Effects of Maternal Stress on Breast Milk Production and the Microbiota of Very Premature Infants
Abstract
:1. Introduction
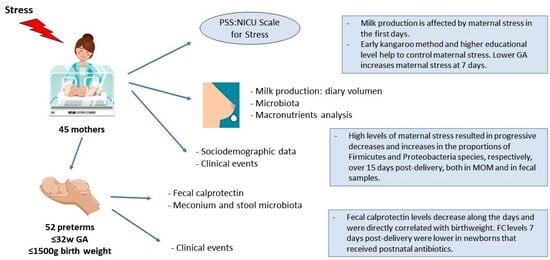
2. Material and Methods
2.1. Study Design
2.2. Study Population
- -
- Sociodemographic data: mother’s age (≥35 years and <35 years); educational level (primary, secondary, higher); type of delivery (vaginal or cesarean section); GA; twinhood; newborn sex and bodyweight.
- -
- Data on the clinical status of premature newborns: respiratory support (invasive mechanical ventilation [IMV], non-invasive mechanical ventilation [NIMV]) required or not; hemodynamically significant patent ductus arteriosus; sepsis; antibiotics received by newborn; antibiotics received by mother prior to delivery; days of parenteral nutrition; complete enteral nutrition; degree of feeding tolerance; start date of milk fortification; and evolution of newborn’s bodyweight.
- -
- Initiation of kangaroo method: early (from birth to ≤4 days), intermediate (5–7 days), or late (≥8 days).
- -
- Exposure to stress in mothers of premature newborns, measured using the parental stress scale (PSS:NICU).
- -
- Breast milk parameters, determined by nutritional analysis and study of microbiota.
- -
- Fecal calprotectin (FC) and microbiota, evaluated in fecal samples from premature newborns.
2.3. Inclusion and Exclusion Criteria
2.4. Outcome Measures
2.4.1. Anthropometric Assessment
2.4.2. Parental Stress Scale
2.4.3. Nutritional Analysis of Macronutrients in MOM
2.4.4. FC
2.4.5. Analysis of Microbiota in Newborn Stool and MOM
2.5. Data Collection
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Study Participants
3.2. Macronutrient Content in MOM
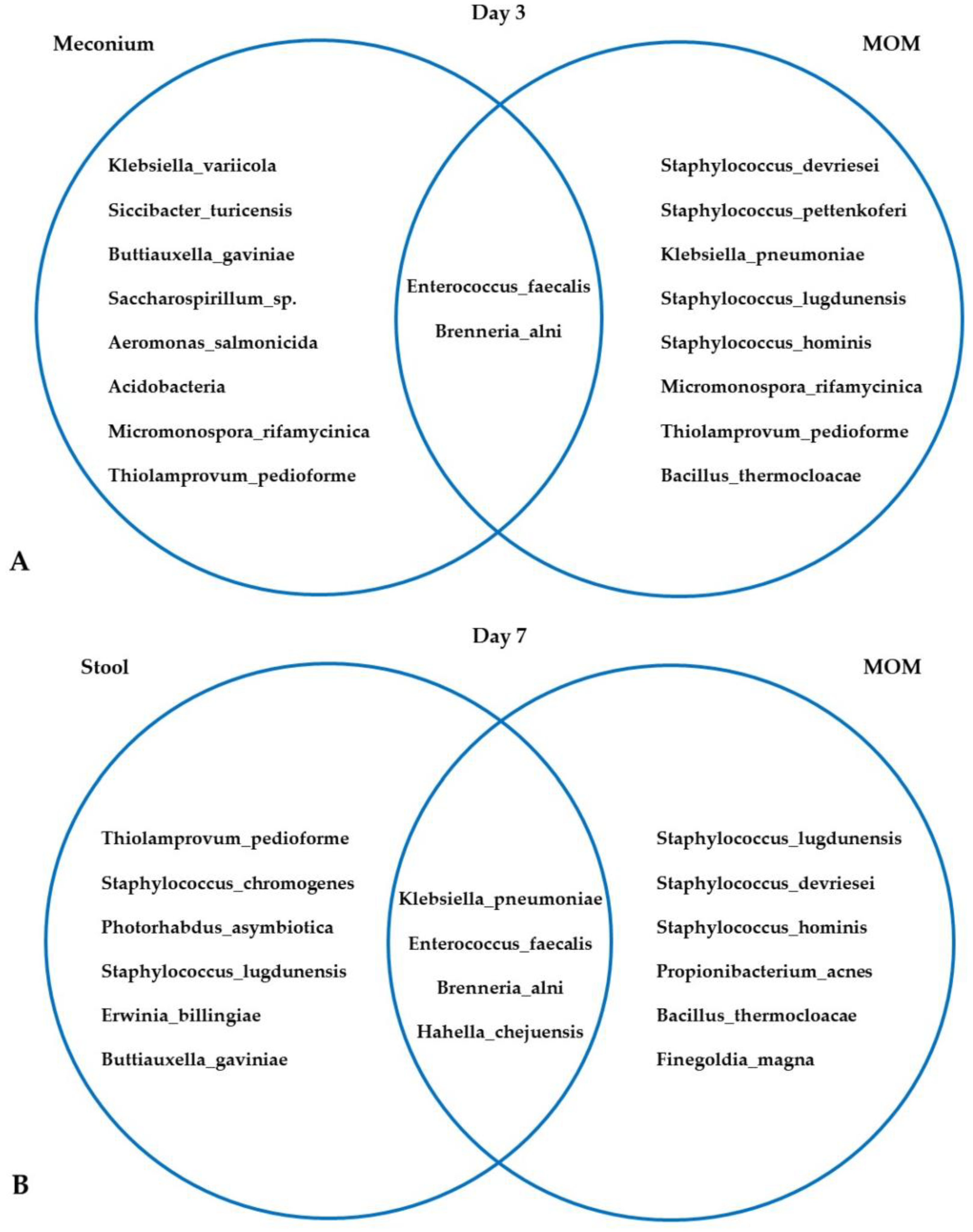
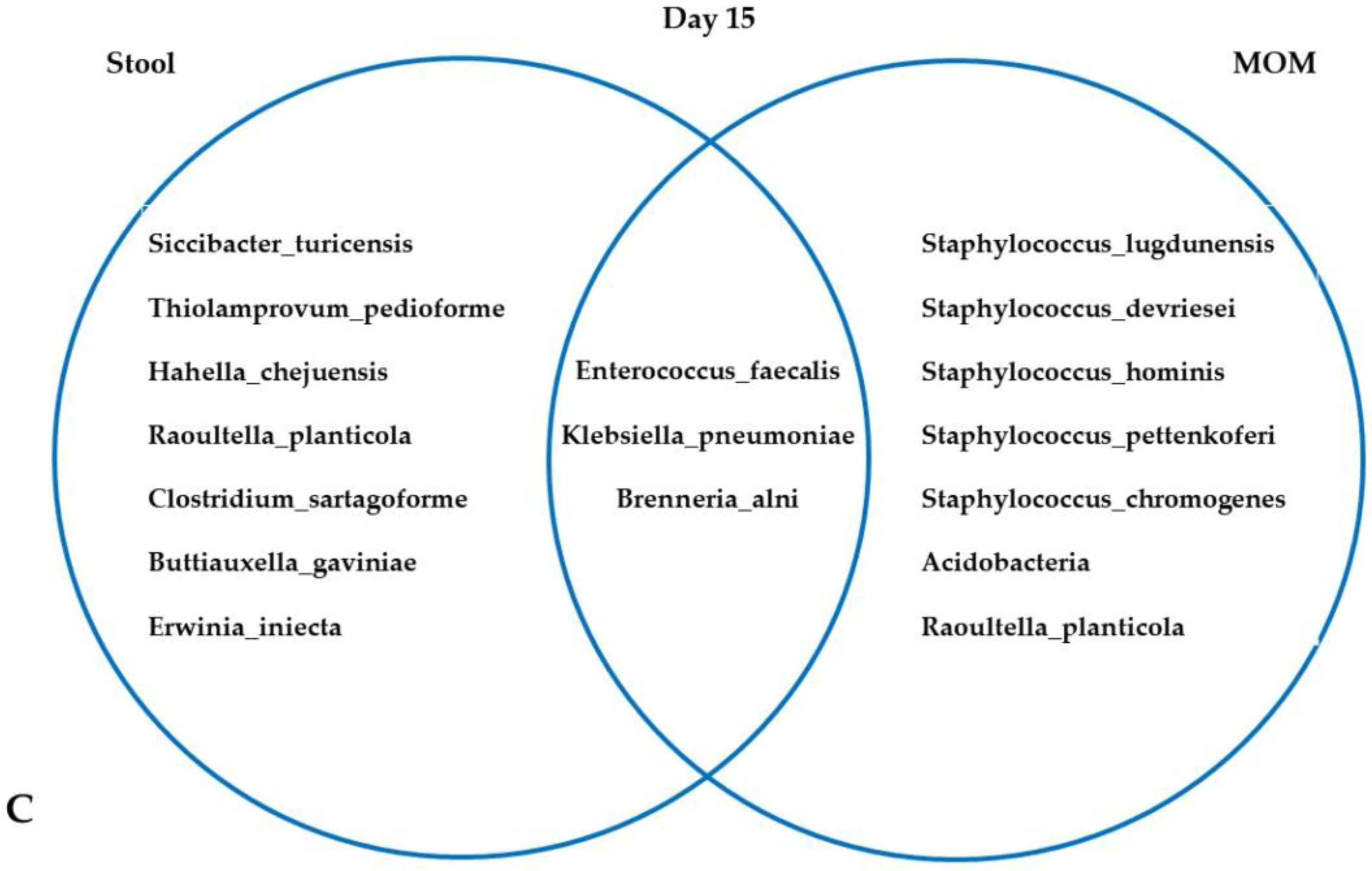
3.3. Microbiota in MOM and Neonatal Stool Samples
4. Discussion
4.1. Parental Stress and MOM
4.2. FC, Microbiota, MOM, and Maternal Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Kramer, M.S. “Breast is best”: The evidence. Early Hum. Dev. 2010, 86, 729–732. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Sadauskaite-Kuehne, V.; Ludvigsson, J.; Padaiga, Z.; Jasinskiene, E.; Samuelsson, U. Longer Breastfeeding in an independent predictive factor against development of type 1 diabetes in chilhood. Diabetes Metab. Res. Rev. 2004, 20, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Alshaer, W.; Hasan, H.; Albakri, K.A.; Alkhafaji, E.; Issa, N.N.; Al-Holy, M.A.; Abderrahman, S.M.; et al. Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects. Biomedicines 2022, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Chernausek, S.D.; Garman, L.D.; Pezant, N.P.; Plows, J.F.; Kharoud, H.K.; Demerath, E.W.; Fields, D.A. Human Milk Exosomal MicroRNA: Associations with Maternal Overweight/Obesity and Infant Body Composition at 1 Month of Life. Nutrients 2021, 13, 1091. [Google Scholar] [CrossRef] [PubMed]
- Sivanandan, S.; Sankar, M.J. Kangaroo mother care for preterm or low birthweight infants: A systematic review and meta-analysis. BMJ Glob. Health 2023, 8, e010728. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Díaz-Rossello, J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2016, 2016, CD002771. [Google Scholar] [CrossRef]
- Adejuyigbe, E.A.; Agyeman, I.; Anand, P.; Anyabolu, H.C.; Arya, S.; Assenga, E.N.; Badhal, S.; Brobby, N.W.; Chellani, H.K.; Chopra, N.; et al. Evaluation of the impact of continuous Kangaroo Mother Care (KMC) initiated immediately after birth compared to KMC initiated after stabilization in newborns with birthweight 1.0 to < 1.8 kg on neurodevelopmental outcomes: Protocol for a follow-up study. Trials 2023, 24, 265. [Google Scholar] [CrossRef]
- Cristóbal-Cañadas, D.; Parrón-Carreño, T.; Nievas-Soriano, B.J. Effect of the Kangaroo Mother Method after Preterm Delivery on Maternal Stress and Anxiety in the Context of the COVID-19 Pandemic—A Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 16432. [Google Scholar] [CrossRef]
- Samsudin, S.; Chui, P.L.; Kamar, A.B.A.; Abdullah, K.L. Maternal Kangaroo care education program in the neonatal intensive care unit improved mothers’ perceptions, knowledge, perceived barriers and stress relates to premature infant. Nurs. Open 2023, 10, 349–357. [Google Scholar] [CrossRef]
- Cañizo Vázquez, D.; Salas García, S.; Izquierdo Renau, M.; Iglesias-Platas, I. Availability of Donor Milk for Very Preterm Infants Decreased the Risk of Necrotizing Enterocolitis without Adversely Impacting Growth or Rates of Breastfeeding. Nutrients 2019, 11, 1895. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Masi, A.C.; Embleton, N.D.; Lamb, C.A.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 2021, 70, 2273–2282. [Google Scholar] [CrossRef]
- Moukarzel, S.; Bode, L. Human Milk Oligosaccharides and the Preterm Infant: A Journey in Sickness and in Health. Clin. Perinatol. 2017, 44, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Ahearn-Ford, S.; Berrington, J.E.; Stewart, C.J. Development of the gut microbiome in early life. Exp. Physiol. 2022, 107, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.L.; Hair, A.B.; Preidis, G.A. A systematic review of associations between gut microbiota composition and growth failure in preterm neonates. Gut Microbes 2023, 15, 2190301. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.; Hain, T.; Chao, C.M.; Rudloff, S. Respiratory and Intestinal Microbiota in Pediatric Lung Diseases-Current Evidence of the Gut-Lung Axis. Int. J. Mol. Sci. 2022, 23, 6791. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xia, X.; Sun, L.; Wang, H.; Li, Q.; Yang, Z.; Ren, J. Microbial Diversity and Correlation between Breast Milk and the Infant Gut. Foods 2023, 12, 1740. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, L.; Wang, Y.; Li, J.; Zhou, Q.; Peng, C.; Li, Y.; Cheng, R.; He, F.; Shen, X. The Effect of Breast Milk Microbiota on the Composition of Infant Gut Microbiota: A Cohort Study. Nutrients 2022, 14, 5397. [Google Scholar] [CrossRef]
- Weiss, S.J.; Hamidi, M. Maternal stress during the third trimester of pregnancy and the neonatal microbiome. J. Matern. Fetal Neonatal Med. 2023, 36, 2214835. [Google Scholar] [CrossRef]
- Yeramilli, V.; Cheddadi, R.; Shah, J.; Brawner, K.; Martin, C. A Review of the Impact of Maternal Prenatal Stress on Offspring Microbiota and Metabolites. Metabolites 2023, 13, 535. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Apanasewicz, A.; Danel, D.P.; Babiszewska, M.; Piosek, M.; Orczyk-Pawiłowicz, M. Maternal Distress and Social Support Are Linked to Human Milk Immune Properties. Nutrients 2021, 13, 1857. [Google Scholar] [CrossRef] [PubMed]
- Juncker, H.; Naninck, E.; Schipper, L.; Lucassen, P.; van Goudoever, J.; de Rooij, S.; Korosi, A. Maternal stress in the postpartum period is associated with altered human milk fatty acid composition. Clin. Nutr. 2022, 41, 2517–2528. [Google Scholar] [CrossRef]
- Nagel, E.M.; Howland, M.A.; Pando, C.; Stang, J.; Mason, S.M.; Fields, D.A.; Demerath, E.W. Maternal Psychological Distress and Lactation and Breastfeeding Outcomes: A Narrative Review. Clin. Ther. 2022, 44, 215–227. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Miles, M.S.; Funk, S.G.; Carlson, J. Parental Stressor Scale: Neonatal intensive care unit. Nurs. Res. 1993, 42, 148–152. [Google Scholar] [CrossRef]
- Kubicka, Z.; Fiascone, J.; Williams, D.; Zahr, E.; Ditzel, A.; Perry, D.; Rousseau, T.; Lacy, M.; Arzuaga, B. Implementing modified family integrated care in a U.S. neonatal intensive care unit: Nursing perspectives and effects on parents. J. Perinatol. 2023, 43, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Shriber, L. Parent Stress in the Neonatal Intensive Care Unit and the Influence of Parent and Infant Characteristics. Am. J. Occup. Ther. 2004, 58, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Mikulic, I.M. El estrés en padres de bebés prematuros internados en la Unidad de Cuidados Intensivos Neonatales: Traducción y adaptación de la escala Parental Stressor Scale: Neonatal Intensive Care Unit (PSS: NICU-M.S. Miles y D. Holditch Davis, 1987; M.S. Miles y S.G. Funk, 1998). Anuario de Investigaciones 2012, XIX, 19–26. [Google Scholar]
- Fusch, G.; Rochow, N.; Choi, A.; Fusch, S.; Poeschl, S.; Ubah, A.O.; Lee, S.Y.; Raja, P.; Fusch, C. Rapid measurement of macronutrients in breast milk: How reliable are infrared milk analyzers? Clin. Nutr. 2015, 34, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Mangel, L.; Ovental, A.; Batscha, N.; Arnon, M.; Yarkoni, I.; Dollberg, S.; Eidelman, A.I.; Lubetzky, R.; Sever, O.; Mimouni, F.B.; et al. Higher Fat Content in Breastmilk Expressed Manually: A Randomized Trial. Breastfeed. Med. 2015, 10, 352–354. [Google Scholar] [CrossRef]
- Alarcón Cavero, T.; Dauria, G.; Delgado Palacio, S.; Del Campo Moreno, R.; Ferrer Martínez, M. Microbiota. Procedimientos en Microbiología Clínica. Recomendaciones de la Sociedad Española de Enfermedades Infecciosas Y Microbiología Clínica; Cercenado Mansilla E., Cantón Moreno R, Ed.; Madrid, Spain, 2016. Available online: https://seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimientomicrobiologia59mod.pdf (accessed on 20 March 2023).
- López Causapé, C.; González Candelas, F.; Tomás Carmona, M.; Oliver Palomo, A. Aplicaciones de las Técnicas de Secuenciación Masiva en la Microbiología Clínica. Recomendaciones de la Sociedad Española de Enfermedades Infecciosas Y Microbiología Clínica; Cercenado Mansilla E., Cantón Moreno R, Ed.; Madrid, Spain, 2021. Available online: https://seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimiento71.pdf (accessed on 2 April 2023).
- 16S Metagenomic Sequencing Library Preparation; 2013, Cercenado Mansilla E, Cantón Moreno R (publishers). Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 26 July 2023).
- Pla, L. Biodiversidad: Inferencia basada en el índice de Shannon y la riqueza. INCI 2006, 31, 583–590. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442006000800008&lng=es (accessed on 20 May 2023).
- Haegeman, B.; Hamelin, J.; Moriarty, J.; Neal, P.; Dushoff, J.; Weitz, J.S. Robust estimation of microbial diversity in theory and in practice. ISME J. 2013, 7, 1092–1101. [Google Scholar] [CrossRef]
- Ionio, C.; Ciuffo, G.; Landoni, M. Parent–Infant Skin-to-Skin Contact and Stress Regulation: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 4695. [Google Scholar] [CrossRef]
- Dewey, K.G. Maternal and Fetal Stress Are Associated with Impaired Lactogenesis in Humans. J. Nutr. 2001, 131, 3012S–3015S. [Google Scholar] [CrossRef]
- Fang, Y.; Boelens, M.; Windhorst, D.A.; Raat, H.; van Grieken, A. Factors associated with parenting self-efficacy: A systematic review. J. Adv. Nurs. 2021, 77, 2641–2661. [Google Scholar] [CrossRef]
- Soffer, G.P.; Siri, M.; Mangel, L.; Mandel, D.; Lubetzky, R. Impact of Maternal Anxiety on Human Milk Macronutrients Content: A Prospective Observational Study. Breastfeed. Med. 2020, 15, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, C.J.; Kang, N.M. Maternal Factors Affecting the Macronutrient Composition of Transitional Human Milk. Int. J. Environ. Res. Public Health 2022, 19, 3308. [Google Scholar] [CrossRef] [PubMed]
- Ziomkiewicz, A.; Babiszewska, M.; Apanasewicz, A.; Piosek, M.; Wychowaniec, P.; Cierniak, A.; Barbarska, O.; Szołtysik, M.; Danel, D.; Wichary, S. Psychosocial stress and cortisol stress reactivity predict breast milk composition. Sci. Rep. 2021, 11, 11576. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, J.; Geng, S.; Wang, J.; Liu, J.; Zhang, J.; Sheng, X. Fecal Calprotectin Concentrations in Healthy Children Aged 1–18 Months. PLoS ONE 2015, 10, e0119574. [Google Scholar] [CrossRef] [PubMed]
- Campeotto, F.; Butel, M.J.; Kalach, N.; Derrieux, S.; Aubert-Jacquin, C.; Barbot, L.; Francoual, C.; Dupont, C.; Kapel, N. High faecal calprotectin concentrations in newborn infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F353–F355. [Google Scholar] [CrossRef]
- Kapel, N.; Campeotto, F.; Kalach, N.; Baldassare, M.; Butel, M.-J.; Dupont, C. Faecal Calprotectin in Term and Preterm Neonates. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 542–547. [Google Scholar] [CrossRef]
- Rougé, C.; Butel, M.-J.; Piloquet, H.; Ferraris, L.; Legrand, A.; Vodovar, M.; Voyer, M.; de la Cochetière, M.-F.; Darmaun, D.; Rozé, J.-C. Fecal Calprotectin Excretion in Preterm Infants during the Neonatal Period. PLoS ONE 2010, 5, e11083. [Google Scholar] [CrossRef]
- Groer, M.; Ashmeade, T.; Louis-Jacques, A.; Beckstead, J.; Ji, M. Relationships of Feeding and Mother’s Own Milk with Fecal Calprotectin Levels in Preterm Infants. Breastfeed. Med. 2016, 11, 207–212. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, J.Y.; Chung, C.; Oh, S.H.; Do, H.-J.; Seo, J.-H.; Lim, J.Y.; Park, C.-H.; Woo, H.-O.; Youn, H.-S. Dynamic Changes of Fecal Calprotectin and Related Clinical Factors in Neonates. Front. Pediatr. 2020, 8, 326. [Google Scholar] [CrossRef]
| At Birth | ||
|---|---|---|
| Mothers’ age (years) median (interquartile range) | 35 (31.5–38) | |
| Gestational age (w) | 31 (29–32) | |
| Prenatal antibiotics (n, %) | 17, 37.8% | |
| Educational level (n, %) | Primary studies | 4, 9% |
| Secondary studies | 18, 40% | |
| Higher education | 23, 51% | |
| Type of delivery (n, %) | Vaginal | 17, 33% |
| Caesarean section | 35, 67% | |
| Twinhood (n, %) | 8, 15.4% | |
| Sex of newborn: M/F (n, %) | M (28, 56.8%) F (24, 46.2%) |
| Time (Days) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Birth | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| Neonatal weight (g), median (interquartile range), and percentile | 1375 (1012–1667) p33 | 1240 (927–1467) p15 | 1280 (990–1515) p12 | 1520 (1125–1755) p14 | |||||||||||||
| Neonatal antibiotics in first 48 h (n, %) | 34, 65.4% | ||||||||||||||||
| Duration of neonatal antibiotic treatment | 7 days | ||||||||||||||||
| Respiratory support (n, %) | Invasive | 2, 3.8% | 0, 0% | 1, 1.92% | |||||||||||||
| Non-invasive | 38, 73.1% | 16, 30.8% | 7, 13.5% | ||||||||||||||
| Significant patent ductus arteriosus (n, %) | 2, 3.8% | 3, 5.8% | 3, 5.8% | ||||||||||||||
| Sepsis (n, %) | 7, 13.5% | 4, 7.7% | 3, 5.8% | ||||||||||||||
| Nutrition (n, %) | Parenteral | 51, 98.1% | 24, 46.2% | 3, 5.8% | |||||||||||||
| Well tolerated | 46, 88.5% | 51, 98.1% | 50, 96.2% | ||||||||||||||
| Complete enteral nutrition | 4, 7.7% | 39, 75% | 48, 92.3% | ||||||||||||||
| Fortification | 0, 0% | 19, 36.5% | 45, 86.5% | ||||||||||||||
| Initiation of kangaroo method (n, %) | Early | 16, 30.8% | |||||||||||||||
| Intermediate | 20, 38.5% | ||||||||||||||||
| Late | 16, 30.8% | ||||||||||||||||
| FC (mcg/g stool), median (interquartile range) | 70 (20.25–148.75) | 26 (7.25–60.75) | 42 (28–72.75) | ||||||||||||||
| FC levels (mcg/g stool) in newborns with vs. without antibiotics, median (interquartile range) | 83.5 (22–168) vs. 64 (19–129) | 17 (6–39) vs. 54 (17–94) p = 0.027 | 49 (28–73) vs. 38 (29–54) | ||||||||||||||
| FC (mcg/g stool) in stool samples according to body weight: <1000 g; 1001–1500 g; >1500 g, median (interquartile range) | 122 (55–246.5); 70 (21–127); 21 (12.5–120.5) p = 0.044 | 24.5 (14.5–45); 21.5 (6–54); 42.5 (8–78.5) | 40 (7.5–71.5); 43 (29.5–91.5); 47.5 (30–66.5) | ||||||||||||||
| Day 3 | Day 7 | Day 15 | ||||||
|---|---|---|---|---|---|---|---|---|
| Breastfeeding volume | TS | p | Breastfeeding volume | TS | p | Breastfeeding volume | TS | p |
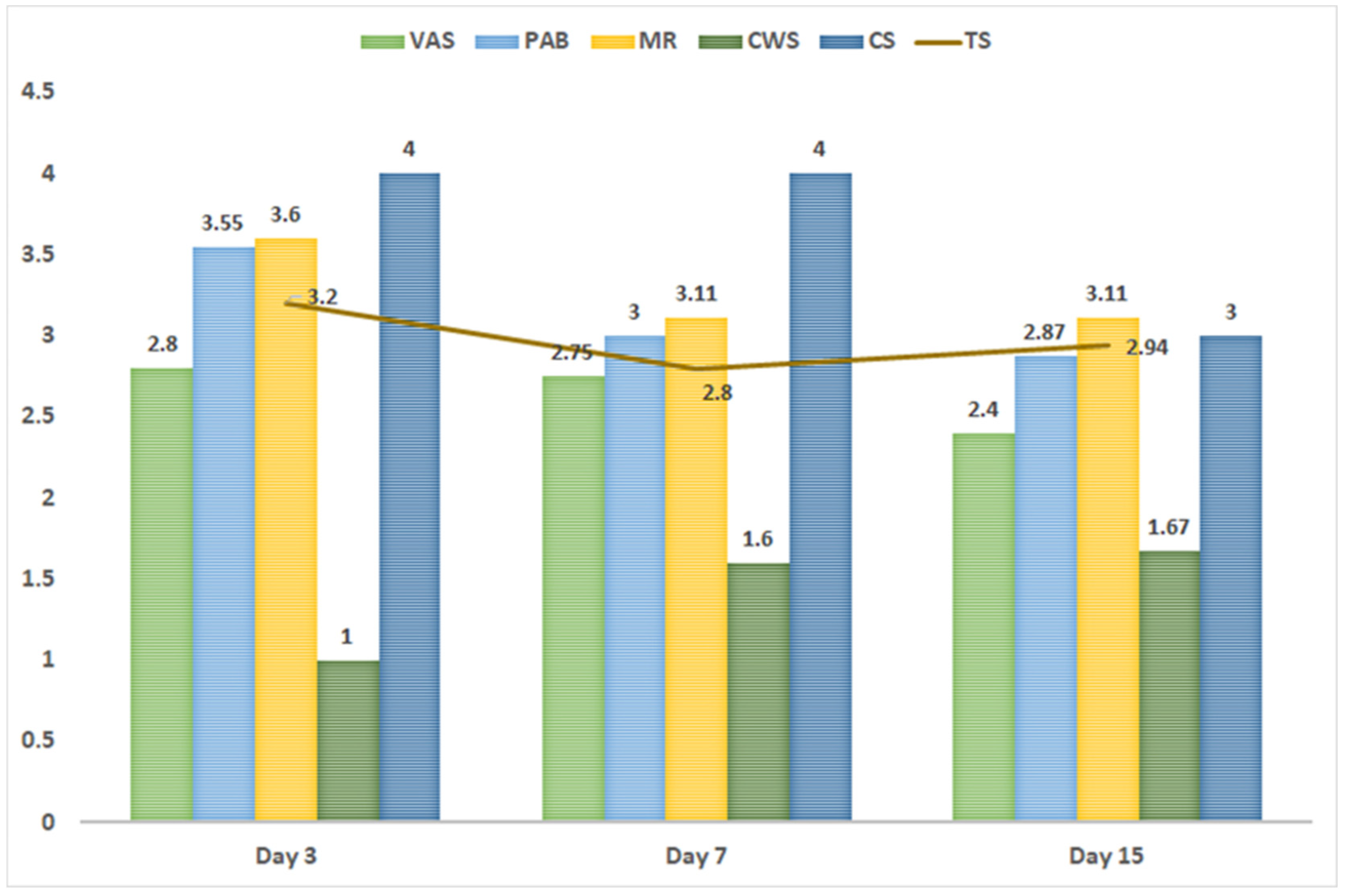
| 22.5 mL (5–61) | 3.2 (2.52–3.64) | 0.012 | 150 mL (45–340) | 2.8 (2.3–3.87) | NS | 275 mL (130–530) | 2.94 (2–3.8) | NS |
| Initiation of Kangaroo method | Decrease stress | p | Initiation of Kangaroo method | Decrease stress | p | Initiation of Kangaroo method | Decrease stress | p |
| Early | −0.65 (−1.00–−0.38) | 0.011 | Intermediate | 0.02 (−0.41–0.34) | NS | Late | −0.38 (−0.66–−0.12) | NS |
| TS with Primary Educational level | TS with Secondary and Higher Educational level | p | TS with Primary Educational level | TS with Secondary and Higher Educational level | p | TS with Primary Educational level | TS with Secondary and Higher Educational level | p |
| 3.64 (2.72–4.01) | 3.2 (2.4–3.73) | NS | 4.43 (3.48–4.58) | 2.79 (2.25–3.78) | 0.032 | 4.3 (3.14–4.51) | 2.89 (1.84–3.7) | 0.04 |
| Day 3 | Day 7 | Day 15 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Stress (<3.2) | High Stress (≥3.2) | p | Low Stress (<2.8) | High Stress (≥2.8) | p | Low Stress (<2.94) | High Stress (≥2.94) | p | ||
| Mothers’ age (y) median (interquartile range) | 35 (29.5–38.5) | 35 (33–36) | NS | 36 (35–39) | 33.5 (26–36) | 0.03 | 36 (32–39) | 34 (26–36) | NS | |
| Gestational age (w) median (interquartile range) | 31 (29–32) | 30 (27–31) | NS | 31 (30–32) | 30 (26–31) | 0.031 | 31 (29–32) | 30 (27–31) | NS | |
| Type of delivery | Vaginal | 33.3% | 66.7% | NS | 31.2% | 68.8% | NS | 43.7% | 56.3% | NS |
| Caesarean section | 57.7% | 42.3% | 59.2% | 40.8% | 53.8% | 46.2% | ||||
| Twinhood (Yes) | 57.2% | 42.8% | NS | 87.5% | 12.5% | 0.015 | 87.5% | 12.5% | 0.018 | |
| Initiation of kangaroo method | Early | 50% | 50% | NS | 66.7% | 33.3% | NS | 58.3% | 41.7% | NS |
| Intermediate | 52.9% | 47.1% | 47.1% | 52.9% | 43.7% | 56.3% | ||||
| Late | 38.4% | 61.6% | 30.7% | 69.3% | 46.1% | 53.9% | ||||
| MOM volume median (interquartile range) | 40 mL (11–122.5) | 20 mL (5–48) | 0.046 | 180 mL (117.5–370) | 82.5 mL (44–340) | NS | 392.5 mL (197.5–550) | 220 mL (85–440) | NS | |
| Day 3 | Day 7 | Day 15 | |||||
|---|---|---|---|---|---|---|---|
| Stress | Low (<3.2) | High (≥3.2) | Low (<2.8) | High (≥2.8) | Low (<2.94) | High (≥2.94) | |
| Shannon index | Feces | 1 (0.48–1.86) | 1.56 (1.19–1.65) | 0.71 (0.31–1.24) | 1.25 (0.76–1.36) | 1.13 (0.77–1.35) | 0.91 (0.33–1.08) |
| MOM | 0.99 (0.60–1.39) | 0.76 (0.61–1.01) | 0.75 (0.46–1.21) | 0.65 (0.38–1.14) | 1.19 (0.60–1.34) | 0.99 (0.34–1.37) | |
| Day 3 | Day 7 | Day 15 | ||||
|---|---|---|---|---|---|---|
| Meconium | MOM | Feces | MOM | Feces | MOM | |
| Proteobacteria | 62.5% | 30.4% | 50% | 30% | 71.4% | 34.6% |
| Firmicutes | 37.5% | 69.6% | 35% | 70% | 17.9% | 65.4% |
| Actinobacteria | N/A | N/A | 5% | N/A | 3.6% | N/A |
| Bacteroidetes | N/A | N/A | 5% | N/A | 3.6% | N/A |
| Phyla in MOM | Stress 3 Days | Stress 7 Days | Stress 15 Days | |||
|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | |
| Proteobacteria | 41.7% | 12.5% | 16.7% | 37.5% | 26.7% | 44.4% |
| Firmicutes | 58.3% | 87.5% | 83.3% | 62.5% | 73.3% | 55.6% |
| Phyla in feces | ||||||
| Proteobacteria | 75% | 33.3% | 66.7% | 40% | 90% | 70% |
| Firmicutes | 25% | 66.7% | 22.2% | 40% | 10% | 20% |
| Actinobacteria | N/A | N/A | 11.1% | N/A | N/A | N/A |
| Bacteroidetes | N/A | N/A | N/A | 20% | N/A | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Tuñas, M.d.C.; Pérez-Muñuzuri, A.; Trastoy-Pena, R.; Pérez del Molino, M.L.; Couce, M.L. Effects of Maternal Stress on Breast Milk Production and the Microbiota of Very Premature Infants. Nutrients 2023, 15, 4006. https://doi.org/10.3390/nu15184006
Fernández-Tuñas MdC, Pérez-Muñuzuri A, Trastoy-Pena R, Pérez del Molino ML, Couce ML. Effects of Maternal Stress on Breast Milk Production and the Microbiota of Very Premature Infants. Nutrients. 2023; 15(18):4006. https://doi.org/10.3390/nu15184006
Chicago/Turabian StyleFernández-Tuñas, María del Carmen, Alejandro Pérez-Muñuzuri, Rocío Trastoy-Pena, María Luisa Pérez del Molino, and María L. Couce. 2023. "Effects of Maternal Stress on Breast Milk Production and the Microbiota of Very Premature Infants" Nutrients 15, no. 18: 4006. https://doi.org/10.3390/nu15184006
APA StyleFernández-Tuñas, M. d. C., Pérez-Muñuzuri, A., Trastoy-Pena, R., Pérez del Molino, M. L., & Couce, M. L. (2023). Effects of Maternal Stress on Breast Milk Production and the Microbiota of Very Premature Infants. Nutrients, 15(18), 4006. https://doi.org/10.3390/nu15184006