Evaluation of Tibial Hemodynamic Response to Glucose Tolerance Test in Young Healthy Males and Females
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Heart Rate
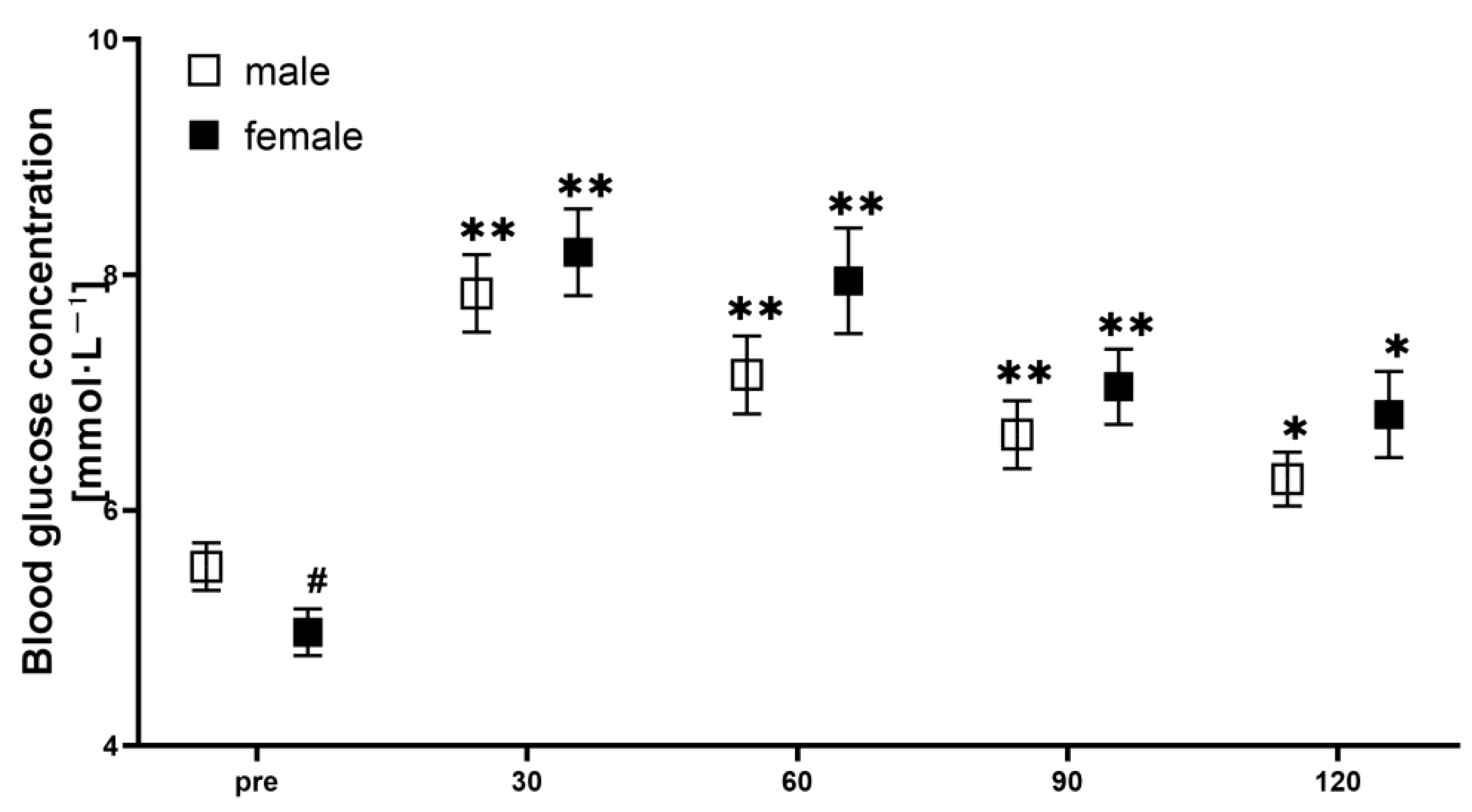
2.4. Blood Glucose
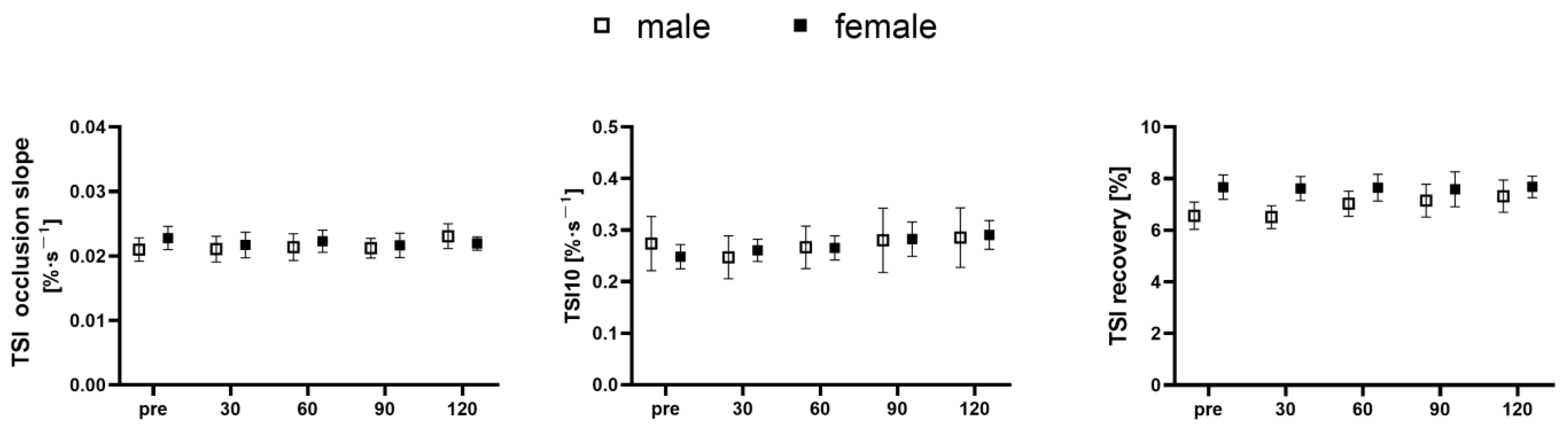
2.5. NIRS Testing
2.6. Data Analysis
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Chen, X.W.; Huang, X.; Song, B.L.; Wang, Y.; Wang, Y. Regulation of glucose and lipid metabolism in health and disease. Sci. China Life Sci. 2019, 62, 1420–1458. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Agius, L. The biochemistry of diabetes. Biochem. J. 1988, 250, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Wareham, N.J.; Byrne, C.D.; Carr, C.; Day, N.E.; Boucher, B.J.; Hales, C.N. Glucose intolerance is associated with altered calcium homeostasis: A possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism 1997, 46, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Gann, P.H.; Lowe, W.; Liu, K.; Colangelo, L.; Dyer, A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000, 283, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Permert, J.; Ihse, I.; Jorfeldt, L.; Von Schenck, H.; Arnqvist, H.; Larsson, J. Pancreatic cancer is associated with impaired glucose metabolism. Eur. J. Surg. 1993, 159, 101–107. [Google Scholar]
- Karner, C.M.; Long, F. Glucose metabolism in bone. Bone 2018, 115, 2–7. [Google Scholar] [CrossRef]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Leibson, C.L.; Achenbach, S.J.; Therneau, T.M.; Khosla, S. Fracture risk in type 2 diabetes: Update of a population-based study. J. Bone. Miner. Res. 2008, 23, 1334–1342. [Google Scholar] [CrossRef]
- Bartoli, E.; Fra, G.P.; Carnevale Schianca, G.P. The oral glucose tolerance test (OGTT) revisited. Eur. J. Intern. Med. 2011, 22, 8–12. [Google Scholar] [CrossRef]
- Soares, R.N.; Reimer, R.A.; Murias, J.M. Changes in vascular responsiveness during a hyperglycemia challenge measured by near-infrared spectroscopy vascular occlusion test. Microvasc. Res. 2017, 111, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Westberg-Rasmussen, S.; Starup-Linde, J.; Hermansen, K.; Holst, J.J.; Hartmann, B.; Vestergaard, P.; Gregersen, S. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone 2017, 97, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Paldanius, P.M.; Ivaska, K.K.; Hovi, P.; Andersson, S.; Vaananen, H.K.; Kajantie, E.; Makitie, O. The effect of oral glucose tolerance test on serum osteocalcin and bone turnover markers in young adults. Calcif. Tissue Int. 2012, 90, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Viljakainen, H.; Ivaska, K.K.; Paldanius, P.; Lipsanen-Nyman, M.; Saukkonen, T.; Pietilainen, K.H.; Andersson, S.; Laitinen, K.; Makitie, O. Suppressed bone turnover in obesity: A link to energy metabolism? A case-control study. J. Clin. Endocrinol. Metab. 2014, 99, 2155–2163. [Google Scholar] [CrossRef]
- Soares, R.N.; Reimer, R.A.; Alenezi, Z.; Doyle-Baker, P.K.; Murias, J.M. Near-infrared spectroscopy can detect differences in vascular responsiveness to a hyperglycaemic challenge in individuals with obesity compared to normal-weight individuals. Diab. Vasc. Dis. Res. 2018, 15, 55–63. [Google Scholar] [CrossRef]
- Zhang, C.; Modlesky, C.M.; McCully, K.K. Measuring tibial hemodynamics and metabolism at rest and after exercise using near-infrared spectroscopy. Appl. Physiol. Nutr. Metab. 2021, 46, 1354–1362. [Google Scholar] [CrossRef]
- Binzoni, T.; Leung, T.S.; Courvoisier, C.; Giust, R.; Tribillon, G.; Gharbi, T.; Delpy, D.T. Blood volume and haemoglobin oxygen content changes in human bone marrow during orthostatic stress. J. Physiol. Anthropol. 2006, 25, 1–6. [Google Scholar] [CrossRef]
- Draghici, A.E.; Taylor, J.A. Mechanisms of bone blood flow regulation in humans. J. Appl. Physiol. 2021, 130, 772–780. [Google Scholar] [CrossRef]
- Draghici, A.E.; Potart, D.; Hollmann, J.L.; Pera, V.; Fang, Q.; DiMarzio, C.A.; Andrew Taylor, J.; Niedre, M.J.; Shefelbine, S.J. Near infrared spectroscopy for measuring changes in bone hemoglobin content after exercise in individuals with spinal cord injury. J. Orthop. Res. 2018, 36, 183–191. [Google Scholar] [CrossRef]
- Zhang, C.; McCully, K.K. The case for measuring long bone hemodynamics with Near-infrared spectroscopy. Front. Physiol. 2020, 11, 1698. [Google Scholar] [CrossRef]
- Meertens, R.; Casanova, F.; Knapp, K.M.; Thorn, C.; Strain, W.D. Use of near-infrared systems for investigations of hemodynamics in human in vivo bone tissue: A systematic review. J. Orthop. Res. 2018, 36, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Huebschmann, A.G.; Huxley, R.R.; Kohrt, W.M.; Zeitler, P.; Regensteiner, J.G.; Reusch, J.E.B. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia 2019, 62, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.J.; Fryer, S.M.; Ryan, T.; Stoner, L. The validity and reliability of continuous-wave near-infrared spectroscopy for the assessment of leg blood volume during an orthostatic challenge. Atherosclerosis 2016, 251, 234–239. [Google Scholar] [CrossRef]
- Barstow, T.J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, J.R.; Figueroa, A.; Gonzales, J.U. Reactive hyperemia half-time response is associated with skeletal muscle oxygen saturation changes during cycling exercise. Microvasc. Res. 2023, 149, 104569. [Google Scholar] [CrossRef]
- Ihsan, M.; Labidi, M.; Racinais, S. Skeletal muscle oxidative adaptations following localized heat therapy. Eur. J. Appl. Physiol. 2023, 123, 1629–1635. [Google Scholar] [CrossRef]
- Tai, M.M. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 1994, 17, 152–154. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Levinger, I.; Seeman, E.; Jerums, G.; McConell, G.K.; Rybchyn, M.S.; Cassar, S.; Byrnes, E.; Selig, S.; Mason, R.S.; Ebeling, P.R.; et al. Glucose-loading reduces bone remodeling in women and osteoblast function in vitro. Physiol. Rep. 2016, 4, e12700. [Google Scholar] [CrossRef]
- Booth, S.L.; Centi, A.; Smith, S.R.; Gundberg, C. The role of osteocalcin in human glucose metabolism: Marker or mediator? Nat. Rev. Endocrinol. 2013, 9, 43–55. [Google Scholar] [CrossRef]
- Riddle, R.C.; Clemens, T.L. Bone Cell Bioenergetics and Skeletal Energy Homeostasis. Physiol. Rev. 2017, 97, 667–698. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Pessin, J.E. Insulin regulation of glucose uptake: A complex interplay of intracellular signalling pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Shimazu, J.; Makinistoglu, M.P.; Maurizi, A.; Kajimura, D.; Zong, H.; Takarada, T.; Lezaki, T.; Pessin, J.E.; Hinoi, E.; et al. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell 2015, 161, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Muller-Delp, J.M. Aging-induced adaptations of microvascular reactivity. Microcirculation 2006, 13, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Egan, J.M. Diabetes and altered glucose metabolism with aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 333–347. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Vehkavaara, S.; Westerbacka, J.; Hakala-Ala-Pietilä, T.; Virkamäki, A.; Hovatta, O.; Yki-Järvinen, H. Effect of Estrogen Replacement Therapy on Insulin Sensitivity of Glucose Metabolism and Preresistance and Resistance Vessel Function in Healthy Postmenopausal Women1. J. Clin. Endocrinol. Metab. 2000, 85, 4663–4670. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A vascular hormone in health and disease. J. Endocrinol. 2013, 217, R47–R71. [Google Scholar] [CrossRef]
- Grossmann, M. Testosterone and glucose metabolism in men: Current concepts and controversies. J. Endocrinol. 2014, 220, R37–R55. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- van Beekvelt, M.C.P.; Borghuis, M.S.; van Engelen, B.G.M.; Wevers, R.A.; Colier, W.N.J.M. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin. Sci. 2001, 101, 21–28. [Google Scholar] [CrossRef]
| Total | Males | Females | p | |
|---|---|---|---|---|
| N | 28 | 14 | 14 | - |
| Age (years) | 22.1 (2.6) | 22.3 (2.9) | 22.0 (2.4) | 0.839 |
| Height, cm | 166.5 (7.9) | 172.5 (4.4) | 160.5 (5.7) | <0.001 |
| Weight, kg | 60.0 (12.4) | 67.6 (10.9) | 52.3 (8.4) | <0.001 |
| BMI, kg/m2 | 21.5 (3.7) | 22.8 (4.1) | 20.2 (2.8) | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, S.; Ding, S.; Zhang, C. Evaluation of Tibial Hemodynamic Response to Glucose Tolerance Test in Young Healthy Males and Females. Nutrients 2023, 15, 4062. https://doi.org/10.3390/nu15184062
Chen S, Wang S, Ding S, Zhang C. Evaluation of Tibial Hemodynamic Response to Glucose Tolerance Test in Young Healthy Males and Females. Nutrients. 2023; 15(18):4062. https://doi.org/10.3390/nu15184062
Chicago/Turabian StyleChen, Si, Shubo Wang, Shuqiao Ding, and Chuan Zhang. 2023. "Evaluation of Tibial Hemodynamic Response to Glucose Tolerance Test in Young Healthy Males and Females" Nutrients 15, no. 18: 4062. https://doi.org/10.3390/nu15184062
APA StyleChen, S., Wang, S., Ding, S., & Zhang, C. (2023). Evaluation of Tibial Hemodynamic Response to Glucose Tolerance Test in Young Healthy Males and Females. Nutrients, 15(18), 4062. https://doi.org/10.3390/nu15184062







