Associations between Milk Intake and Sleep Disorders in Chinese Adults: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
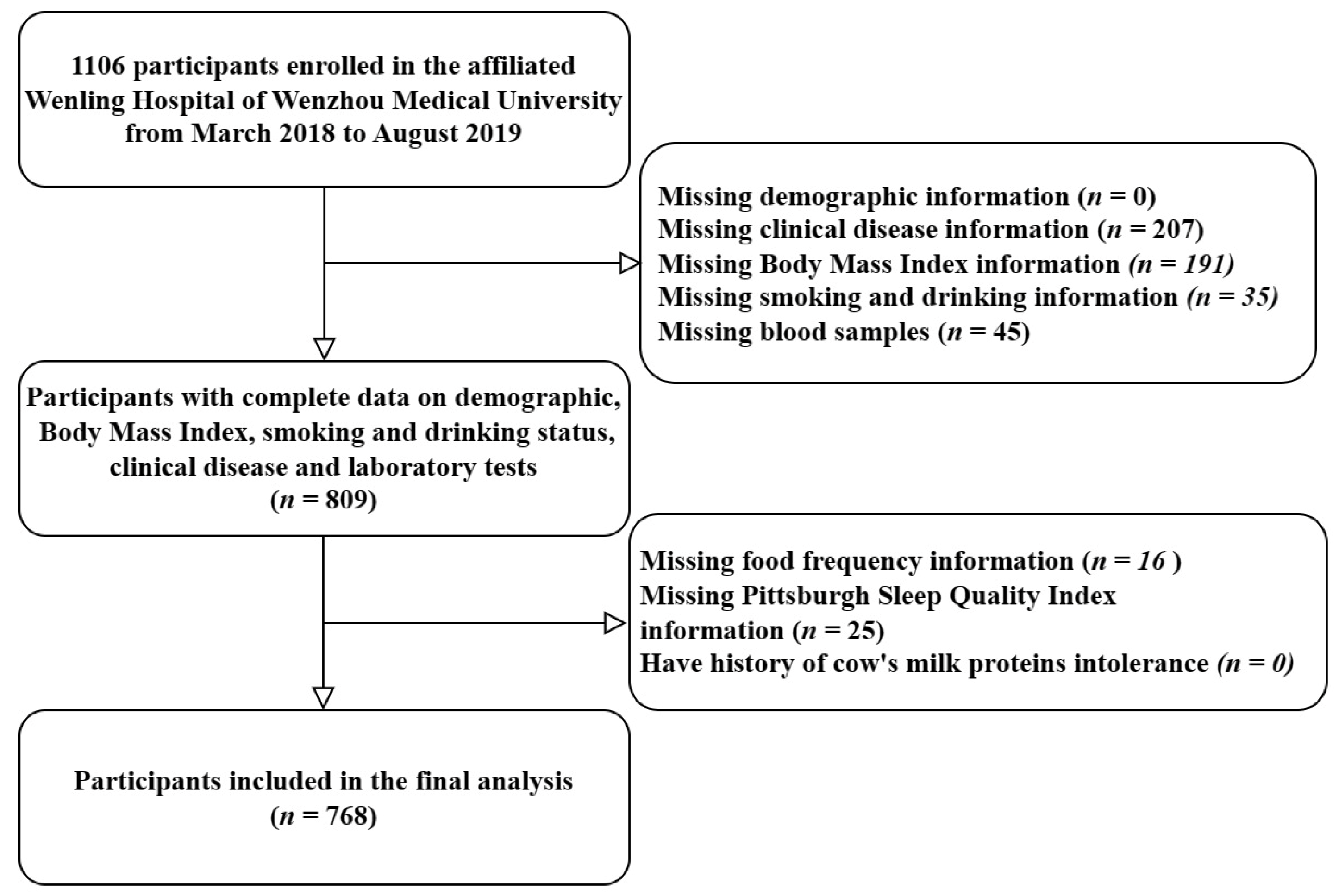
2.2. Participants
2.3. Clinical and Biochemical Measurements
2.4. Dietary Assessment
2.5. Sleep Quality
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association between Milk Intake and Sleep Disorders
3.3. Association between Milk Intake and Biochemical Parameters
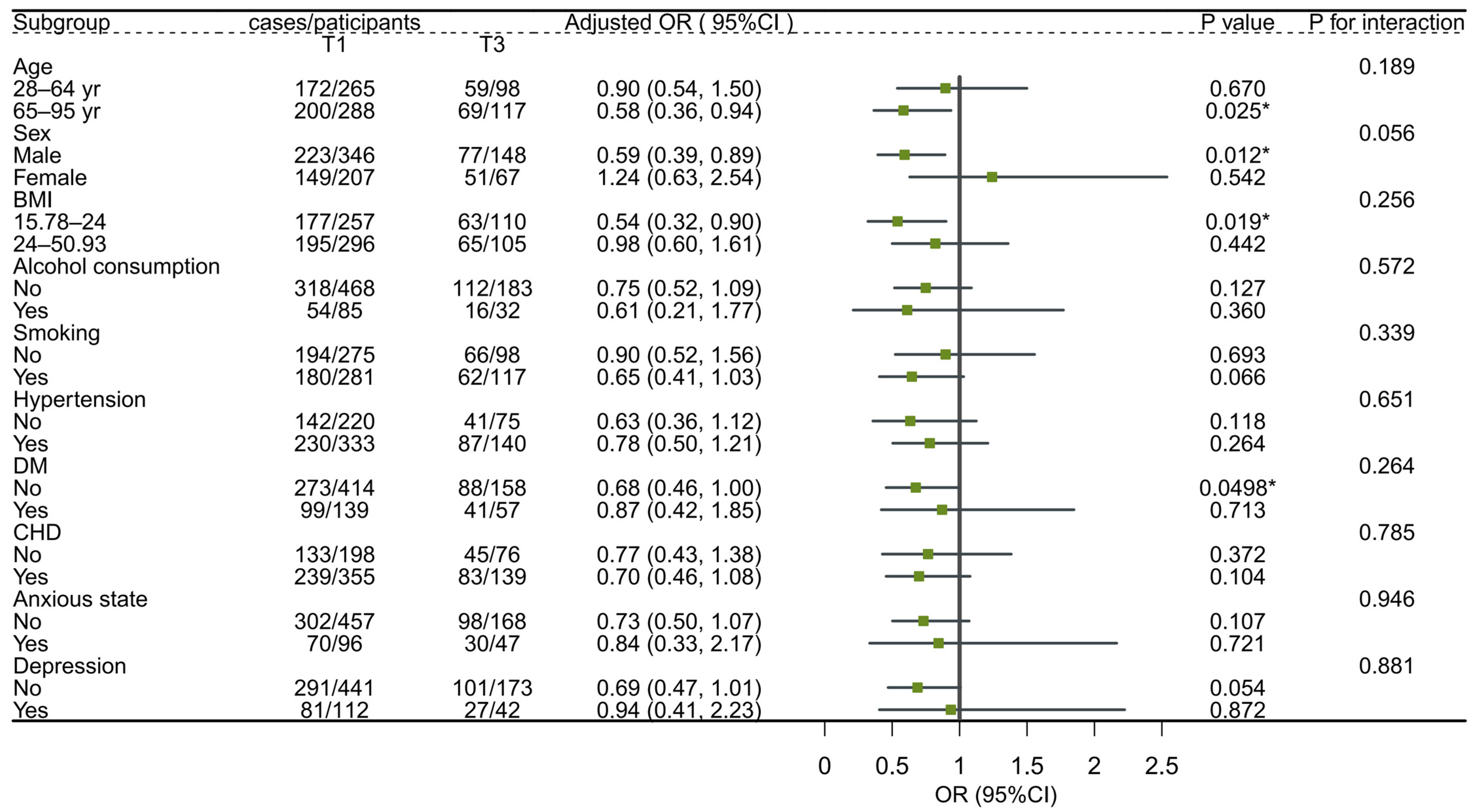
3.4. Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inoue, M.; Matsumura, K.; Sugimori, N.; Hamazaki, K.; Tsuchida, A.; Inadera, H. Dietary intake of yogurt and cheese in children at age 1 year and sleep duration at age 1 and 3 years: The Japan Environment and Children’s Study. BMC Pediatr. 2022, 22, 624. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, C.; Zhu, Y.; Tang, X.; Ling, L. The Obesity−Related Dietary Pattern Is Associated with Higher Risk of Sleep Disorders: A Cross−Sectional Study from NHANES. Nutrients 2022, 14, 3987. [Google Scholar] [CrossRef] [PubMed]
- Al Lihabi, A. A literature review of sleep problems and neurodevelopment disorders. Front. Psychiatry 2023, 14, 1122344. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.; Ren, Z.; Liu, X.; Niu, X. Sleep disorder, Mediterranean diet, and all-cause and cause-specific mortality: A prospective cohort study. BMC Public Health 2023, 23, 904. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.C.; Baylin, A.; Cantoral, A.; Rojo, M.M.T.; Burgess, H.J.; O’Brien, L.M.; Olascoaga, L.T.; Peterson, K.E. Dietary Patterns in Relation to Prospective Sleep Duration and Timing among Mexico City Adolescents. Nutrients 2020, 12, 2305. [Google Scholar] [CrossRef]
- Milagres, M.P.; Minim, V.P.; Minim, L.A.; Simiqueli, A.A.; Moraes, L.E.; Martino, H.S. Night milking adds value to cow’s milk. J. Sci. Food Agric. 2014, 94, 1688–1692. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rakhshandeh, H.; Sadeghnia, H.R. Potentiating Effects of Lactuca sativa on Pentobarbital−Induced Sleep. Iran. J. Pharm. Res. 2013, 12, 401–406. [Google Scholar]
- Lin, H.-H.; Tsai, P.-S.; Fang, S.-C.; Liu, J.-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174. [Google Scholar]
- Zeng, Y.; Yang, J.; Du, J.; Pu, X.; Yang, X.; Yang, S.; Yang, T. Strategies of Functional Foods Promote Sleep in Human Being. Curr. Signal Transduct. Ther. 2014, 9, 148–155. [Google Scholar] [CrossRef]
- Kitano, N.; Tsunoda, K.; Tsuji, T.; Osuka, Y.; Jindo, T.; Tanaka, K.; Okura, T. Association between difficulty initiating sleep in older adults and the combination of leisure−time physical activity and consumption of milk and milk products: A cross−sectional study. BMC Geriatr. 2014, 14, 118. [Google Scholar] [CrossRef]
- Jansen, E.C.; Corcoran, K.; Perng, W.; Dunietz, G.L.; Cantoral, A.; Zhou, L.; Téllez-Rojo, M.M.; Peterson, E.K. Relationships of beverage consumption and actigraphy−assessed sleep parameters among urban−dwelling youth from Mexico. Public Health Nutr. 2021, 25, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Peña, I.J.I.D.; Kim, H.J.; de la Peña, J.B.; Kim, M.; Botanas, C.J.; You, K.Y.; Woo, T.; Lee, Y.S.; Jung, J.-C.; Kim, K.-M.; et al. Cheong. A tryptic hydrolysate from bovine milk αs1−casein enhances pentobarbital−induced sleep in mice via the GABAA receptor. Behav. Brain Res. 2016, 313, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Tambalis, K.D.; Panagiotakos, D.B.; Psarra, G.; Sidossis, L.S. Recommended dairy intake is associated with healthy dietary habits, better physical fitness, less obesity and a healthier lifestyle profile in school age children. Br. J. Nutr. 2022, 128, 2046–2053. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, J.M.; Hu, R.Y.; Gong, W.W.; Yu, M. Sleep duration and behavioral correlates in middle and high school students: A cross-sectional study in Zhejiang province, China. Sleep Med. 2021, 79, 55–61. [Google Scholar] [CrossRef]
- Yim, H.R.; Yun, H.J.; Lee, J.H. An Investigation on Korean Adolescents’ Dietary Consumption: Focused on Sociodemographic Characteristics, Physical Health, and Mental Health. Int. J. Environ. Res. Public Health. 2021, 18, 9773. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, L.; Sun, Y.; Yang, W.; Wang, J.; Wu, S.; Gao, X. Adherence to the Dietary Approaches to Stop Hypertension Diet and Hyperuricemia: A Cross−Sectional Study. Arthritis Care Res. 2021, 73, 603–611. [Google Scholar] [CrossRef]
- Siena, L.A.; A Ortiz, J.P.A.; Leblanc, O.; Pessino, S. PnTgs1−like expression during reproductive development supports a role for RNA methyltransferases in the aposporous pathway. BMC Plant Biol. 2014, 14, 297. [Google Scholar] [CrossRef]
- Luo, T.Y.; Liu, X.H.; Dai, T.Y.; Liu, X.M.; Zhang, Q.; Dong, J.Z. Ideal Cardiovascular Health Metrics and Coronary Artery Calcification in Northern Chinese Population: A Cross−sectional Study. Biomed. Environ. Sci. 2016, 29, 475–483. [Google Scholar]
- Zhuang, M.; Yuan, Z.; Lin, L.; Hu, B.; Wang, X.; Yang, Y.; Chen, X.; Jin, L.; Lu, M.; Ye, W. Reproducibility and relative validity of a food frequency questionnaire developed for adults in Taizhou, China. PLoS ONE 2012, 7, e48341. [Google Scholar] [CrossRef]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double−blind, Randomized, Placebo−controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef]
- Nsengimana, A.; Mugabo, E.; Niyonsenga, J.; Hategekimana, J.C.; Biracyaza, E.; Mutarambirwa, R.; Ngabo, E.; Nduwayezu, R. Sleep quality among undergraduate medical students in Rwanda: A comparative study. Sci. Rep. 2023, 13, 265. [Google Scholar] [CrossRef]
- van Egmond, L.; Tan, X.; Sjögren, P.; Cederholm, T.; Benedict, C. Association between Healthy Dietary Patterns and Self−Reported Sleep Disturbances in Older Men: The ULSAM Study. Nutrients 2019, 11, 1029. [Google Scholar] [CrossRef]
- Komada, Y.; Okajima, I.; Kuwata, T. The Effects of Milk and Dairy Products on Sleep: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9440. [Google Scholar] [CrossRef]
- Peña, I.J.I.D.; Hong, E.; de la Peña, J.B.; Kim, H.J.; Botanas, C.J.; Hong, Y.S.; Hwang, Y.S.; Moon, B.S.; Cheong, J.H.; Cicero, A.F.; et al. Milk Collected at Night Induces Sedative and Anxiolytic−Like Effects and Augments Pentobarbital−Induced Sleeping Behavior in Mice. J. Med. Food 2015, 18, 1255–1261. [Google Scholar] [CrossRef]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. Consumption of OLL1073R−1 yogurt improves psychological quality of life in women healthcare workers: Secondary analysis of a randomized controlled trial. BMC Gastroenterol. 2021, 21, 237. [Google Scholar] [CrossRef]
- Valtonen, M.; Niskanen, L.; Kangas, A.-P.; Koskinen, T. Effect of melatonin−rich night−time milk on sleep and activity in elderly institutionalized subjects. Nord. J. Psychiatry 2005, 59, 217–221. [Google Scholar] [CrossRef]
- Karunanithi, D.; Radhakrishna, A.; Sivaraman, K.P.; Biju, V.M.N. Quantitative determination of melatonin in milk by LC−MS/MS. J. Food Sci. Technol. 2014, 51, 805–812. [Google Scholar] [CrossRef]
- Sangsopha, J.; Moongngarm, A.; Johns, N.P.; Grigg, N.P. Optimization of pasteurized milk with soymilk powder and mulberry leaf tea based on melatonin, bioactive compounds and antioxidant activity using response surface methodology. Heliyon 2019, 5, e02939. [Google Scholar] [CrossRef]
- Ma, J.; Liu, T.; Qu, J. The effect of orbital implantation on peripheral blood melatonin and sex hormone levels in child patients with congenital eyeball dysplasia. Exp. Ther. Med. 2017, 14, 2211–2215. [Google Scholar] [CrossRef][Green Version]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Moreno−Fernandez, J.; Diaz−Castro, J.; Alférez, M.J.; Nestares, T.; Ochoa, J.J.; Sánchez−Alcover, A.; López−Aliaga, I. Fermented goat milk consumption improves melatonin levels and influences positively the antioxidant status during nutritional ferropenic anemia recovery. Food Funct. 2016, 7, 834–842. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Sangsopha, J.; Johns, N.P.; Johns, J.; Moongngarm, A. Dietary sources of melatonin and benefits from production of high melatonin pasteurized milk. J. Food Sci. Technol. 2020, 57, 2026–2037. [Google Scholar] [CrossRef]
- Madrid−Valero, J.J.; Sánchez−Romera, J.F.; Martínez−Selva, J.M.; Ordoñana, J.R. Phenotypic, Genetic and Environmental Architecture of the Components of Sleep Quality. Behav. Genet. 2022, 52, 236–245. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Cohen, B.E.; Neylan, T.C.; Whooley, M.A. Gender differences in the prospective associations of self−reported sleep quality with biomarkers of systemic inflammation and coagulation: Findings from the Heart and Soul Study. J. Psychiatr. Res. 2013, 47, 1228–1235. [Google Scholar] [CrossRef]
- Yasuda, J.; Yoshizaki, T.; Yamamoto, K.; Yoshino, M.; Ota, M.; Kawahara, T.; Kamei, A. Association of Frequency of Milk or Dairy Product Consumption with Subjective Sleep Quality during Training Periods in Japanese Elite Athletes: A Cross−Sectional Study. J. Nutr. Sci. Vitaminol. 2019, 65, 177–183. [Google Scholar] [CrossRef]
- Min, C.; Kim, H.J.; Park, I.S.; Park, B.; Kim, J.H.; Sim, S.; Choi, H.G. The association between sleep duration, sleep quality, and food consumption in adolescents: A cross−sectional study using the Korea Youth Risk Behavior Web−based Survey. BMJ Open 2018, 8, e022848. [Google Scholar] [CrossRef]
- Mozaffarian, N.; Heshmat, R.; Ataie-Jafari, A.; Motlagh, M.E.; Ziaodini, H.; Shafiee, G.; Taheri, M.; Mansourian, M.; Qorbani, M.; Kelishadi, R. Association of sleep duration and snack consumption in children and adolescents: The CASPIAN−V study. Food Sci. Nutr. 2020, 8, 1888–1897. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Xia, J.; Zhao, L.; Yang, Y. Consumption of meat and dairy products in China: A review. Proc. Nutr. Soc. 2016, 75, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Umesawa, M.; Yoshioka, T.; Iso, H. Systolic Blood Pressure and Objective Hearing Thresholds among Japanese Middle−Aged Adults: A Facility−Based Retrospective Cohort Study. Otol. Neurotol. 2023, 44, e268–e272. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.C.; Irwin, C.; Vincent, G.E.; Khalesi, S. The Relationship between Diet and Sleep in Older Adults: A Narrative Review. Curr. Nutr. Rep. 2021, 10, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Morishima, H.O.; Kumano-go, T.; Suganuma, N.; Matsumoto, H.; Adachi, H.; Sigedo, Y.; Mikami, A.; Kai, T.; Masuyama, A.; et al. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur. J. Clin. Nutr. 2009, 63, 100–105. [Google Scholar] [CrossRef]
- Chu, H.S.; Oh, J.; Lee, K. The Relationship between Living Arrangements and Sleep Quality in Older Adults: Gender Differences. Int. J. Environ. Res. Public Health 2022, 19, 3893. [Google Scholar] [CrossRef]
- Sakaniwa, R.; Noguchi, M.; Imano, H.; Shirai, K.; Tamakoshi, A.; Iso, H. Impact of modifiable healthy lifestyle adoption on lifetime gain from middle to older age. Age Ageing 2022, 51, afac080. [Google Scholar] [CrossRef]
- Lee, M.-N.; Kim, S.-D.; Choi, Y.-S. The Relationship between Physical Activity and Health−Related Quality of Life (HINT−Eight) in Middle−Aged Korean Women. J. Environ. Public Health 2022, 2022, 4555547. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, J.; Lee, S.-M. Combined treatment of isoflavone supplementation and exercise restores the changes in hepatic protein expression in ovariectomized rats—A proteomics approach. J. Int. Soc. Sports Nutr. 2014, 11, 29. [Google Scholar] [CrossRef]
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef]
- Oh, N.S.; Kim, K.; Oh, S.; Kim, Y. Enhanced Production of Galactooligosaccharides Enriched Skim Milk and Applied to Potentially Synbiotic Fermented Milk with Lactobacillus rhamnosus 4B15. Food Sci. Anim. Resour. 2019, 39, 725–741. [Google Scholar] [CrossRef]
- Min, Q.Q.; Qin, L.Q.; Sun, Z.Z.; Zuo, W.T.; Zhao, L.; Xu, J.Y. Effects of Metformin Combined with Lactoferrin on Lipid Accumulation and Metabolism in Mice Fed with High−Fat Diet. Nutrients 2018, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Zhou, J.; Kim, J.E.; Campbell, W.W. Incorporating Milk Protein Isolate into an Energy−Restricted Western−Style Eating Pattern Augments Improvements in Blood Pressure and Triglycerides, but Not Body Composition Changes in Adults Classified as Overweight or Obese: A Randomized Controlled Trial. Nutrients 2020, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Gandhi, S.; Shamshoum, H.; Trottier, S.K.; Mutch, D.M.; Reimer, R.A.; Shearer, J.; LeBlanc, P.J.; Wright, D.C. Exercise and Dairy Protein have Distinct Effects on Indices of Liver and Systemic Lipid Metabolism. Obesity 2020, 28, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wat, E.; Tandy, S.; Kapera, E.; Kamili, A.; Chung, R.W.; Brown, A.; Rowney, M.; Cohn, J.S. Dietary phospholipid−rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high−fat diet. Atherosclerosis 2009, 205, 144–150. [Google Scholar] [CrossRef]
- Santesso, N.; Akl, E.A.; Bianchi, M.; Mente, A.; Mustafa, R.; Heels−Ansdell, D.; Schünemann, H.J. Effects of higher− versus lower−protein diets on health outcomes: A systematic review and meta−analysis. Eur. J. Clin. Nutr. 2012, 66, 780–788. [Google Scholar] [CrossRef]
- Budoff, M. Triglycerides and Triglyceride−Rich Lipoproteins in the Causal Pathway of Cardiovascular Disease. Am. J. Cardiol. 2016, 118, 138–145. [Google Scholar] [CrossRef]
- Peila, R.; Xue, X.; Feliciano, E.M.C.; Allison, M.; Sturgeon, S.; Zaslavsky, O.; Stone, K.L.; Ochs-Balcom, H.M.; Mossavar-Rahmani, Y.; Crane, T.E.; et al. Association of sleep duration and insomnia with metabolic syndrome and its components in the Women’s Health Initiative. BMC Endocr. Disord. 2022, 22, 228. [Google Scholar] [CrossRef]
- Shigiyama, F.; Kumashiro, N.; Tsuneoka, Y.; Igarashi, H.; Yoshikawa, F.; Kakehi, S.; Funato, H.; Hirose, T. Mechanisms of sleep deprivation−induced hepatic steatosis and insulin resistance in mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E848–E858. [Google Scholar] [CrossRef]
- Kim, C.E.; Shin, S.; Lee, H.-W.; Lim, J.; Lee, J.-K.; Shin, A.; Kang, D. Association between sleep duration and metabolic syndrome: A cross−sectional study. BMC Public Health 2018, 18, 720. [Google Scholar] [CrossRef]
- Fan, L.; Hao, Z.; Gao, L.; Qi, M.; Feng, S.; Zhou, G. Non−linear relationship between sleep duration and metabolic syndrome: A population−based study. Medicine 2020, 99, e18753. [Google Scholar] [CrossRef]
- Spira, A.P.; Beaudreau, S.A.; Stone, K.L.; Kezirian, E.J.; Lui, L.-Y.; Redline, S.; Ancoli-Israel, S.; Ensrud, K.; Stewart, A. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Rahat, H.; Carreau, A.M.; Garcia−Reyes, Y.; Halbower, A.; Pyle, L.; Nadeaum, K.J.; Cree−Green, M. Poor Sleep Is Related to Metabolic Syndrome Severity in Adolescents with PCOS and Obesity. J. Clin. Endocrinol. Metab. 2020, 105, e1827–e1834. [Google Scholar] [CrossRef] [PubMed]


| Value | Milk Intake | p | |
|---|---|---|---|
| Rare (≤62.5 mL Per Week), n = 553 | Regularly (>62.5 mL Per Week), n = 215 | ||
| Socio demographics | |||
| Male, % | 346 (62.23) | 148 (68.84) | 0.123 |
| Age (≥65 years), % | 288 (52.08) | 117 (54.42) | 0.615 |
| BMI (≥24 kg/m2), % | 296 (53.53) | 105 (48.84) | 0.277 |
| Lifestyle risk factors | |||
| alcohol consumption, % | 85 (15.29) | 32 (14.88) | 0.955 |
| smoking, % | 279 (50.18) | 117 (54.42) | 0.364 |
| Salt intake (<6 g/d), % | 155 (28.17) | 63 (29.30) | 0.793 |
| Fruit intake, % | 324 (58.27) | 178 (82.79) | <0.001 *** |
| Vegetable intake, % | 548 (98.56) | 214 (99.53) | 0.870 |
| Red meat intake, % | 531 (95.50) | 208 (96.74) | 0.794 |
| Seafood intake, % | 498 (89.57) | 200 (93.02) | 0.253 |
| Egg intake, % | 446 (80.22) | 193 (89.77) | <0.01 ** |
| Soy product intake, % | 370 (66.55) | 150 (69.77) | 0.500 |
| Nuts intake, % | 149 (26.80) | 82 (38.14) | <0.01 ** |
| Sugar-sweetened beverages intake, % | 83 (14.93) | 53 (24.65) | <0.01 ** |
| Clinical assessment | |||
| Hypertension, % | 333 (60.22) | 140 (65.12) | 0.242 |
| Diabetes mellitus, % | 139 (25.14) | 56 (26.05) | 0.867 |
| CHD, % | 355 (64.20) | 139 (64.65) | 0.973 |
| Anxious state, % | 96 (17.36) | 47 (21.86) | 0.182 |
| Depression, % | 112 (20.25) | 42 (19.53) | 0.806 |
| PSQI Global Scores | 6 (4, 9) | 6 (3, 8) | 0.068 |
| Sleep Disorders, % | 372 (67.27) | 128 (59.53) | 0.053 |
| Low Sleep Quality, % | 138 (24.95) | 45 (20.93) | 0.280 |
| Low Sleep Efficiency, % | 278 (50.27) | 112 (52.09) | 0.709 |
| Long Sleep Latency, % | 141 (25.50) | 44 (20.47) | 0.218 |
| Short Sleep Duration, % | 333 (60.22) | 140 (65.12) | 0.242 |
| Sleep Disturbances, % | 520 (94.03) | 184 (85.58) | <0.001 *** |
| Daytime Dysfunction, % | 395 (71.43) | 141 (65.58) | 0.134 |
| Used Sleep Medication, % | 20 (3.62) | 9 (4.19) | 0.872 |
| Medication factors | |||
| Anti-hypertensive drugs, % | 371 (67.09) | 146 (67.91) | 0.896 |
| Hypoglycemic drugs, % | 129 (23.33) | 49 (22.79) | 0.950 |
| Biochemical Indicators | |||
| ALT, U/L | 18.87 (13.80, 27.50) | 20.00 (14.60, 28.60) | 0.284 |
| AST, U/L | 21.40 (17.70, 27.50) | 22.80 (18.50, 29.75) | 0.127 |
| LDH, U/L | 190.00 (165.00, 220.00) | 187.00 (164.00, 217.00) | 0.693 |
| FBG, mmol/L | 5.19 (4.73, 6.15) | 5.14 (4.63, 6.19) | 0.497 |
| TC, mmol/L | 4.35 (3.58, 5.13) | 4.24 (3.53, 5.19) | 0.757 |
| TG, mmol/L | 1.44 (1.11, 2.08) | 1.34 (1.00, 1.85) | 0.021 * |
| HDL-C, mmol/L | 1.00 (0.85, 1.18) | 1.04 (0.88, 1.22) | 0.139 |
| LDL-C, mmol/L | 2.96 (2.32, 3.52) | 2.93 (2.33, 3.50) | 0.879 |
| FFA, µmol/L | 364.00 (247.00, 514.00) | 361.00 (244.50, 542.00) | 0.863 |
| Milk Intake | Cases/ Participants | Crude Model | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| Sleep Disorder | Rare | 372/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 128/215 | 0.72 (0.52, 0.99) | 0.045 * | 0.73 (0.53, 1.02) | 0.064 | 0.74 (0.53, 1.05) | 0.092 | 0.72 (0.51, 1.03) | 0.068 | |
| PSQI Component | ||||||||||
| Low Sleep Quality | Rare | 138/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 45/215 | 0.80 (0.54, 1.16) | 0.240 | 0.82 (0.55, 1.20) | 0.307 | 0.85 (0.56, 1.27) | 0.427 | 0.84 (0.55, 1.28) | 0.422 | |
| Low Sleep Efficiency | Rare | 278/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 112/215 | 1.08 (0.79, 1.48) | 0.650 | 1.09 (0.79, 1.51) | 0.605 | 0.97 (0.69, 1.38) | 0.881 | 0.97 (0.69, 1.38) | 0.871 | |
| Long Sleep Latency | Rare | 141/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 44/215 | 0.92 (0.67, 1.26) | 0.608 | 0.95 (0.69, 1.31) | 0.746 | 0.85 (0.60, 1.19) | 0.346 | 0.85 (0.60, 1.19) | 0.334 | |
| Short Sleep Duration | Rare | 333/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 140/215 | 1.23 (0.89, 1.72) | 0.211 | 1.27 (0.92, 1.78) | 0.153 | 1.24 (0.88, 1.77) | 0.218 | 1.22 (0.86, 1.74) | 0.259 | |
| Sleep Disturbances | Rare | 520/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 184/215 | 0.38 (0.22, 0.63) | <0.001 *** | 0.38 (0.22, 0.64) | <0.001 *** | 0.50 (0.28, 0.87) | 0.014 * | 0.49 (0.28, 0.87) | 0.015 * | |
| Daytime Dysfunction | Rare | 395/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 141/215 | 0.76 (0.55, 1.07) | 0.114 | 0.79 (0.56, 1.11) | 0.163 | 0.84 (0.59, 1.21) | 0.349 | 0.83 (0.58, 1.19) | 0.316 | |
| Used Sleep Medication | Rare | 20/553 | Reference | Reference | Reference | Reference | ||||
| Regularly | 9/215 | 1.16 (0.50, 2.53) | 0.710 | 1.17 (0.49, 2.58) | 0.704 | 1.20 (0.48, 2.81) | 0.677 | 1.08 (0.42, 2.60) | 0.876 | |
| Milk Intake | Crude Model | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI)v | p Value | ||
| PSQI Global Score | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.029 (−0.061, 0.002) | 0.069 | −0.025 (−0.056, 0.006) | 0.109 | −0.021 (−0.053, 0.011) | 0.198 | −0.023 (−0.055, 0.009) | 0.155 | |
| Scores of PSQI Components | |||||||||
| Low Sleep Quality | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.056 (−0.093, −0.018) | <0.01 ** | −0.053 (−0.090, −0.016) | <0.01 ** | −0.044 (−0.082, −0.006) | 0.024 * | −0.045 (−0.083, −0.007) | 0.020 * | |
| Low Sleep Efficiency | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.007 (−0.059, 0.074) | 0.830 | 0.009 (−0.056, 0.075) | 0.780 | −0.006 (−0.073, 0.062) | 0.864 | −0.007 (−0.074, 0.060) | 0.838 | |
| Long Sleep Latency | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.028 (−0.083, 0.026) | 0.304 | −0.029 (−0.083, 0.024) | 0.286 | −0.031 (−0.087, 0.025) | 0.277 | −0.031 (−0.087, 0.024) | 0.269 | |
| Short Sleep Duration | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.002 (−0.058, 0.055) | 0.950 | 0.003 (−0.054, 0.059) | 0.927 | 0.010 (−0.049, 0.068) | 0.749 | 0.007 (−0.052, 0.066) | 0.811 | |
| Sleep Disturbances | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.074 (−0.103, −0.044) | <0.001 *** | −0.071 (−0.101, −0.042) | <0.001 *** | −0.057 (−0.087, −0.026) | <0.001 *** | −0.059 (−0.090, −0.029) | <0.001 *** | |
| Daytime Dysfunction | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.030 (−0.008, 0.020) | 0.236 | −0.023 (−0.073, 0.026) | 0.358 | −0.007 (−0.059, 0.044) | 0.784 | −0.011 (−0.062, 0.040) | 0.677 | |
| Used Sleep Medication | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.004 (−0.025, 0.017) | 0.706 | −0.004 (−0.024, 0.017) | 0.721 | −0.005 (−0.027, 0.016) | 0.622 | −0.008 (−0.029, 0.013) | 0.466 | |
| Milk Intake | Crude Model | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | ||
| ALT, U/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.002 (−0.014, 0.011) | 0.764 | −0.002 (−0.015, 0.010) | 0.734 | −0.004 (−0.017, 0.009) | 0.547 | −0.003 (−0.016, 0.010) | 0.655 | |
| AST, U/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.004 (−0.018, 0.010) | 0.585 | −0.005 (−0.020, 0.009) | 0.475 | −0.005 (−0.020, 0.010) | 0.544 | −0.003 (−0.018, 0.012) | 0.699 | |
| LDH, U/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.005 (−0.019, 0.010) | 0.515 | −0.006 (−0.021, 0.007) | 0.429 | −0.003 (−0.018, 0.012) | 0.710 | −0.001 (−0.016, 0.013) | 0.850 | |
| FBG, mmol/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.004 (−0.016, 0.023) | 0.718 | 0.006 (−0.013, 0.026) | 0.531 | 0.012 (−0.008, 0.033) | 0.233 | 0.006 (−0.011, 0.023) | 0.502 | |
| TC, mmol/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.004 (−0.001, 0.010) | 0.134 | 0.004 (−0.001, 0.010) | 0.134 | 0.004 (−0.002, 0.010) | 0.159 | 0.005 (−0.001, 0.011) | 0.128 | |
| TG, mmol/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | −0.011 (−0.037, 0.004) | 0.015 * | −0.017 (−0.033, −0.004) | 0.045 * | −0.019 (−0.036, 0.002) | 0.030 * | −0.020 (−0.037, −0.003) | 0.023 * | |
| HDL, mmol/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.016 (−0.006, 0.037) | 0.147 | 0.016 (−0.005, 0.037) | 0.132 | 0.020 (−0.002, 0.041) | 0.072 | 0.021 (−0.001, 0.042) | 0.059 | |
| LDL, mmol/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.004 (−0.021, 0.030) | 0.727 | 0.010 (−0.015, 0.034) | 0.439 | 0.005 (−0.020, 0.031) | 0.685 | 0.007 (−0.018, 0.033) | 0.580 | |
| FFA, µmol/L | Rare, n = 553 | ||||||||
| Regularly, n = 215 | 0.004 (−0.013, 0.020) | 0.644 | 0.004 (−0.012, 0.021) | 0.604 | 0.001 (−0.016, 0.018) | 0.923 | −0.002 (−0.018, 0.015) | 0.853 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Lao, J.; Jiang, Q.; Lin, W.; Chen, X.; Zhu, C.; He, S.; Xie, W.; Wang, F.; Yang, B.; et al. Associations between Milk Intake and Sleep Disorders in Chinese Adults: A Cross-Sectional Study. Nutrients 2023, 15, 4079. https://doi.org/10.3390/nu15184079
Xu J, Lao J, Jiang Q, Lin W, Chen X, Zhu C, He S, Xie W, Wang F, Yang B, et al. Associations between Milk Intake and Sleep Disorders in Chinese Adults: A Cross-Sectional Study. Nutrients. 2023; 15(18):4079. https://doi.org/10.3390/nu15184079
Chicago/Turabian StyleXu, Jinzhong, Jiaying Lao, Qingxi Jiang, Wenhui Lin, Xiyi Chen, Chongrong Zhu, Shencong He, Wenbo Xie, Fan Wang, Bo Yang, and et al. 2023. "Associations between Milk Intake and Sleep Disorders in Chinese Adults: A Cross-Sectional Study" Nutrients 15, no. 18: 4079. https://doi.org/10.3390/nu15184079
APA StyleXu, J., Lao, J., Jiang, Q., Lin, W., Chen, X., Zhu, C., He, S., Xie, W., Wang, F., Yang, B., & Liu, Y. (2023). Associations between Milk Intake and Sleep Disorders in Chinese Adults: A Cross-Sectional Study. Nutrients, 15(18), 4079. https://doi.org/10.3390/nu15184079






