The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol Development
2.2. Literature Search Strategy
2.3. Inclusion and Exclusion Criteria
- (a)
- Study Design: Randomized controlled trials (RCTs), prospective cohort studies, cross-sectional studies, and case-control studies were included. Animal studies, case reports, reviews, and conference abstracts were excluded.
- (b)
- Participants: Studies involving adult human participants (aged 18 years or older) diagnosed with IBS according to recognized criteria (e.g., Rome criteria) were considered eligible.
- (c)
- Interventions: Studies investigating the effects of dietary polyphenols, minerals, fibers, and fruits as part of the intervention for IBS management were included.
- (d)
- Outcome measures: The primary outcomes of interest were changes in IBS symptoms, including abdominal pain, bloating, and altered bowel habits. Secondary outcomes included gut motility, gut microbiota composition, and quality of life measures.
- (e)
- Only articles written in English were included in our systematic review.
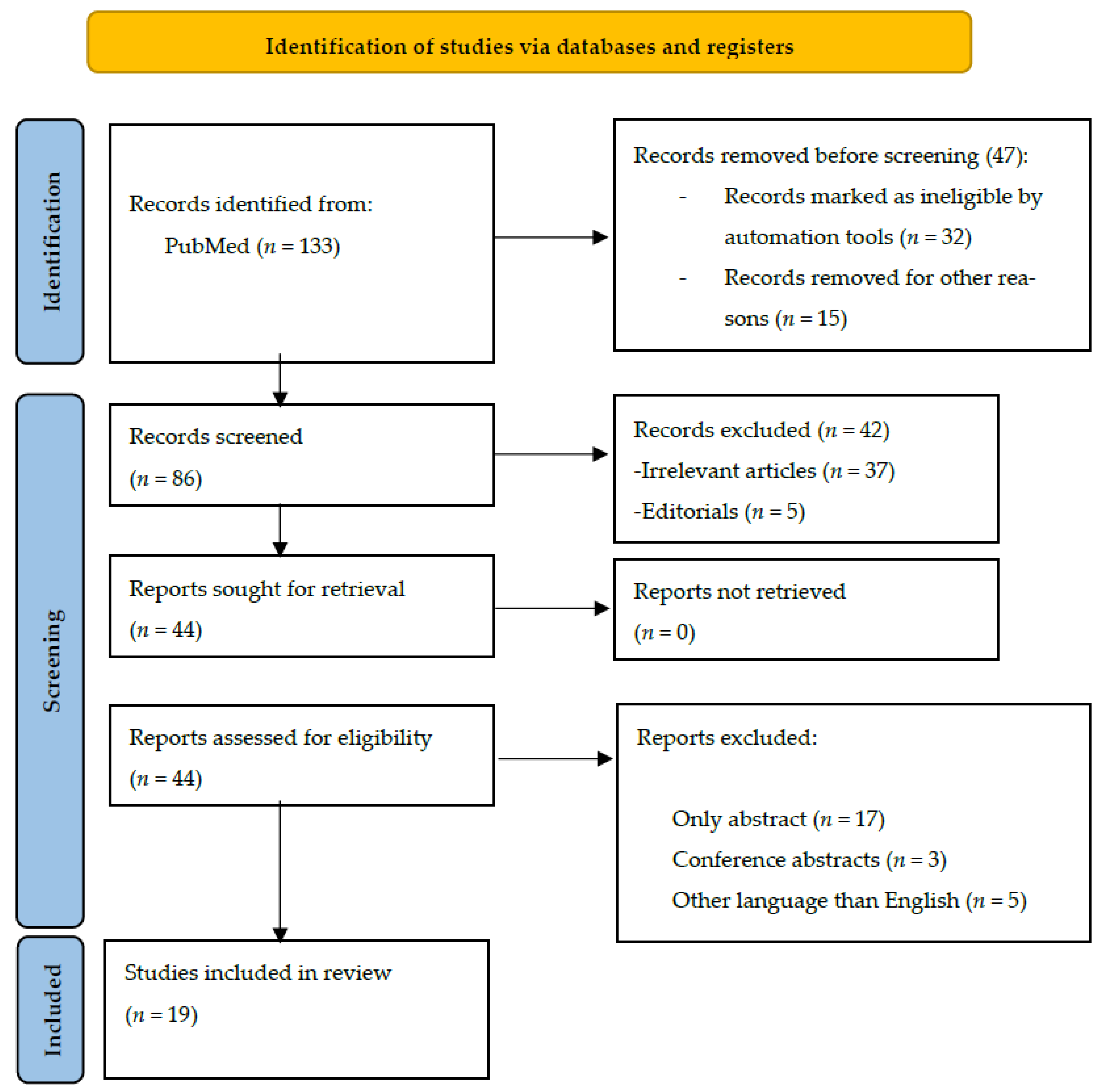
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Synthesis and Analysis
2.8. Limitations
3. Results
3.1. Polyphenols and Polysaccharides
| Author (Year) | Participants | Intervention | Main Outcome |
|---|---|---|---|
| Trifan et al. (2019) [18] | 60 patients with IBS-D | Gelsectan or placebo for 28 days, then crossed over for another 28 days. BSFS IBS QoL | Significantly more normal stools in the Gelsectan group. Overall subjective improvements when it comes to abdominal pain, bloating, and QoL. |
| Portincasa et al. (2016) [19] | 121 patients with mild-to-moderate IBS | CU–FEO vs. placebo for 30 days IBS-SSS IBS-QoL | Reduction in the severity of IBS, as well as symptom improvement. More symptom-free patients after 30 days. |
| Storsrud et al. (2015) [20] | 68 patients with IBS | Aloe barbadensis Mill vs. placebo for 28 days IBS-SSS HADS | No difference when it comes to the response to treatment and adequate relief during at least half of the study period. Higher reduction in the severity of the symptoms in the treatment group. |
| Lauche et al. (2016) [21] | 32 patients with IBS-D | Curry, pomegranate, and turmeric vs. placebo IBS-SSS IBS QoL HADS | No difference when it comes to symptom intensity between the two groups. More adverse effects in the treatment group. |
| Brown et al. (2015) [22] | 16 patients with IBS-C | Blended querbacho, conker tree, and Mentha balsamea Willd extracts vs. placebo for 14 days | Significant reduction in constipation and bloating. |
| Mosaffa-Jahromi et al. (2016) [23] | 120 patients with IBS | Anise oil vs. peppermint oil vs. placebo for 28 days IBS-SSS IBS QoL | Significant differences in the number of patients free of symptoms at the end of the trial. Anise oil beneficial for pain, bloating, and reflux. Peppermint oil beneficial for bloating. |
| Cash et al. (2016) [24] | 72 patients with IBS | Peppermint oil vs. placebo for 28 days TISS | Peppermint oil was superior to placebo in decreasing the TISS score. |
| Capello et al. (2007) [25] | 57 patients with IBS | Peppermint oil vs. placebo for 28 days | 75% of the patients in the treatment group reported a significant reduction in symptoms. The effect was also observed in the follow-up period (one month after the end of the trial). |
| Liu et al. (1997) [26] | 110 patients with IBS | Peppermint oil vs. placebo for 28 days | 79% of the patients in the treatment group reported an improvement in abdominal pain, 83% less distension, 83% reduced stool frequency, and 79% less flatulence. |
| Merat et al. (2010) [27] | 90 patients with IBS | Peppermint oil vs. placebo for 8 weeks | 84% of the patients in the treatment group reported only occasional or no abdominal pain at the end of the trial. |
| Weerts et al. (2020) [28] | 190 patients with IBS | Small intestinal-release peppermint oil vs. ileocolonic-release peppermint oil vs. placebo for 8 weeks IBS-SSS IBS-QoL EuroQoL-5D GAD-7 PHQ-9 | Small intestinal-release peppermint oil led to a better improvement in abdominal pain and discomfort. No difference in the abdominal pain response. |
| Al-Jassim (2019) [29] | 45 patients with IBS-C | Brewer’s yeast vs. ginger vs. placebo for 20 days | Significant reduction in abdominal pain in the Brewer’s yeast group. Significant reduction in abdominal distension and constipation in the Brewer’s yeast and ginger groups compared with placebo. |
| Jalili et al. (2015) [30] | 67 patients with IBS | Soy isoflavones vs. placebo for 6 weeks IBS-SSS IBS-QoL | Significantly better QoL for patients receiving soy isoflavones. No differences when it comes to symptom severity. |
| van Tilburg et al. (2014) [31] | 45 patients with IBS | Ginger vs. placebo for 28 days IBS-SSS | No significant differences between the groups. |
3.2. Fruits, Fibers, and Minerals
| Author | Participants | Intervention | Main Findings |
|---|---|---|---|
| Chang (2010) [39] | 76 patients, 60 with IBS-C, and 16 healthy | Kiwifruit vs. placebo for 4 weeks | Statistically significant improvements in defecation frequency and colon transit time. No significant differences in fecal volume change, life stress, and post-defecation feelings. |
| Eady (2019) [40] | 32 female patients, 23 with IBS-C, and 9 with functional constipation | Kiwifruit vs. Metamucil® for 4 weeks each, with a 4-week washout period bowel movements BSSS | Statistically significant improvements in the mean number of complete spontaneous bowel movements, BSSS, and indigestion. No significant differences in the other parameters measured. |
| Chen (2018) [41] | 20 female patients, 10 with IBS and 10 healthy | Kiwifruit vs. lactulose, fructose, lactose, and apple CH4 and H2 production | Statistically significant differences in the AUC for CH4 and H2 compared to lactulose and positive breath tests. No significant difference in the AUC for CH4 and H2 compared to fructose, lactose, or apple. |
| Riezzo (2023) [42] | 18 female patients with IBS-D | Tritordeum-based diet for 12 weeks IBS-SSS BSSS Anthropometric and BIA parameters IBS-QoL SF-36 SCL-90-R SAS SDS QPF/R | Statistically significant differences in almost all parameters measured. 16 out of 18 patients responded to the diet. |
| Roth (2022) [43] | 105 patients, 86 with IBS, and 19 with non-IBS functional gastrointestinal disorders | SSRD vs. control for 4 weeks Minerals IBS-SSS Extraintestinal IBS-SSS Lipid intake | A lower intake of micronutrients was recorded at baseline compared to the national guidelines. SSRD increased vitamin, selenium, and fat intake and reduced symptom severity and sodium intake. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable bowel syndrome: A clinical review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, D.L.; Baban, A.; Bancila, I.; Barboi, O.; Bataga, S.; Chira, A.; Chirila, I.; Cijevschi Prelipcean, C.; Ciobanu, L.; Cozma-Petrut, A.; et al. Romanian Guidelines for Nonpharmacological Therapy of IBS. J. Gastrointestin Liver Dis. 2021, 30, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Andrés-Lacueva, C. Polyphenols and health: Current state and progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef]
- Natsume, M. Polyphenols: Inflammation. Curr. Pharm. Des. 2018, 24, 191–202. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Roca-Saavedra, P.; Mendez-Vilabrille, V.; Miranda, J.M.; Nebot, C.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Food additives, contaminants and other minor components: Effects on human gut microbiota-a review. J. Physiol. Biochem. 2018, 74, 69–83. [Google Scholar] [CrossRef]
- Huskisson, E.; Maggini, S.; Ruf, M. The role of vitamins and minerals in energy metabolism and well-being. J. Int. Med. Res. 2007, 35, 277–289. [Google Scholar] [CrossRef]
- Zhang, F.F.; Barr, S.I.; McNulty, H.; Li, D.; Blumberg, J.B. Health effects of vitamin and mineral supplements. BMJ 2020, 369, m2511. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, C.J.; de Wit, N.J.; Muris, J.W.; Whorwell, P.J.; Knottnerus, J.A.; Hoes, A.W. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009, 339, b3154. [Google Scholar] [CrossRef]
- Garg, P. Inflammation in Irritable Bowel Syndrome (IBS): Role of Psyllium Fiber Supplementation in Decreasing Inflammation and Physiological Management of IBS. Turk. J. Gastroenterol. 2021, 32, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.; Morden, A.; Bischof, D.; King, E.A.; Kosztowski, M.; Wick, E.C.; Stein, E.M. The role of fiber supplementation in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Bek, S.; Teo, Y.N.; Tan, X.H.; Fan, K.H.R.; Siah, K.T.H. Association between irritable bowel syndrome and micronutrients: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1485–1497. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Environ. Sci. 2014. Available online: https://web.archive.org/web/20210716121605id_/http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 21 June 2023).
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Trifan, A.; Burta, O.; Tiuca, N.; Petrisor, D.C.; Lenghel, A.; Santos, J. Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial. United Eur. Gastroenterol. J. 2019, 7, 1093–1101. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Scribano, M.L.; Kohn, A.; Caporaso, N.; Festi, D.; Campanale, M.C.; Di Rienzo, T.; Guarino, M.; Taddia, M.; et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J. Gastrointest in Liver Dis. 2016, 25, 151–157. [Google Scholar] [CrossRef]
- Storsrud, S.; Pontén, I.; Simren, M. A pilot study of the effect of Aloe barbadensis mill. Extract (AVH200®) in patients with irritable bowel syndrome: A randomized, double-blind, placebo-controlled study. J. Gastrointest. Liver Dis. 2015, 24, 275–280. [Google Scholar] [CrossRef]
- Lauche, R.; Kumar, S.; Hallmann, J.; Lüdtke, R.; Rampp, T.; Dobos, G.; Langhorst, J. Efficacy and safety of ayurvedic herbs in diarrhoea-predominant irritable bowel syndrome: A randomised controlled crossover trial. Complement. Ther. Med. 2016, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Scott-Hoy, B.; Jennings, L. Efficacy of a Quebracho, Conker tree, and M. balsamea Willd blended extract in a randomized study in patients with irritable bowel syndrome with constipation. J. Gastroenterol. Hepatol. Res. 2015, 4, 1762–1767. [Google Scholar] [CrossRef][Green Version]
- Mosaffa-Jahromi, M.; Lankarani, K.B.; Pasalar, M.; Afsharypuor, S.; Tamaddon, A.-M. Efficacy and safety of enteric coated capsules of anise oil to treat irritable bowel syndrome. J. Ethnopharmacol. 2016, 194, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Cash, B.; Epstein, M.; Shah, S.; Cash, B.D.; Epstein, M.S.; Shah, S.M. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis. Sci. 2016, 61, 560–571. [Google Scholar] [CrossRef]
- Cappello, G.; Spezzaferro, M.; Grossi, L.; Manzoli, L.; Marzio, L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig. Liver Dis. 2007, 39, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Chen, G.H.; Yeh, H.Z.; Huang, C.K.; Poon, S.K. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: A prospective, randomized trial. J. Gastroenterol. 1997, 32, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Merat, S.; Khalili, S.; Mostajabi, P.; Ghorbani, A.; Ansari, R.; Malekzadeh, R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig. Dis. Sci. 2010, 55, 1385–1390. [Google Scholar] [CrossRef]
- Weerts, Z.Z.R.; Masclee, A.A.; Witteman, B.J.; Clemens, C.H.; Winkens, B.; Brouwers, J.R.; Frijlink, H.W.; Muris, J.W.; De Wit, N.J.; Essers, B.A.; et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology 2020, 158, 123–136. [Google Scholar] [CrossRef]
- Al-Jassim, Z.G. Using brewer’s yeast and ginger in the management of constipation-predominant irritable bowel syndrome: A randomized double-blind placebo-controlled trial. Asian J. Pharm. Clin. Res. 2019, 12, 372–376. [Google Scholar] [CrossRef]
- Jalili, M.; Vahedi, H.; Janani, L.; Poustchi, H.; Malekzadeh, R.; Hekmatdoost, A. Soy isoflavones supplementation for patients with irritable bowel syndrome: A randomized double blind clinical trial. Middle East. J. Dig. Dis. 2015, 7, 170–176. [Google Scholar]
- van Tilburg, M.A.; Palsson, O.S.; Ringel, Y.; Whitehead, W.E. Is ginger effective for the treatment of irritable bowel syndrome? A double blind randomized controlled pilot trial. Complement. Ther. Med. 2014, 22, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Gómez-Guillén, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Izadi-Darbandi, A.; Bahmani, K.; Farhadpour, M.; Ebrahimi, M.; Ramshini, H.; Esmaeili, Z. Assessment of phenolic profile, and antioxidant activity in developed breeding populations of fennel (Foeniculum vulgare Mill). Biocatalysis and Agricultural Biotechnology 2023, 48, 102639. [Google Scholar] [CrossRef]
- López, A.; de Tangil, M.S.; Vega-Orellana, O.; Ramírez, A.S.; Rico, M. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Chia, S.C.; Lee, S.H.; Lim, J.; Leow, M.K.; Chan, E.C.Y.; Henry, C.J. Polyphenol-rich curry made with mixed spices and vegetables benefits glucose homeostasis in Chinese males (Polyspice Study): A dose-response randomized controlled crossover trial. Eur. J. Nutr. 2019, 58, 301–313. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [PubMed]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef]
- León-González, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual brewing yeast as a source of polyphenols: Extraction, identification and quantification by chromatographic and chemometric tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Y.T.; Lu, Y.T.; Liu, Y.S.; Liu, J.F. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac. J. Clin. Nutr. 2010, 19, 451–457. [Google Scholar]
- Eady, S.L.; Wallace, A.J.; Butts, C.A.; Hedderley, D.; Drummond, L.; Ansell, J.; Gearry, R.B. The effect of ‘Zesy002’ kiwifruit (Actinidia chinensis var. chinensis) on gut health function: A randomised cross-over clinical trial. J. Nutr. Sci. 2019, 8, e18. [Google Scholar] [CrossRef]
- Chen, A.G.Y.; Offereins, M.S.L.; Mulder, C.J.; Frampton, C.M.; Gearry, R.B. A Pilot Study of the Effect of Green Kiwifruit on Human Intestinal Fermentation Measured by Hydrogen and Methane Breath Testing. J. Med. Food. 2018, 21, 1295–1298. [Google Scholar] [CrossRef]
- Riezzo, G.; Prospero, L.; Orlando, A.; Linsalata, M.; D’Attoma, B.; Ignazzi, A.; Giannelli, G.; Russo, F. A Tritordeum-Based Diet for Female Patients with Diarrhea-Predominant Irritable Bowel Syndrome: Effects on Abdominal Bloating and Psychological Symptoms. Nutrients 2023, 15, 1361. [Google Scholar] [CrossRef]
- Roth, B.; Larsson, E.; Ohlsson, B. Poor intake of vitamins and minerals is associated with symptoms among patients with irritable bowel syndrome. J. Gastroenterol. Hepatol. 2022, 37, 1253–1262. [Google Scholar] [CrossRef]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Katsirma, Z.; Dimidi, E.; Rodriguez-Mateos, A.; Whelan, K. Fruits and their impact on the gut microbiota, gut motility and constipation. Food Funct. 2021, 12, 8850–8866. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Walter, J. The Fiber Gap and the Disappearing Gut Microbiome: Implications for Human Nutrition. Trends Endocrinol. Metab. 2016, 27, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules. 2021, 26, 6802. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Black, C.J.; Moayyedi, P.; Quigley, E.M.; Ford, A.C. Peppermint oil in irritable bowel syndrome. Gastroenterology 2020, 159, 395–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarioni, G.; Popa, S.L.; Ismaiel, A.; Pop, C.; Dumitrascu, D.I.; Brata, V.D.; Duse, T.A.; Incze, V.; Surdea-Blaga, T. The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review. Nutrients 2023, 15, 4070. https://doi.org/10.3390/nu15184070
Chiarioni G, Popa SL, Ismaiel A, Pop C, Dumitrascu DI, Brata VD, Duse TA, Incze V, Surdea-Blaga T. The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review. Nutrients. 2023; 15(18):4070. https://doi.org/10.3390/nu15184070
Chicago/Turabian StyleChiarioni, Giuseppe, Stefan Lucian Popa, Abdulrahman Ismaiel, Cristina Pop, Dinu Iuliu Dumitrascu, Vlad Dumitru Brata, Traian Adrian Duse, Victor Incze, and Teodora Surdea-Blaga. 2023. "The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review" Nutrients 15, no. 18: 4070. https://doi.org/10.3390/nu15184070
APA StyleChiarioni, G., Popa, S. L., Ismaiel, A., Pop, C., Dumitrascu, D. I., Brata, V. D., Duse, T. A., Incze, V., & Surdea-Blaga, T. (2023). The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review. Nutrients, 15(18), 4070. https://doi.org/10.3390/nu15184070







