Effects of Whey Protein Supplementation on Inflammatory Marker Concentrations in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
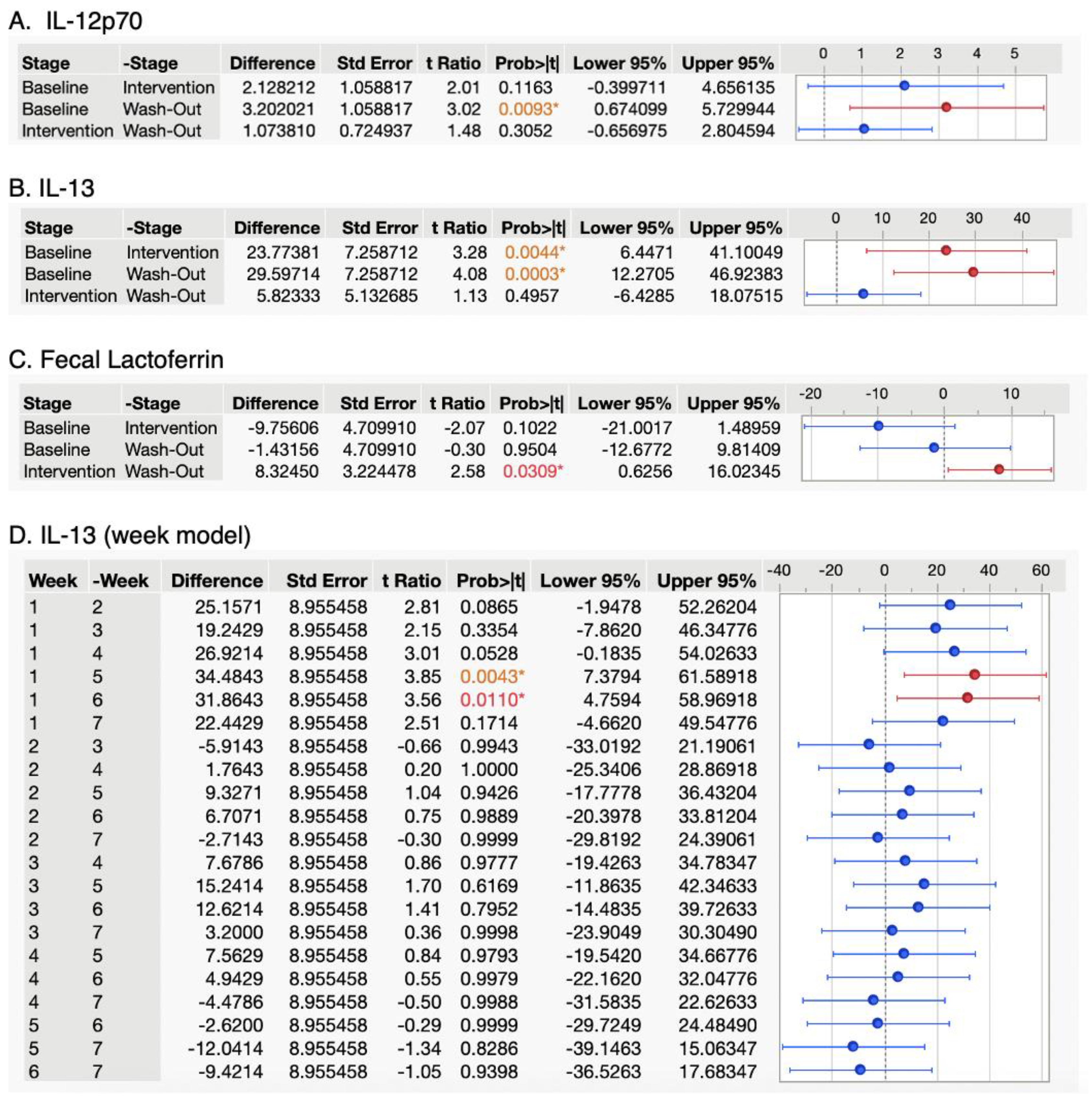
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Gabalawy, H.; Guenther, L.C.; Bernstein, C.N. Epidemiology of Immune-Mediated Inflammatory Diseases: Incidence, Prevalence, Natural History, and Comorbidities. J. Rheumatol. Suppl. 2010, 85, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-Grade Inflammation, Diet Composition and Health: Current Research Evidence and Its Translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Chen, B.; Gao, P.; Song, B.; Zhang, S.; Pang, X.; Hettinga, K.; Lyu, J. Comparison of Whey Proteome and Glycoproteome in Bovine Colostrum and Mature Milk. J. Agric. Food Chem. 2023, 71, 10863–10876. [Google Scholar] [CrossRef]
- Cardinale, V.; Lepore, E.; Basciani, S.; Artale, S.; Nordio, M.; Bizzarri, M.; Unfer, V. Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome. Nutrients 2022, 14, 3220. [Google Scholar] [CrossRef]
- Conneely, O.M. Antiinflammatory Activities of Lactoferrin. J. Am. Coll. Nutr. 2001, 20, 389S–395S; discussion 396S–397S. [Google Scholar] [CrossRef]
- Foisy-Sauvé, M.; Ahmarani, L.; Delvin, E.; Sané, A.T.; Spahis, S.; Levy, E. Glycomacropeptide Prevents Iron/Ascorbate-Induced Oxidative Stress, Inflammation and Insulin Sensitivity with an Impact on Lipoprotein Production in Intestinal Caco-2/15 Cells. Nutrients 2020, 12, 1175. [Google Scholar] [CrossRef]
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tomé, D.; Leonil, J. Sequential Release of Milk Protein-Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk Bioactive Peptide Database: A Comprehensive Database of Milk Protein-Derived Bioactive Peptides and Novel Visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Dávalos, L.E.; Jiménez, M.; Salinas, E. Glycomacropeptide Bioactivity and Health: A Review Highlighting Action Mechanisms and Signaling Pathways. Nutrients 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Feeney, S.; Ryan, J.T.; Kilcoyne, M.; Joshi, L.; Hickey, R. Glycomacropeptide Reduces Intestinal Epithelial Cell Barrier Dysfunction and Adhesion of Entero-Hemorrhagic and Entero-Pathogenic Escherichia Coli in vitro. Foods 2017, 6, 93. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Mazurier, J.; Spik, G.; Legrand, D. Lactoferrin: A Multifunctional Glycoprotein Involved in the Modulation of the Inflammatory Process. Clin. Chem. Lab. Med. 1999, 37, 281–286. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of Whey and Soy Protein Supplementation on Inflammatory Cytokines in Older Adults: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [CrossRef]
- Abraham, B.P.; Kane, S. Fecal Markers: Calprotectin and Lactoferrin. Gastroenterol. Clin. N. Am. 2012, 41, 483–495. [Google Scholar] [CrossRef]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.-K.; Seo, S.J. Particulate Matter Induces Pro-Inflammatory Cytokines via Phosphorylation of P38 MAPK Possibly Leading to Dermal Inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Drouin, R.; Pouliot, Y.; Gauthier, S.; Poubelle, P.E. A Bovine Whey Protein Extract Stimulates Human Neutrophils to Generate Bioactive IL-1Ra through a NF-KappaB- and MAPK-Dependent Mechanism. J. Nutr. 2010, 140, 382–391. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.P.; Vale, V.L.C.; Silva, M.d.C.; de Oliveira Araújo, I.B.; Trindade, S.C.; Moura-Costa, L.F.; de Rodrigues, G.C.; Sales, T.S.; dos Santos, H.A.; de Carvalho-Filho, P.C.; et al. MAPK Involvement in Cytokine Production in Response to Corynebacterium Pseudotuberculosis Infection. BMC Microbiol. 2014, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Van Eldik, L.J. The P38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging Dis. 2010, 1, 199–211. [Google Scholar]
- Dalziel, J.E.; Anderson, R.C.; Bassett, S.A.; Lloyd-West, C.M.; Haggarty, N.W.; Roy, N.C. Influence of Bovine Whey Protein Concentrate and Hydrolysate Preparation Methods on Motility in the Isolated Rat Distal Colon. Nutrients 2016, 8, 809. [Google Scholar] [CrossRef]
- Jamshidi, S.; Mohsenpour, M.A.; Masoumi, S.J.; Fatahi, S.; Nasimi, N.; Zahabi, E.S.; Pourrajab, B.; Shidfar, F. Effect of Whey Protein Consumption on IL-6 and TNF-α: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. 2022, 16, 102372. [Google Scholar] [CrossRef]
- Vincent, F.B.; Nim, H.T.; Lee, J.P.W.; Morand, E.F.; Harris, J. Effect of Storage Duration on Cytokine Stability in Human Serum and Plasma. Cytokine 2019, 113, 453–457. [Google Scholar] [CrossRef]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine Levels in the Serum of Healthy Subjects. Mediators Inflamm. 2013, 2013, 434010. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, H.-S.; Youn, J.-C.; Shin, E.-C.; Park, S. Serum Cytokine Profiles in Healthy Young and Elderly Population Assessed Using Multiplexed Bead-Based Immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef]
- Biancotto, A.; Wank, A.; Perl, S.; Cook, W.; Olnes, M.J.; Dagur, P.K.; Fuchs, J.C.; Langweiler, M.; Wang, E.; McCoy, J.P. Baseline Levels and Temporal Stability of 27 Multiplexed Serum Cytokine Concentrations in Healthy Subjects. PLoS ONE 2013, 8, e76091. [Google Scholar] [CrossRef]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for Evaluation of Chronic Inflammatory Status in Ageing Research: Reliability and Phenotypic Characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. IL-13 Effector Functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.E. The Role of IL-13 and Its Receptor in Allergy and Inflammatory Responses. J. Allergy Clin. Immunol. 1998, 102, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Minty, A.; Chalon, P.; Derocq, J.M.; Dumont, X.; Guillemot, J.C.; Kaghad, M.; Labit, C.; Leplatois, P.; Liauzun, P.; Miloux, B.; et al. Interleukin-13 Is a New Human Lymphokine Regulating Inflammatory and Immune Responses. Nature 1993, 362, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Roldán, N.R.; Jiménez, M.; Cervantes-García, D.; Marín, E.; Salinas, E. Glycomacropeptide Administration Attenuates Airway Inflammation and Remodeling Associated to Allergic Asthma in Rat. Inflamm. Res. 2016, 65, 273–283. [Google Scholar] [CrossRef]
- Reyes-Pavón, D.; Cervantes-García, D.; Bermúdez-Humarán, L.G.; Córdova-Dávalos, L.E.; Quintanar-Stephano, A.; Jiménez, M.; Salinas, E. Protective Effect of Glycomacropeptide on Food Allergy with Gastrointestinal Manifestations in a Rat Model through Down-Regulation of Type 2 Immune Response. Nutrients 2020, 12, 2942. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, C.; Ming, Z.; Cao, J.; Yan, Y.; Zhao, P.; Pang, G.; Deng, Z.; Yao, Y.; Chen, Q. Molecular Mechanisms by Which Casein Glycomacropeptide Maintains Internal Homeostasis in Mice with Experimental Ulcerative Colitis. PLoS ONE 2017, 12, e0181075. [Google Scholar] [CrossRef]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) Family Cytokines: Role in Immune Pathogenesis and Treatment of CNS Autoimmune Disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic Biology and Role of Interleukin-17 in Immunity and Inflammation. Periodontol. 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Berger, A. Th1 and Th2 Responses: What Are They? BMJ 2000, 321, 424. [Google Scholar] [CrossRef]
- Wong, S.H.; Francis, N.; Chahal, H.; Raza, K.; Salmon, M.; Scheel-Toellner, D.; Lord, J.M. Lactoferrin Is a Survival Factor for Neutrophils in Rheumatoid Synovial Fluid. Rheumatol. Oxf. Engl. 2009, 48, 39–44. [Google Scholar] [CrossRef] [PubMed]
| Serving Size | 59 g |
|---|---|
| Calories | 230 |
| Nutrients | |
| Protein | 35 g |
| Carbs | 19 g (16 g added sugar) |
| Fat | 0 g |
| Cholesterol | 5 mg |
| Sodium | 220 mg |
| Calcium | 223 mg |
| Iron | 1 mg |
| Potassium | 192 mg |
| Marker | Range of Quantification (pg/mL) Unless Other Stated | Limit of Detection (pg/mL) Unless Other Stated | Proportion of Values below LOQ |
|---|---|---|---|
| IFN-γ | 1.31–20,000 | 0.86 | 60/98 |
| IL-1β | 1.6–25,000 | 0.52 | 31/98 |
| IL-1RA | 1.6–25,000 | 1.29 | 0/98 |
| IL-2 | 0.64–10,000 | 0.28 | 60/98 |
| IL-3 | 1.3–20,000 | 0.28 | 98/98 |
| IL-4 | 0.64–10,000 | 0.2 | 38/98 |
| IL-5 | 0.64–10,000 | 0.17 | 1/98 |
| IL-6 | 0.64–10,000 | 0.14 | 34/98 |
| IL-7 | 0.64–10,000 | 0.14 | 7/98 |
| IL-8 | 0.64–10,000 | 0.52 | 7/98 |
| IL-9 | 0.64–10,000 | 3.05 | 41/98 |
| IL-10 | 2.6–40,000 | 0.91 | 28/98 |
| IL-12p70 | 3–50,000 | 0.88 | 45/98 |
| IL-13 | 6.4–100,000 | 2.58 | 25/98 |
| IL-17A | 1.3–20,000 | 0.71 | 42/98 |
| TNF-α | 6.4–100,000 | 5.39 | 7/98 |
| Fecal calprotectin | 4.77–840 [ng/cm3] | 2.27 [ng/cm3] | 0/98 |
| Fecal lactoferrin | 0.37–240 [ng/cm3] | 0.37 [ng/cm3] | 0/98 |
| Total | N/A | N/A | 523/1862 |
| Inflammatory Marker 1 | Study Stage (Number of Weeks) | ||
|---|---|---|---|
| Baseline, 2 (1 Week) | Intervention (3 Weeks) | Washout (3 Weeks) | |
| IFN-γ | 5.56 ± 2.77 | 3.11 ± 0.83 | 3.79 ± 0.83 |
| Il-1β | 8.21 ± 1.92 | 7.30 ± 1.01 | 7.79 ± 1.17 |
| IL-1RA | 3.48 ± 0.72 | 3.70 ± 0.49 | 3.66 ± 0.71 |
| IL-2 | 1.28 ± 0.39 | 1.19 ± 0.21 | 1.01 ± 0.11 |
| IL-4 | 3.74 ± 2.29 | 4.12 ± 1.89 | 3.57 ± 1.48 |
| IL-5 | 4.39 ± 0.80 | 3.60 ± 0.33 | 3.96 ± 0.42 |
| IL-6 | 1.99 ± 1.01 | 2.07 ± 0.55 | 1.62 ± 0.46 |
| IL-7 | 2.59 ± 0.40 | 2.97 ± 0.26 | 2.81 ± 0.29 |
| IL-8 | 3.23 ± 0.51 | 3.71 ± 0.38 | 3.55 ± 0.38 |
| IL-9 | 32.7 ± 9.8 | 24.6 ± 4.7 | 29.8 ± 5.4 |
| IL-10 | 7.65 ± 1.72 | 8.46 ± 1.16 | 7.75 ± 1.12 |
| IL-12p70 3 | 7.27 ± 2.59 A | 5.04 ± 0.76 AB | 3.96 ± 0.46 B |
| IL-13 3,4 | 52.7 ± 14.3 A | 28.9 ± 5.8 B | 23.1 ± 4.3 B |
| IL-17A | 5.88 ± 1.73 | 6.30 ± 1.18 | 5.65 ± 1.09 |
| TNF-α | 24.3 ± 3.70 | 20.5 ± 2.19 | 18.9 ± 2.08 |
| Fecal calprotectin | 22.2 ± 7.6 | 31.9 ± 4.7 | 27.8 ± 4.6 |
| Fecal lactoferrin 3 | 0.09 ± 0.03 B | 0.23 ± 0.05 A | 0.15 ± 0.02 B |
| Cytokine | Significance in the Stage Model | Significance in the Week Model |
|---|---|---|
| IFN-γ | 0.20 1 | 0.47 |
| Il-1β | 0.74 | 0.86 |
| IL-1RA | 0.93 | 0.85 |
| IL-2 | 0.40 | 0.22 |
| IL-3 | NA | NA |
| IL-4 | 0.65 | 0.70 |
| IL-5 | 0.11 | 0.39 |
| IL-6 | 0.39 | 0.39 |
| IL-7 | 0.50 | 0.64 |
| IL-8 | 0.51 | 0.87 |
| IL-9 | 0.10 | 0.21 |
| IL-10 | 0.38 | 0.79 |
| IL-12p70 | 0.01 | 0.07 |
| IL-13 | 0.0005 | 0.0071 |
| IL-17A | 0.81 | 0.57 |
| TNF-α | 0.28 | 0.42 |
| Fecal calprotectin | 0.41 | 0.25 |
| Fecal lactoferrin | 0.02 | 0.13 |
| Effect Size: | 0.2 1 |
|---|---|
| α | 0.05 |
| Sample size | 14 |
| Number of groups | 1 |
| Number of measurements 2 | 3 |
| Correlation among repeated measures 3 | 0.55 2 |
| Computed current study power | 0.35 |
| Computed minimum ideal sample size for future studies 4 | 38 |
| Cytokine | This Study | Kleiner et al., 2013 [28] | Kim et al., 2019 [29] 1 | Biancotto et al., 2013 [30] |
|---|---|---|---|---|
| IFN-γ | 5.51 2 | ~160 | 13.1–10.3 | 543–823 3 |
| Il-1β | 8.21 | <3.2 | 2.04–2.52 | 4.6–4.3 |
| IL-1RA | 3.50 | 279–305 | ||
| IL-2 | 1.30 | 14 | 5.13–5.18 | N/A |
| IL-3 | N/A 4 | <12 | ||
| IL-4 | 3.70 | ~8 | 33.4–36 | |
| IL-5 | 4.40 | 5.8–8 | ||
| IL-6 | 1.98 | ~11 | 2.91–2.57 | 18.3–24.9 |
| IL-7 | 2.50 | 13.5 | 52.2–59.5 | |
| IL-8 | 3.22 | 29.3 | 23.9–27.6 | 38.3–44.2 |
| IL-9 | 32.7 | 23.3 | 113.5–573.3 | |
| IL-10 | 7.65 | 12.6 | 3.06–6.16 | 2.7 |
| IL-12p70 | 7.04 | 34.8 | 7.4–12.2 | 42.3–53.4 |
| IL-13 | 52.7 | ~15 | 11.2–14.1 | |
| IL-17A | 5.88 | 208–202 | ||
| TNF-α | 24.3 | ~30 | 3.21–4.94 | 37–68.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adler, S.; Olsen, W.; Rackerby, B.; Spencer, R.; Dallas, D.C. Effects of Whey Protein Supplementation on Inflammatory Marker Concentrations in Older Adults. Nutrients 2023, 15, 4081. https://doi.org/10.3390/nu15184081
Adler S, Olsen W, Rackerby B, Spencer R, Dallas DC. Effects of Whey Protein Supplementation on Inflammatory Marker Concentrations in Older Adults. Nutrients. 2023; 15(18):4081. https://doi.org/10.3390/nu15184081
Chicago/Turabian StyleAdler, Samuel, Wyatt Olsen, Bryna Rackerby, Rachel Spencer, and David C. Dallas. 2023. "Effects of Whey Protein Supplementation on Inflammatory Marker Concentrations in Older Adults" Nutrients 15, no. 18: 4081. https://doi.org/10.3390/nu15184081
APA StyleAdler, S., Olsen, W., Rackerby, B., Spencer, R., & Dallas, D. C. (2023). Effects of Whey Protein Supplementation on Inflammatory Marker Concentrations in Older Adults. Nutrients, 15(18), 4081. https://doi.org/10.3390/nu15184081







