grupo Interdisciplinario de Investigación en Sechium edule en México, A.C. (GISeM): México City, Mexico, 2010. [Google Scholar]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- MinJin, K.; YouChul, C.; SangSuk, K.; ChanKyu, L.; KyungJin, P.; YoungHun, C.; Taejin, P.; SeungYoung, K.; ChangGu, H. Anti-inflammatory effect of Sechium edule extract in LPS-stimulated RAW 264.7 cells via p-JNK and p-p38 down-regulation. Int. Korean Soc. Biotechnol. Bioeng. Geogr. Inf. Sci. 2019, 34, 99–106. [Google Scholar]
- Lombardo-Earl, G.; Roman-Ramos, R.; Zamilpa, A.; Herrera-Ruiz, M.; Rosas-Salgado, G.; Tortoriello, J.; Jiménez-Ferrer, E. Extracts and fractions from edible roots of Sechium edule (Jacq.) Sw. with antihypertensive activity. Evid. Based Complement. Altern. Med. 2014, 2014, 594326. [Google Scholar] [CrossRef]
- Arista-Ugalde, T.L.; Santiago-Osorio, E.; Monroy-García, A.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Cadena-Iñiguez, J.; Gavia-García, G.; Mendoza-Núñez, V.M. Antioxidant and anti-inflammatory effect of the consumption of powdered concentrate of Sechium edule var. nigrum spinosum in Mexican older adults with metabolic syndrome. Antioxidants 2022, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Habibi-Rezaei, M.; Arese, M.; Saso, L.; Moosavi-Movahedi, A.A. Nrf2 modulation in breast cancer. Biomedicines 2022, 10, 2668. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular modulators of the NRF2/KEAP1 signaling pathway in prostate cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.M. Nrf2 as a potential therapeutic target for traumatic brain injury. J. Integr. Neurosci. 2023, 22, 81. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in ovarian cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Ruiz-Posadas, L.M.; Soto-Hernández, M.; Aguirre-Medina, J.F.; Avendaño-Arrazate, C.H.; Arévalo-Galarza, L. Infraspecific variation of Sechium edule (Jacq.) Sw. in the state of Veracruz, Mexico. Genet. Resour. Crop. Evol. 2008, 55, 835–847. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. The consumption of Sechium edule (chayote) has antioxidant effect and prevents telomere attrition in older adults with metabolic syndrome. Redox Rep. 2023, 28, 2207323. [Google Scholar] [CrossRef]
- NCEP. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Secretaría de Salud. Toma de Medidas Clínicas y Antropométricas en el Adulto Mayor; Subsecretaría de Prevención y Protección de La Salud: Mexico City, Mexico, 2002. [Google Scholar]
- Secretaría de Salud. Norma Oficial Mexicana NOM-030-SSA-1999. In Para la Prevención, Tratamiento y Control de la Hipertensión Arterial; Secretaría de Salud: Mexico City, Mexico, 1999. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Primer-BLAST-NCBI-NIH (Primer-BLAST). Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 1 October 2022).
- Agbabiaka, T.; Wider, B.; Watson, L.K.; Goodman, C. Concurrent use of prescription drugs and herbal medicinal products in older adults: A systematic review. Drugs Aging 2017, 34, s40017–s40266. [Google Scholar] [CrossRef]
- Wu, C.H.; Ou, T.T.; Chang, C.H.; Chang, X.Z.; Yang, M.Y.; Wang, C.J. The polyphenol extract from Sechium edule shoots inhibits lipogenesis and stimulates lipolysis via activation of AMPK signals in HepG2 cells. J. Agric. Food Chem. 2014, 62, 750–759. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, J.; Lee, M.J.; Kim, Y. Abnormalities of plasma cytokines and spleen in senile APP/PS1/Tau transgenic mouse model. Sci. Rep. 2015, 5, 15703. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Chen, M.J.; Yeh, C.T.; Yen, G.C. Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. New Biotechnol. 2011, 28, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kotakeyama, Y.; Li, J.; Pan, Y.; Matsuura, A.; Ohya, Y.; Yoshida, M.; Xiang, L.; Qi, J. Cucurbitacin B exerts antiaging effects in yeast by regulating autophagy and oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 4517091. [Google Scholar] [CrossRef]
- Liu, Z.; Kumar, M.; Kabra, A. Cucurbitacin B exerts neuroprotection in a murine Alzheimer’s disease model by modulating oxidative stress, inflammation, and neurotransmitter levels. Front. Biosci. 2022, 27, 71. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Park, G. Cucurbitacins attenuate microglial activation and protect from neuroinflammatory injury through Nrf2/ARE activation and STAT/NF-κB inhibition. Neurosci. Lett. 2015, 609, 129–136. [Google Scholar] [CrossRef]
- Qin, S.; Chen, J.; Tanigawa, S.; Hou, D.X. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Mol. Nutr. Food Res. 2013, 57, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.H.; Zhu, J.X.; Feng, H.; Ni, J.; Zhang, N.; Chen, S.; Liu, H.J.; Yang, Z.; Deng, W.; Tang, Q.Z. Myricetin possesses potential protective effects on diabetic cardiomyopathy through inhibiting IκBα/NFκB and enhancing Nrf2/HO-1. Oxid. Med. Cell. Longev. 2017, 2017, 8370593. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Deng, Y.; Xiao, R.; Xu, F.; Li, M.; Gong, Q.; Gao, J. Anti-fatigue effect of phlorizin on exhaustive exercise-induced oxidative injury mediated by Nrf2/ARE signaling pathway in mice. Eur. J. Pharmacol. 2022, 918, 174563. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Yeh, C.T.; Yen, G.C. Involvement of p38 MAPK and Nrf2 in phenolic acid-induced P-form phenol sulfotransferase expression in human hepatoma HepG2 cells. Carcinogenesis 2006, 27, 1008–1017. [Google Scholar] [CrossRef]
- Ji, L.L. Exercise-induced modulation of antioxidant defense. Ann. N. Y. Acad. Sci. 2002, 959, 82–92. [Google Scholar] [CrossRef]
- Jinhwan, L.; Ulrike, L. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar]
- Rao, G.; Xia, E.; Nadakavukaren, M.J.; Richardson, A. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J. Nutr. 1990, 120, 602–609. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Med. J. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (Chayote) on oxidative stress and pro-inflammatory markers in older adults with metabolic syndrome: An exploratory study. Antioxidants 2019, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Gavia-García, G.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (chayote) on telomerase levels and antioxidant capacity in older adults with metabolic syndrome. Antioxidants 2020, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Oh-Ishi, S.; Kizaki, T.; Yamashita, H.; Nagata, N.; Suzuki, K.; Taniguchi, N.; Ohno, H. Alterations of superoxide dismutase iso-enzyme activity, content, and mRNA expression with aging in rat skeletal muscle. Mech. Ageing Dev. 1995, 84, 65–76. [Google Scholar] [CrossRef]
- Marasco, M.R.; Conteh, A.M.; Reissaus, C.A.; Cupit, J.E., 5th; Appleman, E.M.; Mirmira, R.G.; Linnemann, A.K. Interleukin-6 reduces β-cell oxidative stress by linking autophagy with the antioxidant response. Diabetes 2018, 67, 1576–1588. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Nakayama, H.; Yoshida, R.; Hirosue, A.; Nagata, M.; Tanaka, T.; Kawahara, K.; Sakata, J.; Arita, H.; Nakashima, H.; et al. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br. J. Cancer 2016, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Wruck, C.J.; Streetz, K.; Pavic, G.; Götz, M.E.; Tohidnezhad, M.; Brandenburg, L.O.; Varoga, D.; Eickelberg, O.; Herdegen, T.; Trautwein, C.; et al. Nrf2 induces interleukin-6 (IL-6) expression via an antioxidant response element within the IL-6 promoter. J. Biol. Chem. 2011, 286, 4493–4499. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Elhady, S.S. Cucurbitacin E glucoside alleviates concanavalin A-induced hepatitis through enhancing SIRT1/Nrf2/HO-1 and inhibiting NF-ĸB/NLRP3 signaling pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef]
- Li, J.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Protective effects of naringenin and apigenin in ameliorating skin damage via mediating the Nrf2 and NF-κB pathways in mice. Foods 2023, 12, 2120. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, D.H.; Kim, J.H.; Hong, S.; Choi, D.; Kim, Y.J.; Kwak, M.K.; Jung, Y. Caffeic acid phenethyl ester-mediated Nrf2 activation and IκB kinase inhibition are involved in NFκB inhibitory effect: Structural analysis for NFκB inhibition. Eur. J. Pharmacol. 2010, 643, 21–28. [Google Scholar] [CrossRef]
- Lampiasi, N.; Montana, G. An in vitro inflammation model to study the Nrf2 and NF-κB crosstalk in presence of ferulic acid as modulator. Immunobiology 2018, 223, 349–355. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Su, Z.Y.; Kong, A.N.T. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P+ cells through epigenetics modifications. AAPS J. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Li, P.; Wu, Z.; Chen, Y.; Fu, Y.; Wu, H.; Ye, Y.; Wang, J.; Yang, Z.; et al. The protective effects of myricetin against acute liver failure via inhibiting inflammation and regulating oxidative stress via Nrf2 signaling. Nat. Prod. Res. 2023, 37, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, F.; Dianat, M.; Badavi, M.; Radan, M.; Mard, S.A. Gallic acid suppresses inflammation and oxidative stress through modulating Nrf2-HO-1-NF-κB signaling pathways in elastase-induced emphysema in rats. Environ Sci. Pollut. Res. 2021, 28, 56822–56834. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Guo, Y.; Xu, L.; Wang, H. Phlorizin exerts potent effects against aging induced by D-galactose in mice and PC12 cells. Food Funct. 2021, 12, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, S.; Zhao, A.; Mi, Y.; Zhang, C. Protective effect of rutin on ferroptosis-induced oxidative stress in aging laying hens through Nrf2/HO-1 signaling. Cell. Biol. Int. 2023, 47, 598–611. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]
- Varì, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Li, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
| Gene | Primer Name | Primer Sequence |
|---|---|---|
| SOD1 | SOD-F | GGTGGGCCAAAGGATGAAGA |
| SOD-R | ATAGACACATCGGCCACACC | |
| GPX1 | GPX-F | ACACCCAGATGAACGAGCTG |
| GPX-R | CTTCGTTCTTGGCGTTCTCC | |
| CAT | CAT-F | TGAAGATGCGGCGAGACTTT |
| CAT-R | GAGGGGTACTTTCCTGTGGC | |
| NFE2L2 | NRF2-F | AGGTTGCCCACATTCCCAAA |
| NRF2-R | ACGTAGCCGAAGAAACCTCA | |
| IL-6 | IL6-F | CCACCGGGAACGAAAGAGAA |
| IL6-R | GAGAAGGCAACTGGACCGAA | |
| IL-8 | IL8-F | GAAGAGAGCTCTGTCTGGACC |
| IL8-R | TGAATTCTCAGCCCTCTTCAAAAAC | |
| TNF-α | TNF-F | GAAGAGAGCTCTGTCTGGACC |
| TNF-R | TGAATTCTCAGCCCTCTTCAAAAAC | |
| NFKB-p50 | NFKBP50-F | GGAGGCCGAACGCGG |
| NFKBP50-R | AAACATTTGTTCAGGCCTTCCC | |
| NFKB-p65 | NFKB65-F | CAGTGTGTGAAGAAGCGGGA |
| NFKB65-R | CCACGCTGCTCTTCTTGGAA | |
| β-ACTIN | ACTIN-F | GAGCACAGAGCCTCGCC |
| ACTIN-R | CGCGGCGATATCATCATCCA |
| Parameter | Placebo n = 20 | Experimenta ln = 26 | p-Value |
|---|---|---|---|
| Age (years) | 68.4 ± 5.7 | 67.9 ± 6.6 | |
| SBP (mmHg) | |||
| Baseline | 132.5 ± 15.5 | 141.4 ± 12.1 | |
| 6 months | 132.3 ± 12.1 | 131.2 ± 11.2 * | 0.01 |
| DBP (mmHg) | |||
| Baseline | 90.2 ± 9.9 | 95.0 ± 9.8 | |
| 6 months | 85.6 ± 6.4 | 83.8 ± 8.0 * | 0.001 |
| Weight (kg) | |||
| Baseline | 76.5 ± 14.6 | 74.4 ± 16.5 | |
| 6 months | 76.2 ± 12.8 | 72.4 ± 16.4 * | 0.01 |
| Waist circumference (cm) | |||
| Baseline | 103.6 ± 11.4 | 99.9 ± 12.2 | |
| 6 months | 104.2 ± 11.9 | 102.1 ± 12.1 | 0.55 |
| Parameter | Placebo n = 20 | Experimental n = 26 | p-Value |
|---|---|---|---|
| Glucose (mg/dL) | |||
| Baseline | 137.3 ± 61.3 | 140.7 ± 48.1 | |
| 6 months | 139.4 ± 69.1 | 140.0 ± 57.3 | 0.91 |
| HDL-c (mg/dL) | |||
| Baseline | 47.8 ± 9.5 | 42.8 ± 7.5 | |
| 6 months | 47.4 ± 7.5 | 47.4 ± 7.4 * | 0.01 |
| Triglycerides (mg/dL) | |||
| Baseline | 169.6 ± 36.6 | 174.1 ± 54.8 | |
| 6 months | 125.7 ± 30.4 | 149.2 ± 45.6 | 0.19 |
| Parameter | Placebo n = 20 | Experimental n = 26 | p-Value |
|---|---|---|---|
| SOD (U/mL) | |||
| Baseline | 176.8 ± 10.3 | 167.1 ± 11.9 | |
| 6 months | 174.6 ± 4.9 | 180.6 ± 7.6 * | 0.05 |
| GPx (U/L) | |||
| Baseline | 5740 ± 938 | 5221 ± 822 | |
| 6 months | 5023 ± 1885 | 5559 ± 2007 | 0.33 |
| CAT (U/mL) | |||
| Baseline | 1.2 ± 0.1 | 1.0 ± 0.2 | |
| 6 months | 1.2 ± 0.2 | 1.3 ± 0.2 * | 0.01 |
| TOS (µmol H2O2 Equiv./L) | |||
| Baseline | 24.5 ± 2.3 | 28.9 ± 3.6 | |
| 6 months | 28.0 ± 3.7 | 23.7 ± 3.4 * | 0.01 |
| TAS (mmol/L) | |||
| Baseline | 1.2 ± 0.1 | 1.1 ± 0.1 | |
| 6 months | 1.1 ± 0.2 | 1.4 ± 0.1 * | 0.01 |
| OSI | |||
| Baseline | 19.2 ± 2.1 | 24.1 ± 3.8 | |
| 6 months | 26.1 ± 7.0 | 17.7 ± 4.0 * | 0.01 |
| Parameter | Placebo n = 20 | Experimental n = 26 | p-Value |
|---|---|---|---|
| IL-6 (pg/dL) | |||
| Baseline | 10.7 ± 2.1 | 10.7 ± 1.1 | |
| 6 months | 11.0 ± 0.9 | 12.3 ± 2.0 * | 0.03 |
| IL-8 (pg/dL) | |||
| Baseline | 27.6 ± 4.4 | 37.7 ± 9.9 | |
| 6 months | 25.8 ± 6.0 | 30.9 ± 11.1 | 0.12 |
| TNF-α (pg/dL) | |||
| Baseline | 8.4 ± 1.2 | 8.2 ± 0.6 | |
| 6 months | 8.8 ± 1.6 | 9.2 ± 1.1 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavia-García, G.; Hernández-Álvarez, D.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M.; Rosado-Pérez, J. The Supplementation of Sechium edule var. nigrum spinosum (Chayote) Promotes Nrf2-Mediated Antioxidant Protection in Older Adults with Metabolic Syndrome. Nutrients 2023, 15, 4106. https://doi.org/10.3390/nu15194106
Gavia-García G, Hernández-Álvarez D, Arista-Ugalde TL, Aguiñiga-Sánchez I, Santiago-Osorio E, Mendoza-Núñez VM, Rosado-Pérez J. The Supplementation of Sechium edule var. nigrum spinosum (Chayote) Promotes Nrf2-Mediated Antioxidant Protection in Older Adults with Metabolic Syndrome. Nutrients. 2023; 15(19):4106. https://doi.org/10.3390/nu15194106
Chicago/Turabian StyleGavia-García, Graciela, David Hernández-Álvarez, Taide Laurita Arista-Ugalde, Itzen Aguiñiga-Sánchez, Edelmiro Santiago-Osorio, Víctor Manuel Mendoza-Núñez, and Juana Rosado-Pérez. 2023. "The Supplementation of Sechium edule var. nigrum spinosum (Chayote) Promotes Nrf2-Mediated Antioxidant Protection in Older Adults with Metabolic Syndrome" Nutrients 15, no. 19: 4106. https://doi.org/10.3390/nu15194106
APA StyleGavia-García, G., Hernández-Álvarez, D., Arista-Ugalde, T. L., Aguiñiga-Sánchez, I., Santiago-Osorio, E., Mendoza-Núñez, V. M., & Rosado-Pérez, J. (2023). The Supplementation of Sechium edule var. nigrum spinosum (Chayote) Promotes Nrf2-Mediated Antioxidant Protection in Older Adults with Metabolic Syndrome. Nutrients, 15(19), 4106. https://doi.org/10.3390/nu15194106






