The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome
Abstract
1. Introduction
1.1. Background on Irritable Bowel Syndrome
1.2. Current Treatment Modalities for IBS
1.3. Importance of the Gut Microbiome and Inflammation in IBS Pathogenesis
1.4. Bovine Kappa-Casein Glycomacropeptide: A Potential Nutritional Intervention in IBS Management
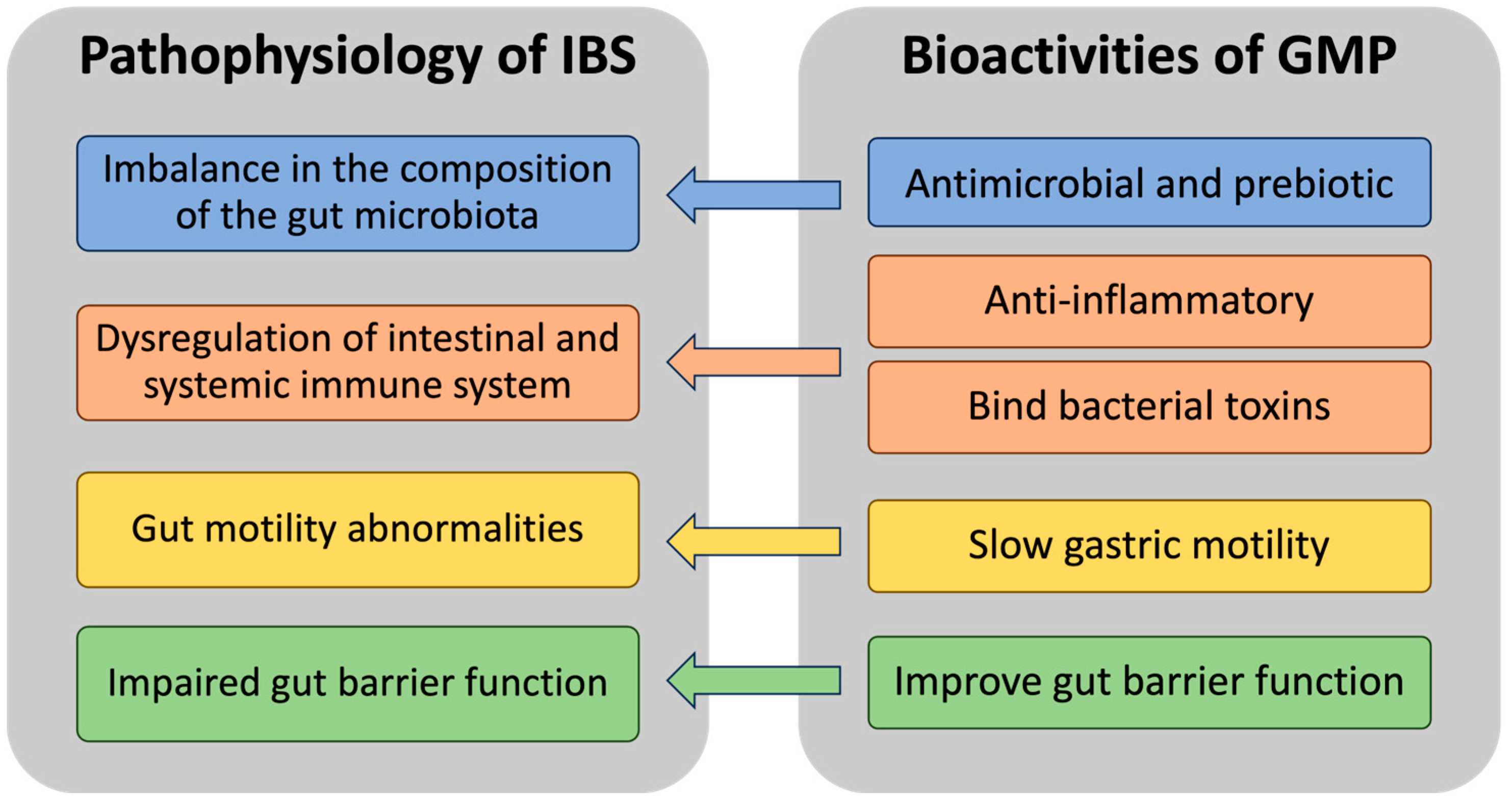
2. GMP and the Microbiome in IBS
2.1. Gut Microbiome in IBS
2.2. GMP as an Antimicrobial Agent
2.3. GMP as a Prebiotic
2.4. GMP’s Influence on the Gut Microbiome
2.5. Potential Implications of GMP-Induced Microbiota Modulation in IBS
3. GMP and Inflammation in IBS
3.1. Inflammation in IBS
3.2. GMP as an Anti-Inflammatory Agent
3.3. Potential Implications of GMP-Induced Anti-Inflammatory Modulation in IBS
4. GMP’s Toxin Binding, Gut Motility-Decreasing, and Barrier Function-Enhancing Properties in IBS
4.1. Binding Toxin
4.2. Gut Motility
4.3. Barrier Function
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyedmirzaee, S.; Hayatbakhsh, M.M.; Ahmadi, B.; Baniasadi, N.; Bagheri Rafsanjani, A.M.; Nikpoor, A.R.; Mohammadi, M. Serum Immune Biomarkers in Irritable Bowel Syndrome. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Hungin, A.P.S.; Chang, L.; Locke, G.R.; Dennis, E.H.; Barghout, V. Irritable Bowel Syndrome in the United States: Prevalence, Symptom Patterns and Impact. Aliment. Pharmacol. Ther. 2005, 21, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable Bowel Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Locke, G.R. Epidemiology of IBS. Gastroenterol. Clin. N. Am. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global Prevalence of Irritable Bowel Syndrome According to Rome III or IV Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Tornkvist, N.T.; Aziz, I.; Whitehead, W.E.; Sperber, A.D.; Palsson, O.S.; Hreinsson, J.P.; Simrén, M.; Törnblom, H. Health Care Utilization of Individuals with Rome IV Irritable Bowel Syndrome in the General Population. United Eur. Gastroenterol. J. 2021, 9, 1178–1188. [Google Scholar] [CrossRef]
- Saito, Y.A.; Schoenfeld, P.; Locke, G.R., 3rd. The Epidemiology of Irritable Bowel Syndrome in North America: A Systematic Review. Am. J. Gastroenterol. 2002, 97, 1910–1915. [Google Scholar] [CrossRef]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The Kinetics of Gut Microbial Community Composition in Patients with Irritable Bowel Syndrome Following Fecal Microbiota Transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef]
- Lesbros-Pantoflickova, D.; Michetti, P.; Fried, M.; Beglinger, C.; Blum, A.L. Meta-Analysis: The Treatment of Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2004, 20, 1253–1269. [Google Scholar] [CrossRef]
- Cook, I.J.; Irvine, E.J.; Campbell, D.; Shannon, S.; Reddy, S.N.; Collins, S.M. Effect of Dietary Fiber on Symptoms and Rectosigmoid Motility in Patients with Irritable Bowel Syndrome. A Controlled, Crossover Study. Gastroenterology 1990, 98, 66–72. [Google Scholar] [CrossRef]
- Hebden, J.M.; Blackshaw, E.; D’Amato, M.; Perkins, A.C.; Spiller, R.C. Abnormalities of GI Transit in Bloated Irritable Bowel Syndrome: Effect of Bran on Transit and Symptoms. Am. J. Gastroenterol. 2002, 97, 2315–2320. [Google Scholar] [CrossRef]
- Thimister, P.W.L.; Hopman, W.P.M.; Van Roermund, R.F.C.; Willems, H.L.; Rosenbusch, G.; Woestenborghs, R.; Jansen, J.B.M.J. Inhibition of Pancreaticobiliary Secretion by Loperamide in Humans. Hepatology 1997, 26, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Cann, P.A.; Read, N.W.; Holdsworth, C.D.; Barends, D. Role of Loperamide and Placebo in Management of Irritable Bowel Syndrome (IBS). Dig. Dis. Sci. 1984, 29, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Dobrilla, G.; Piazzi, L.; Bensi, G.; Dobrilla, G. Longterm Treatment of Irritable Bowel Syndrome with Cimetropium Bromide: A Double Blind Placebo Controlled Clinical Trial. Gut 1990, 31, 355. [Google Scholar] [CrossRef] [PubMed]
- Passaretti, S.; Guslandi, M.; Imbimbo, B.P.; Daniotti, S.; Tittobello, A. Effects of Cimetropium Bromide on Gastrointestinal Transit Time in Patients with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 1989, 3, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Milo, R. Use of the Peripheral Dopamine Antagonist, Domperidone, in the Management of Gastro-Intestinal Symptoms in Patients with Irritable Bowel Syndrome. Curr. Med. Res. Opin. 1980, 6, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Hovdenak, N.; Wetterhus, S.; Lange, O.J.; Hovde, Ø.; Trondstad, R. The Symptomatic Effect of Cisapride in Patients with Irritable Bowel Syndrome and Constipation. Scand. J. Gastroenterol. 2009, 33, 128–131. [Google Scholar] [CrossRef]
- Schütze, K.; Brandstätter, G.; Dragosics, B.; Judmaier, G.; Hentschel, E. Double-Blind Study of the Effect of Cisapride on Constipation and Abdominal Discomfort as Components of the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 1997, 11, 387–394. [Google Scholar] [CrossRef]
- Myren, J.; Groth, H.; Larssen, S.E.; Larsen, S. The Effect of Trimipramine in Patients with the Irritable Bowel Syndrome. A Double-Blind Study. Scand. J. Gastroenterol. 1982, 17, 871–875. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Kurian, G.; John, J. Symptom Relief with Amitriptyline in the Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 1998, 13, 738–741. [Google Scholar] [CrossRef]
- Fayyaz, M.; Lackner, J.M. Serotonin Receptor Modulators in the Treatment of Irritable Bowel Syndrome. Ther. Clin. Risk Manag. 2008, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Coulie, B.; Szarka, L.A.; Camilleri, M.; Burton, D.D.; McKinzie, S.; Stambler, N.; Cedarbaum, J.M. Recombinant Human Neurotrophic Factors Accelerate Colonic Transit and Relieve Constipation in Humans. Gastroenterology 2000, 119, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fioramonti, J.; Gaultier, E.; Toulouse, M.; Sanger, G.J.; Bueno, L. Intestinal Anti-Nociceptive Behaviour of NK3 Receptor Antagonism in Conscious Rats: Evidence to Support a Peripheral Mechanism of Action. Neurogastroenterol. Motil. 2003, 15, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Ikeura, Y.; Inatomi, N. Effects of Tachykinin NK1 Receptor Antagonists on the Viscerosensory Response Caused by Colorectal Distention in Rabbits. J. Pharmacol. Exp. Ther. 2002, 300, 925–931. [Google Scholar] [CrossRef]
- Sanger, G.J. Neurokinin NK1 and NK3 Receptors as Targets for Drugs to Treat Gastrointestinal Motility Disorders and Pain. Br. J. Pharmacol. 2004, 141, 1303–1312. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-Pituitary-Adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Elsenbruch, S.; Orr, W.C. Diarrhea- and Constipation-Predominant IBS Patients Differ in Postprandial Autonomic and Cortisol Responses. Am. J. Gastroenterol. 2001, 96, 460–466. [Google Scholar] [CrossRef]
- Camilleri, M.; Kim, D.Y.; McKinzie, S.; Kim, H.J.; Thomforde, G.M.; Burton, D.D.; Low, P.A.; Zinsmeister, A.R. A Randomized, Controlled Exploratory Study of Clonidine in Diarrhea-Predominant Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2003, 1, 111–121. [Google Scholar] [CrossRef]
- Bazzocchi, G.; Gionchetti, P.; Almerigi, P.F.; Amadini, C.; Campieri, M. Intestinal Microflora and Oral Bacteriotherapy in Irritable Bowel Syndrome. Dig. Liver Dis. 2002, 34 (Suppl. S2), S48–S53. [Google Scholar] [CrossRef]
- Coutinho, S.V.; Plotsky, P.M.; Sablad, M.; Miller, J.C.; Zhou, H.; Bayati, A.I.; McRoberts, J.A.; Mayer, E.A. Neonatal Maternal Separation Alters Stress-Induced Responses to Viscerosomatic Nociceptive Stimuli in Rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G307–G316. [Google Scholar] [CrossRef]
- Gholamrezaei, A.; Ardestani, S.K.; Emami, M.H. Where Does Hypnotherapy Stand in the Management of Irritable Bowel Syndrome? A Systematic Review. J. Altern. Complement. Med. 2006, 12, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Akiho, H.; Ihara, E.; Nakamura, K. Low-Grade Inflammation Plays a Pivotal Role in Gastrointestinal Dysfunction in Irritable Bowel Syndrome. World J. Gastrointest. Pathophysiol. 2010, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.A.; Ruigómez, A. Increased Risk of Irritable Bowel Syndrome after Bacterial Gastroenteritis: Cohort Study. BMJ 1999, 318, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.; Bielsa, M.V.; Carmona-Sánchez, R.; Hernández, A.; López-Colombo, A.; Vidal, Y.L.; Peláez-Luna, M.; Remes-Troche, J.M.; Tamayo, J.L.; Valdovinos, M.A. Microbiota, Gastrointestinal Infections, Low-Grade Inflammation, and Antibiotic Therapy in Irritable Bowel Syndrome (IBS): An Evidence-Based Review. Rev. Gastroenterol. Méx. 2014, 79, 96–134. [Google Scholar] [CrossRef]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut Dysbiosis and Irritable Bowel Syndrome: The Potential Role of Probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef]
- Casén, C.; Vebø, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in Human Gut Microbiota: A Novel Diagnostic Test for Determining Dysbiosis in Patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Karantanos, T.; Markoutsaki, T.; Gazouli, M.; Anagnou, N.P.; Karamanolis, D.G. Current Insights in to the Pathophysiology of Irritable Bowel Syndrome. Gut Pathog. 2010, 2, 3. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An Irritable Bowel Syndrome Subtype Defined by Species-Specific Alterations in Faecal Microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Collins, S.M. A Role for the Gut Microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef]
- Matricon, J.; Meleine, M.; Gelot, A.; Piche, T.; Dapoigny, M.; Muller, E.; Ardid, D. Review Article: Associations between Immune Activation, Intestinal Permeability and the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2012, 36, 1009–1031. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Therapies Aimed at the Gut Microbiota and Inflammation: Antibiotics, Prebiotics, Probiotics, Synbiotics, Anti-Inflammatory Therapies. Gastroenterol. Clin. N. Am. 2011, 40, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Farrell Jr, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk--Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [PubMed]
- Eigel, W.N.; Butler, J.E.; Ernstrom, C.A.; Farrell, H.M., Jr.; Harwalkar, V.R.; Jenness, R.; Whitney, R.M. Nomenclature of Proteins of Cow’s Milk: Fifth Revision. J. Dairy Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Thomä-Worringer, C.; Sørensen, J.; López-Fandiño, R. Health Effects and Technological Features of Caseinomacropeptide. Int. Dairy J. 2006, 16, 1324–1333. [Google Scholar] [CrossRef]
- Qu, Y.; Kim, B.J.; Koh, J.; Dallas, D.C. Analysis of Bovine Kappa-Casein Glycomacropeptide by Liquid Chromatography–Tandem Mass Spectrometry. Foods 2021, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, N.; Kane, M.; Joshi, L.; Hickey, R.M. Structural and Functional Characteristics of Bovine Milk Protein Glycosylation. Glycobiology 2014, 24, 220–236. [Google Scholar] [CrossRef]
- Arunkumar, A.; Etzel, M.R. Fractionation of Glycomacropeptide from Whey Using Positively Charged Ultrafiltration Membranes. Foods 2018, 7, 166. [Google Scholar] [CrossRef]
- Ney, D.M.; Hull, A.K.; van Calcar, S.C.; Liu, X.; Etzel, M.R. Dietary Glycomacropeptide Supports Growth and Reduces the Concentrations of Phenylalanine in Plasma and Brain in a Murine Model of Phenylketonuria. J. Nutr. 2008, 138, 316–322. [Google Scholar] [CrossRef]
- Furlanetti, A.M.; Prata, L.F. Free and Total GMP (Glycomacropeptide) Contents of Milk during Bovine Lactation. Food Sci. Technol. 2003, 23, 121–125. [Google Scholar] [CrossRef]
- Yvon, M.; Beucher, S.; Guilloteau, P.; Le Huerou-Luron, I.; Corring, T. Effects of Caseinomacropeptide (CMP) on Digestion Regulation. Reprod. Nutr. Dev. 1994, 34, 527–537. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Isoda, H.; Shinmoto, H.; Tanimoto, M.; Dosako, S.; Idota, T.; Nakajima, I. Inhibition by κ-Casein Glycomacropeptide and Lactoferrin of Influenza Virus Hemagglutination. Biosci. Biotechnol. Biochem. 1993, 57, 1214–1215. [Google Scholar] [CrossRef]
- Janer, C.; Díaz, J.; Peláez, C.; Requena, T. The effect of caseinomacropeptide and whey protein concentrate on streptococcus mutans adhesion to polystyrene surfaces and cell aggregation. J. Food Qual. 2004, 27, 233–238. [Google Scholar] [CrossRef]
- Robitaille, G. Growth-Promoting Effects of Caseinomacropeptide from Cow and Goat Milk on Probiotics. J. Dairy Res. 2013, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vara, E.J.; Brokstad, K.A.; Hausken, T.; Lied, G.A. Altered Levels of Cytokines in Patients with Irritable Bowel Syndrome Are Not Correlated with Fatigue. Int. J. Gen. Med. 2018, 11, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Isoda, H.; Tanimoto, M.; Dosako, S.; Idota, T.; Ahiko, K. Inhibition by Lactoferrin and κ-Casein Glycomacropeptide of Binding of Cholera Toxin to Its Receptor. Biosci. Biotechnol. Biochem. 1992, 56, 195–198. [Google Scholar] [CrossRef]
- Brück, W.M.; Graverholt, G.; Gibson, G.R. A Two-Stage Continuous Culture System to Study the Effect of Supplemental Alpha-Lactalbumin and Glycomacropeptide on Mixed Cultures of Human Gut Bacteria Challenged with Enteropathogenic Escherichia coli and Salmonella Serotype Typhimurium. J. Appl. Microbiol. 2003, 95, 44–53. [Google Scholar] [CrossRef]
- Brück, W.M.; Kelleher, S.L.; Gibson, G.R.; Graverholt, G.; Lönnerdal, B.L. The Effects of Alpha-Lactalbumin and Glycomacropeptide on the Association of CaCo-2 Cells by Enteropathogenic Escherichia coli, Salmonella typhimurium and Shigella flexneri. FEMS Microbiol. Lett. 2006, 259, 158–162. [Google Scholar] [CrossRef]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in Irritable Bowel Syndrome and Other Functional Bowel Disorders in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of Butyrate- and Methane-Producing Microorganisms in Patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Keku, T.O.; Chang, Y.H.; Packey, C.D.; Balfour Sartor, R.; Ringel, Y. Molecular Analysis of the Luminal- and Mucosal-Associated Intestinal Microbiota in Diarrhea-Predominant Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G799–G807. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients with Diarrhea-Predominant Irritable Bowel Syndrome and Patients with Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef] [PubMed]
- Rangel, I.; Sundin, J.; Fuentes, S.; Repsilber, D.; de Vos, W.M.; Brummer, R.J. The Relationship between Faecal-Associated and Mucosal-Associated Microbiota in Irritable Bowel Syndrome Patients and Healthy Subjects. Aliment. Pharmacol. Ther. 2015, 42, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Durbán, A.; Abellán, J.J.; Jiménez-Hernández, N.; Salgado, P.; Ponce, M.; Ponce, J.; Garrigues, V.; Latorre, A.; Moya, A. Structural Alterations of Faecal and Mucosa-Associated Bacterial Communities in Irritable Bowel Syndrome. Environ. Microbiol. Rep. 2012, 4, 242–247. [Google Scholar] [CrossRef]
- Rigsbee, L.; Agans, R.; Shankar, V.; Kenche, H.; Khamis, H.J.; Michail, S.; Paliy, O. Quantitative Profiling of Gut Microbiota of Children with Diarrhea-Predominant Irritable Bowel Syndrome. Am. J. Gastroenterol. 2012, 107, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated with Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.S.; Hollister, E.B.; Jacobs, J.; Kirbach, K.; Oezguen, N.; Gupta, A.; Acosta, J.; Luna, R.A.; Aagaard, K.; Versalovic, J.; et al. Differences in Gut Microbial Composition Correlate with Regional Brain Volumes in Irritable Bowel Syndrome. Microbiome 2017, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Jahng, J.; Jung, I.S.; Choi, E.J.; Conklin, J.L.; Park, H. The Effects of Methane and Hydrogen Gases Produced by Enteric Bacteria on Ileal Motility and Colonic Transit Time. Neurogastroenterol. Motil. 2012, 24, 185-e92. [Google Scholar] [CrossRef]
- Villanueva-Millan, M.J.; Leite, G.; Wang, J.; Morales, W.; Parodi, G.; Pimentel, M.L.; Barlow, G.M.; Mathur, R.; Rezaie, A.; Sanchez, M.; et al. Methanogens and Hydrogen Sulfide Producing Bacteria Guide Distinct Gut Microbe Profiles and Irritable Bowel Syndrome Subtypes. Am. J. Gastroenterol. 2022, 117, 2055–2066. [Google Scholar] [CrossRef]
- Singer-Englar, T.; Rezaie, A.; Gupta, K.; Pichetshote, N.; Sedighi, R.; Lin, E.; Chua, K.S.; Pimentel, M. 182—Competitive Hydrogen Gas Utilization by Methane- and Hydrogen Sulfide-Producing Microorganisms and Associated Symptoms: Results of a Novel 4-Gas Breath Test Machine. Gastroenterology 2018, 154, 47. [Google Scholar] [CrossRef]
- Quan, X.; Luo, H.; Liu, Y.; Xia, H.; Chen, W.; Tang, Q. Hydrogen Sulfide Regulates the Colonic Motility by Inhibiting Both L-Type Calcium Channels and BKCa Channels in Smooth Muscle Cells of Rat Colon. PLoS ONE 2015, 10, e0121331. [Google Scholar] [CrossRef] [PubMed]
- Parkes, G.C.; Rayment, N.B.; Hudspith, B.N.; Petrovska, L.; Lomer, M.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Distinct Microbial Populations Exist in the Mucosa-Associated Microbiota of Sub-Groups of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2012, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Crouzet, L.; Gaultier, E.; Del’Homme, C.; Cartier, C.; Delmas, E.; Dapoigny, M.; Fioramonti, J.; Bernalier-Donadille, A. The Hypersensitivity to Colonic Distension of IBS Patients Can Be Transferred to Rats through Their Fecal Microbiota. Neurogastroenterol. Motil. 2013, 25, e272–e282. [Google Scholar] [CrossRef]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Efficacy of an Encapsulated Probiotic Bifidobacterium Infantis 35624 in Women with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and Bifidobacterium in Irritable Bowel Syndrome: Symptom Responses and Relationship to Cytokine Profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Enck, P.; Zimmermann, K.; Menke, G.; Müller-Lissner, S.; Martens, U.; Klosterhalfen, S. A Mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for Treatment of the Irritable Bowel Syndrome—A Randomized Controlled Trial with Primary Care Physicians. Neurogastroenterol. Motil. 2008, 20, 1103–1109. [Google Scholar] [CrossRef]
- Choi, S.C.; Kim, B.J.; Rhee, P.L.; Chang, D.K.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Kim, S.I.; Han, Y.S.; Sim, K.H.; et al. Probiotic Fermented Milk Containing Dietary Fiber Has Additive Effects in IBS with Constipation Compared to Plain Probiotic Fermented Milk. Gut Liver 2011, 5, 22–28. [Google Scholar] [CrossRef][Green Version]
- Clarke, G.; Cryan, J.F.; Dinan, T.G.; Quigley, E.M. Review Article: Probiotics for the Treatment of Irritable Bowel Syndrome—Focus on Lactic Acid Bacteria. Aliment. Pharmacol. Ther. 2012, 35, 403–413. [Google Scholar] [CrossRef]
- O’Sullivan, M.A.; O’Morain, C.A. Bacterial Supplementation in the Irritable Bowel Syndrome. A Randomised Double-Blind Placebo-Controlled Crossover Study. Dig. Liver Dis. 2000, 32, 294–301. [Google Scholar] [CrossRef]
- Gustavo Hermes, R.; Molist, F.; Francisco Pérez, J.; De Segura, A.G.; Ywazaki, M.; Davin, R.; Nofrarías, M.; Korhonen, T.K.; Virkola, R.; Martín-Orúe, S.M. Casein Glycomacropeptide in the Diet May Reduce Escherichia coli Attachment to the Intestinal Mucosa and Increase the Intestinal Lactobacilli of Early Weaned Piglets after an Enterotoxigenic E. coli K88 Challenge. Br. J. Nutr. 2013, 109, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Lu, Z.; Zhang, H.; Zhang, L.; Song, D.; Wang, Y. Effects of Casein Glycomacropeptide Supplementation on Growth Performance, Intestinal Morphology, Intestinal Barrier Permeability and Inflammatory Responses in Escherichia coli K88 Challenged Piglets. Anim. Nutr. 2015, 1, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of Intestinal Infection by Glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.R.; Gibson, G.R.; Formentin, K.; Beer, M.; Greenberg, N.; Rastall, R.A. Caseinoglycomacropeptide Inhibits Adhesion of Pathogenic Escherichia coli Strains to Human Cells in Culture. J. Dairy Sci. 2005, 88, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Brück, W.M.; Redgrave, M.; Tuohy, K.M.; Lönnerdal, B.; Graverholt, G.; Hernell, O.; Gibson, G.R. Effects of Bovine α-Lactalbumin and Casein Glycomacropeptide-Enriched Infant Formulae on Faecal Microbiota in Healthy Term Infants. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 673–679. [Google Scholar] [CrossRef]
- Feeney, S.; Ryan, J.T.; Kilcoyne, M.; Joshi, L.; Hickey, R. Glycomacropeptide Reduces Intestinal Epithelial Cell Barrier Dysfunction and Adhesion of Entero-Hemorrhagic and Entero-Pathogenic Escherichia coli in Vitro. Foods 2017, 6, 93. [Google Scholar] [CrossRef]
- Malinen, E.; Krogius-Kurikka, L.; Lyra, A.; Nikkilä, J.; Jääskeläinen, A.; Rinttilä, T.; Vilpponen-Salmela, T.; von Wright, A.J.; Palva, A. Association of Symptoms with Gastrointestinal Microbiota in Irritable Bowel Syndrome. World J. Gastroenterol. 2010, 16, 4532–4540. [Google Scholar] [CrossRef]
- Inness, V.L.; McCartney, A.L.; Khoo, C.; Gross, K.L.; Gibson, G.R. Molecular Characterisation of the Gut Microflora of Healthy and Inflammatory Bowel Disease Cats Using Fluorescence in Situ Hybridisation with Special Reference to Desulfovibrio Spp. J. Anim. Physiol. Anim. Nutr. 2007, 91, 48–53. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Kollár, P. Analysis of Physiological Parameters of Desulfovibrio Strains from Individuals with Colitis. Open Life Sci. 2019, 13, 481–488. [Google Scholar] [CrossRef]
- Wernlund, P.G.; Hvas, C.L.; Dahlerup, J.F.; Bahl, M.I.; Licht, T.R.; Knudsen, K.E.B.; Agnholt, J.S. Casein Glycomacropeptide Is Well Tolerated in Healthy Adults and Changes Neither High-Sensitive C-Reactive Protein, Gut Microbiota nor Faecal Butyrate: A Restricted Randomised Trial. Br. J. Nutr. 2021, 125, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Ceccarani, C.; Corsello, A.; Zuvadelli, J.; Ottaviano, E.; Dei Cas, M.; Banderali, G.; Zuccotti, G.; Borghi, E.; Verduci, E. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xing, Y.; Liu, H.; Chang, Y.; You, Y.; Dou, Y.; Liu, B.; Wang, Q.; Ma, D.; Chen, L.; et al. Effects of a Formula with ScGOS/LcFOS (9:1) and Glycomacropeptide (GMP) Supplementation on the Gut Microbiota of Very Preterm Infants. Nutrients 2022, 14, 1901. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.E.; Murali, S.; Chaves, I.Z.; Suen, G.; Ney, D.M. Glycomacropeptide Impacts Amylin-Mediated Satiety, Postprandial Markers of Glucose Homeostasis, and the Fecal Microbiome in Obese Postmenopausal Women. J. Nutr. 2023, 153, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide Is a Prebiotic That Reduces Desulfovibrio Bacteria, Increases Cecal Short-Chain Fatty Acids, and Is Anti-Inflammatory in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Muñoz, Y.H.; García, A.; Haro, L.M.; Salinas, E. Novel Mechanisms Underlying the Therapeutic Effect of Glycomacropeptide on Allergy: Change in Gut Microbiota, Upregulation of TGF-β, and Inhibition of Mast Cells. Int. Arch. Allergy Immunol. 2016, 171, 217–226. [Google Scholar] [CrossRef]
- Ntemiri, A.; Ribière, C.; Stanton, C.; Ross, R.P.; O’Connor, E.M.; O’Toole, P.W. Retention of Microbiota Diversity by Lactose-Free Milk in a Mouse Model of Elderly Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhan, B.; Chang, R.; Du, M.; Mao, X. Antidiabetic Effect of Casein Glycomacropeptide Hydrolysates on High-Fat Diet and STZ-Induced Diabetic Mice via Regulating Insulin Signaling in Skeletal Muscle and Modulating Gut Microbiota. Nutrients 2020, 12, 220. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, J.; Jia, Y.; Liu, X.; Yan, Y.; Pang, G. Modulation of Mice Fecal Microbiota by Administration of Casein Glycomacropeptide. Microbiol. Res. 2012, 3, e3. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Tao, S.; Pi, Y.; Han, D.; Ye, H.; Feng, C.; Zhao, J.; Chen, L.; Wang, J. Maternal Supplementation with Combined Galactooligosaccharides and Casein Glycomacropeptides Modulated Microbial Colonization and Intestinal Development of Neonatal Piglets. J. Funct. Foods 2020, 74, 104170. [Google Scholar] [CrossRef]
- Azuma, N.; Yamauchi, K.; Mitsuoka, T. Bifidus Growth-Promoting Activity of a Glycomacropeptide Derived from Human K-Casein. Agric. Biol. Chem. 1984, 48, 2159–2162. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, T.T.; Tang, X.; Han, M.Z.; Leng, X.J.; Mao, X.Y. Developing a Potential Prebiotic of Yogurt: Growth of Bifidobacterium and Yogurt Cultures with Addition of Glycomacropeptide Hydrolysate. Int. J. Food Sci. Technol. 2015, 50, 120–127. [Google Scholar] [CrossRef]
- Ntemiri, A.; Chonchúir, F.N.; O’Callaghan, T.F.; Stanton, C.; Ross, R.P.; O’Toole, P.W. Glycomacropeptide Sustains Microbiota Diversity and Promotes Specific Taxa in an Artificial Colon Model of Elderly Gut Microbiota. J. Agric. Food Chem. 2017, 65, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, N.; O’Callaghan, J.; Buttò, L.F.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Bovine Glycomacropeptide Promotes the Growth of Bifidobacterium longum ssp. infantis and Modulates Its Gene Expression. J. Dairy Sci. 2018, 101, 6730–6741. [Google Scholar] [CrossRef]
- Morozumi, M.; Wada, Y.; Tsuda, M.; Tabata, F.; Ehara, T.; Nakamura, H.; Miyaji, K. Cross-Feeding among Bifidobacteria on Glycomacropeptide. J. Funct. Foods 2023, 103, 105463. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin Dynamics and Enteric Pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Goryca, K.; Karczmarski, J.; Rubel, T.; Wojtowicz, W.; Mlynarz, P.; Marczak, L.; et al. Limited Prolonged Effects of Rifaximin Treatment on Irritable Bowel Syndrome-Related Differences in the Fecal Microbiome and Metabolome. Gut Microbes 2016, 7, 397–413. [Google Scholar] [CrossRef]
- Nagel, R.; Traub, R.J.; Allcock, R.J.N.; Kwan, M.M.S.; Bielefeldt-Ohmann, H. Comparison of Faecal Microbiota in Blastocystis-Positive and Blastocystis-Negative Irritable Bowel Syndrome Patients. Microbiome 2016, 4, 47. [Google Scholar] [CrossRef]
- Chung, C.-S.; Chang, P.-F.; Liao, C.-H.; Lee, T.-H.; Chen, Y.; Lee, Y.-C.; Wu, M.-S.; Wang, H.-P.; Ni, Y.-H. Differences of Microbiota in Small Bowel and Faeces between Irritable Bowel Syndrome Patients and Healthy Subjects. Scand. J. Gastroenterol. 2016, 51, 410–419. [Google Scholar] [CrossRef]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Calvin Coffey, J.; O’Connell, P.R. Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria Can Protect from Enteropathogenic Infection through Production of Acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in Intestinal Barrier Function and Gut Microbiota in High-Fat Diet-Fed Rats Are Dynamic and Region Dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Kim, B.-J.; Koh, J.; Dallas, D.C. Comparison of Solid-Phase Extraction Sorbents for Monitoring the In Vivo Intestinal Survival and Digestion of Kappa-Casein-Derived Caseinomacropeptide. Foods 2023, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Kim, B.J.; Qu, Y.; Dallas, D.C. Mass Spectral Profiling of Caseinomacropeptide Extracted from Feeding Material and Jejunal Fluid Using Three Methods-Ethanol Precipitation, Perchloric Acid Precipitation, and Ultrafiltration. Food Chem. 2023, 398, 133864. [Google Scholar] [CrossRef]
- Koh, J.; Kim, B.J.; Qu, Y.; Huang, H.; Dallas, D.C. Top-Down Glycopeptidomics Reveals Intact Glycomacropeptide Is Digested to a Wide Array of Peptides in Human Jejunum. J. Nutr. 2022, 152, 429–438. [Google Scholar] [CrossRef]
- Chadwick, V.S.; Chen, W.; Shu, D.; Paulus, B.; Bethwaite, P.; Tie, A.; Wilson, I. Activation of the Mucosal Immune System in Irritable Bowel Syndrome. Gastroenterology 2002, 122, 1778–1783. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Hou, X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. Motil. 2016, 22, 181–192. [Google Scholar] [CrossRef]
- Simrén, M.; Öhman, L. Pathogenesis of IBS: Role of Inflammation, Immunity and Neuroimmune Interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Polster, A.; Törnblom, H.; Isaksson, S.; Capronnier, S.; Tessier, A.; Le Nevé, B.; Simrén, M.; Öhman, L. Global Cytokine Profiles and Association with Clinical Characteristics in Patients With Irritable Bowel Syndrome. Am. J. Gastroenterol. 2016, 111, 1165–1176. [Google Scholar] [CrossRef]
- Gonsalkorale, W.M.; Miller, V.; Afzal, A.; Whorwell, P.J. Long Term Benefits of Hypnotherapy for Irritable Bowel Syndrome. Gut 2003, 52, 1623–1629. [Google Scholar] [CrossRef]
- Chang, L. The Role of Stress on Physiologic Responses and Clinical Symptoms in Irritable Bowel Syndrome. Gastroenterology 2011, 140, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Choghakhori, R.; Abbasnezhad, A.; Hasanvand, A.; Amani, R. Inflammatory Cytokines and Oxidative Stress Biomarkers in Irritable Bowel Syndrome: Association with Digestive Symptoms and Quality of Life. Cytokine 2017, 93, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.; Pulido-London, D.; Rodriguez, O.; Morales-Rochlin, N.; Martinez-García, R.; Gutierrez-Ruiz, M.C.; López-Alvarenga, J.C.; Robles-Díaz, G.; Gutiérrez-Reyes, G. Lower Serum IL-10 Is an Independent Predictor of IBS among Volunteers in Mexico. Am. J. Gastroenterol. 2012, 107, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Adeyemo, M.; Karagiannidis, I.; Videlock, E.J.; Bowe, C.; Shih, W.; Presson, A.P.; Yuan, P.Q.; Cortina, G.; Gong, H.; et al. Serum and Colonic Mucosal Immune Markers in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2012, 107, 262–272. [Google Scholar] [CrossRef]
- Hasler, W.L.; Grabauskas, G.; Singh, P.; Owyang, C. Mast Cell Mediation of Visceral Sensation and Permeability in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2022, 34, e14339. [Google Scholar] [CrossRef]
- Zhen, Y.; Chu, C.; Zhou, S.; Qi, M.; Shu, R. Imbalance of Tumor Necrosis Factor-α, Interleukin-8 and Interleukin-10 Production Evokes Barrier Dysfunction, Severe Abdominal Symptoms and Psychological Disorders in Patients with Irritable Bowel Syndrome-Associated Diarrhea. Mol. Med. Rep. 2015, 12, 5239–5245. [Google Scholar] [CrossRef][Green Version]
- Li, F.; Ma, J.; Geng, S.; Wang, J.; Ren, F.; Sheng, X. Comparison of the Different Kinds of Feeding on the Level of Fecal Calprotectin. Early Hum. Dev. 2014, 90, 471–475. [Google Scholar] [CrossRef]
- Melchior, C.; Aziz, M.; Aubry, T.; Gourcerol, G.; Quillard, M.; Zalar, A.; Coëffier, M.; Dechelotte, P.; Leroi, A.-M.; Ducrotté, P. Does Calprotectin Level Identify a Subgroup among Patients Suffering from Irritable Bowel Syndrome? Results of a Prospective Study. United Eur. Gastroenterol. J. 2017, 5, 261–269. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jeong, S.J. Is Fecal Calprotectin Always Normal in Children with Irritable Bowel Syndrome? Intest. Res. 2019, 17, 546–553. [Google Scholar] [CrossRef]
- Chang, M.H.; Chou, J.W.; Chen, S.M.; Tsai, M.C.; Sun, Y.S.; Lin, C.C.; Lin, C.P. Faecal Calprotectin as a Novel Biomarker for Differentiating between Inflammatory Bowel Disease and Irritable Bowel Syndrome. Mol. Med. Rep. 2014, 10, 522–526. [Google Scholar] [CrossRef]
- Waugh, N.; Cummins, E.; Royle, P.; Kandala, N.B.; Shyangdan, D.; Arasaradnam, R.; Clar, C.; Johnston, R. Faecal Calprotectin Testing for Differentiating amongst Inflammatory and Non-Inflammatory Bowel Diseases: Systematic Review and Economic Evaluation. Health Technol. Assess. 2013, 17, xv–xix. [Google Scholar] [CrossRef]
- Thabane, M.; Kottachchi, D.T.; Marshall, J.K. Systematic Review and Meta-Analysis: The Incidence and Prognosis of Post-Infectious Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2007, 26, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dige, A.; Bendix, M.; Wernlund, P.G.; Christensen, L.A.; Dahlerup, J.F.; Agnholt, J. Casein Glycomacropeptide for Active Distal Ulcerative Colitis: A Randomized Pilot Study. Eur. J. Clin. Investig. 2016, 46, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Daddaoua, A.; Puerta, V.; Zarzuelo, A.; Suárez, M.D.; Sánchez De Medina, F.; Martínez-Augustin, O. Bovine Glycomacropeptide Is Anti-Inflammatory in Rats with Hapten-Induced Colitis. J. Nutr. 2005, 135, 1164–1170. [Google Scholar] [CrossRef]
- Requena, P.; Daddaoua, A.; Martínez-Plata, E.; González, M.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine Glycomacropeptide Ameliorates Experimental Rat Ileitis by Mechanisms Involving Downregulation of Interleukin 17. Br. J. Pharmacol. 2008, 154, 825–832. [Google Scholar] [CrossRef]
- Requena, P.; González, R.; López-Posadas, R.; Abadía-Molina, A.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. The Intestinal Antiinflammatory Agent Glycomacropeptide Has Immunomodulatory Actions on Rat Splenocytes. Biochem. Pharmacol. 2010, 79, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- López-Posadas, R.; Requena, P.; González, R.; Suárez, M.D.; Zarzuelo, A.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine Glycomacropeptide Has Intestinal Antiinflammatory Effects in Rats with Dextran Sulfate-Induced Colitis. J. Nutr. 2010, 140, 2014–2019. [Google Scholar] [CrossRef]
- Ortega-González, M.; Capitán-Cañadas, F.; Requena, P.; Ocón, B.; Romero-Calvo, I.; Aranda, C.; Suárez, M.D.; Zarzuelo, A.; Sánchez De Medina, F.; Martínez-Augustin, O. Validation of Bovine Glycomacropeptide as an Intestinal Anti-Inflammatory Nutraceutical in the Lymphocyte-Transfer Model of Colitis. Br. J. Nutr. 2014, 111, 1202–1212. [Google Scholar] [CrossRef]
- Sawin, E.; Aktas, B.; DeWolfe, T.; Stroup, B.; Murali, S.; Steele, J.; Ney, D. Glycomacropeptide Shows Prebiotic and Immune Modulating Properties in Phenylketonuria and Wild Type Mice. FASEB J. 2015, 29, 1010–1012. [Google Scholar] [CrossRef]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-García, D.; Jiménez, M.; Ventura-Juárez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef]
- Cervantes-García, D.; Bahena-Delgado, A.I.; Jiménez, M.; Córdova-Dávalos, L.E.; Palacios, V.R.E.; Sánchez-Alemán, E.; Martínez-Saldaña, M.C.; Salinas, E. Glycomacropeptide Ameliorates Indomethacin-Induced Enteropathy in Rats by Modifying Intestinal Inflammation and Oxidative Stress. Molecules 2020, 25, 2351. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pavón, D.; Cervantes-García, D.; Bermúdez-Humarán, L.G.; Córdova-Dávalos, L.E.; Quintanar-Stephano, A.; Jiménez, M.; Salinas, E. Protective Effect of Glycomacropeptide on Food Allergy with Gastrointestinal Manifestations in a Rat Model through Down-Regulation of Type 2 Immune Response. Nutrients 2020, 12, 2942. [Google Scholar] [CrossRef]
- Mikkelsen, T.L.; Bakman, S.; Sørensen, E.S.; Barkholt, V.; Frøkiær, H. Sialic Acid-Containing Milk Proteins Show Differential Immunomodulatory Activities Independent of Sialic Acid. J. Agric. Food Chem. 2005, 53, 7673–7680. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, D.; Chen, B.; Mao, X. Endotoxin-Binding Peptides Derived from Casein Glycomacropeptide Inhibit Lipopolysaccharide-Stimulated Inflammatory Responses via Blockade of NF-ΚB Activation in Macrophages. Nutrients 2015, 7, 3119–3137. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cheng, X.; Du, M.; Chen, B.; Mao, X. Upregulation of Heme Oxygenase-1 Mediates the Anti-Inflammatory Activity of Casein Glycomacropeptide (GMP) Hydrolysates in LPS-Stimulated Macrophages. Food Funct. 2017, 8, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Foisy-Sauvé, M.; Ahmarani, L.; Delvin, E.; Sané, A.T.; Spahis, S.; Levy, E. Glycomacropeptide Prevents Iron/Ascorbate-Induced Oxidative Stress, Inflammation and Insulin Sensitivity with an Impact on Lipoprotein Production in Intestinal Caco-2/15 Cells. Nutrients 2020, 12, 1175. [Google Scholar] [CrossRef]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial Whey Products Promote Intestinal Barrier Function with Glycomacropeptide Enhanced Activity in Downregulating Bacterial Endotoxin Lipopolysaccharides (LPS)-Induced Inflammation in Vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Li, Z.; Li, W.; Liu, J.; Huang, L.; Wang, Z. Comparative Mass Spectrometry Analysis and Immunomodulatory Effects of Casein Glycomacropeptide O-Glycans in Bovine and Caprine Whey Powder. J. Agric. Food Chem. 2022, 70, 8746–8754. [Google Scholar] [CrossRef]
- Vasilevskaia, L.S.; Stan, E.I.; Chernikov, M.P.; Shlygin, G.K. Inhibiting Action of Glycomacropeptide on Stomach Secretion Induced by Various Humoral Stimulants. Vopr. Pitan. 1977, 4, 21–24. [Google Scholar]
- Stan, E.I.; Chernikov, M.P. Physiological Activity of Kappa-Casein Glycomacropeptide. Vopr. Med. Khim. 1979, 25, 348–352. [Google Scholar]
- Stan, E.I.; Groĭsman, S.D.; Krasil’shchikov, K.B.; Chernikov, M.P. Effect of Kappa-Casein Glycomacropeptide on Gastrointestinal Motility in Dogs. Biull. Eksp. Biol. Med. 1983, 96, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.K.; Bhargava, A.; Buret, A.G. Post-Infectious Irritable Bowel Syndrome: Mechanistic Insights into Chronic Disturbances Following Enteric Infection. World J. Gastroenterol. WJG 2014, 20, 3976. [Google Scholar] [CrossRef] [PubMed]
- Pokkunuri, V.; Pimentel, M.; Morales, W.; Jee, S.-R.; Alpern, J.; Weitsman, S.; Marsh, Z.; Low, K.; Hwang, L.; Khoshini, R.; et al. Role of Cytolethal Distending Toxin in Altered Stool Form and Bowel Phenotypes in a Rat Model of Post-Infectious Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2012, 18, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375. [Google Scholar] [CrossRef]
- Piche, T.; Barbara, G.; Aubert, P.; Bruley des Varannes, S.; Dainese, R.; Nano, J.L.; Cremon, C.; Stanghellini, V.; De Giorgio, R.; Galmiche, J.P.; et al. Impaired Intestinal Barrier Integrity in the Colon of Patients with Irritable Bowel Syndrome: Involvement of Soluble Mediators. Gut 2009, 58, 196–201. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral Mechanisms in Irritable Bowel Syndrome. N. Engl. J. Med. 2013, 368, 578–579. [Google Scholar] [CrossRef]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.R.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G.; Committee, R.F. Intestinal Microbiota in Functional Bowel Disorders: A Rome Foundation Report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Camilleri, M.; Gores, G.J. Therapeutic Targeting of Bile Acids. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G209. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-Based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Heitkemper, M.M.; Chang, L. Do Fluctuations in Ovarian Hormones Affect Gastrointestinal Symptoms in Women with Irritable Bowel Syndrome? Gend. Med. 2009, 6 (Suppl. S2), 152–167. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G.; Quigley, E.M. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal Barrier Dysfunction in Irritable Bowel Syndrome: A Systematic Review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef] [PubMed]
- Guttman, J.A.; Finlay, B.B. Tight Junctions as Targets of Infectious Agents. Biochim. Biophys. Acta 2009, 1788, 832–841. [Google Scholar] [CrossRef] [PubMed]
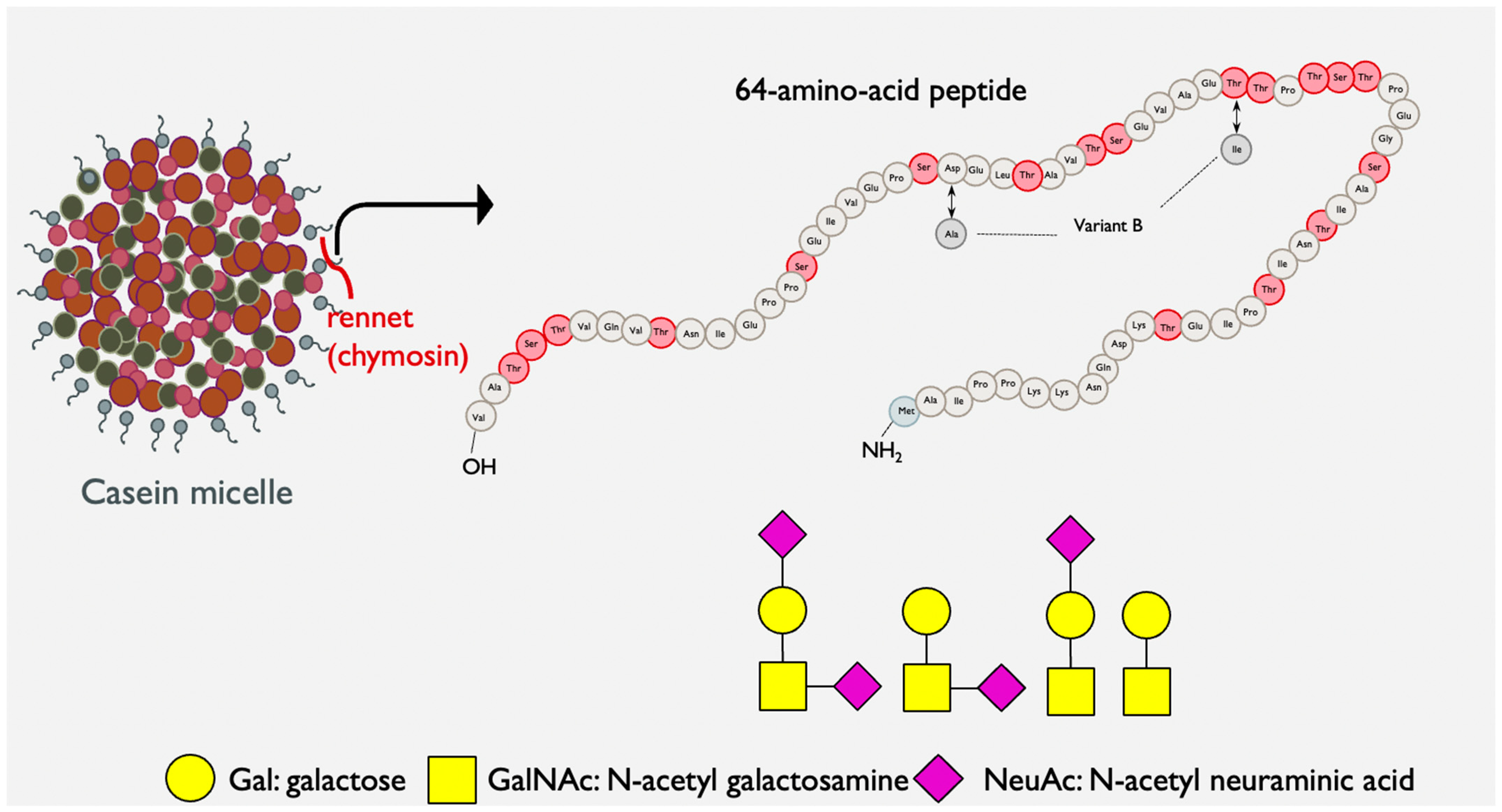
| Study Type | References | GMP Product | Study Model | Effects on Microbiome 1 |
|---|---|---|---|---|
| Clinical Trial | Brück et al., 2006 [86] | α-lactalbumin and GMP-enriched infant formulae | Healthy term infants (n = 85) | (n) gut microbiota |
| Wernlund et al., 2021 [91] | GMP | Healthy adults (n = 25) | (n) gut microbiota | |
| Montanari et al., 2022 [92] | GMP | People with PKU (n = 9) | (+) Agathobacter spp.; (+) Subdoligranulum;(n) for gut microbiota diversity; (n) Short-chain fatty acids (SCFA) | |
| Yu et al., 2022 [93] | scGOS/lcFOS (9:1) and GMP | Very preterm infants (n = 72) | (+) Bifidobacterium | |
| Hansen et al., 2023 [94] | GMP | Obese postmenopausal women (n = 13) | (−) Streptococcus; (−) α diversity | |
| Animal Study | Sawin et al., 2015 [95] | GMP | Wild-type and PKU mice—fed GMP | (−) Proteobacteria; (−) Desulfovibrio; (+) SCFA |
| Jiménez et al., 2016 [96] | GMP | Rats—fed | (+) Lactobacillus; (+) Bifidobacterium; (+) Bacteroides | |
| Ntemiri et al., 2019 [97] | GMP | Mice with humanized fecal microbiota—fed | (n) gut microbiota | |
| Yuan et al., 2020 [98] | GHP | C57BL/6J mice with induced type 2 diabetes—fed | (+) Diversity of gut microbiota; (−) Firmicutes:Bacteroidetes ratio; (+) Bacteroidales_S24-7; (+) Ruminiclostridium; (+) Blautia; (+) Allobaculum; (−) Helicobacteraceae | |
| Chen et al., 2012 [99] | GMP | BALB/c mice—fed | (+) Lactobacillus; (+) Bifidobacteria; (−) Enterobacteriaceae; (−) coliforms; (n) Enterococcus | |
| Gustavo Hermes et al., 2013 [82] | GMP | Piglets—fed | (−) E. coli attachment to intestinal mucosa; (+) Lactobacillus; (−) Enterobacteria; (−) villi with E. coli adherence | |
| Rong et al., 2015 [83] | GMP | Piglets—fed | (−) Intestinal barrier permeability damage caused by E. coli K88 infection; (−) Acute inflammatory response induced by E. coli K88 infection | |
| Wu et al., 2020 [100] | GMP | Sow and piglet model—fed | (+) Prevotella; (+) Fusobacterium; (+) unclassified_f__Prevotellaceae; (+) norank_f__Ruminococcaceae; (+) Christensenellaceae_R-7_group; (+) Ruminococcaceae_UCG-005; (+) Ruminococcaceae_UCG-010 | |
| Cell study | Nakajima et al., 2005 [84] | GMP | Caco-2 cells | (−) Adhesion of Salmonella enteritidis and enterohemorrhagic E. coli O157:H7 to Caco-2 cells |
| Rhoades et al., 2005 [85] | GMP | HT29 cells | (−) Adhesion of pathogenic E. coli (VTEC and EPEC) strains to human HT29 tissue cell cultures; (−) Adhesion of Lactobacillus pentosus (L. pentosus), Lactobacillus acidophilus (L. acidophilus), and L. casei strains; (n) Adhesion of Desulfovibrio desulfuricans or Lactobacillus gasseri (L. gasseri) | |
| Brück et al., 2006 [57] | α-lactalbumin and GMP | Caco-2 cells | (−) Adhesion of Enteropathogenic E. coli (EPEC), Salmonella typhimurium and Shigella flexneri | |
| Feeney et al., 2017 [87] | GMP | HT29 and Caco-2 cells | (−) Epithelial cell barrier dysfunction; (−) pathogen adhesion of Enterohemorrhagic E. coli (EHEC) and Enteropathogenic E. coli (EPEC) | |
| Culture and medium study | Azuma et al., 1984 [101] | GMP | Bacterial culture of B. infantisS12 | (+) B. infantisS12 |
| Brück et al., 2003 [56] | GMP and α-lactalbumin | Bacterial culture | (+) Bifidobacteria; (+) Lactobacilli; (−) Bacteroides; (−) Clostridia; (−) E. coli | |
| Robitaille et al., 2013 [53] | GMP | Bacterial culture | (+) Lactobacillus rhamnosus (L. rhamnosus); (+) Bifidobacterium thermophilum (B. thermophilum) | |
| Tian et al., 2015 [102] | GHP | Yogurt | (+) Bifidobacterium animalis spp. Lactis BB12 (BB-12); (+) Streptococcus thermophilus; (n) Lactobacillus bulgaricus | |
| Ntemiri et al., 2017 [103] | GMP | Artificial colon model | (+) Coprococcus; (+) Clostridium cluster XIVb; (+) Fecal microbiota diversity | |
| O’Riordan et al., 2018 [104] | GMP | Bacterial culture | (+) Bifidobacterium longum ssp. infantis | |
| Morozumi et al., 2023 [105] | GMP | GMP containing medium | (+) Bifidobacterium bifidum; (+) Bifidobacterium breve |
| Study Type | References | GMP Product | Study Model | Effects on Inflammation 1 |
|---|---|---|---|---|
| Clinical Trial | Hvas et al., 2016 [133] | GMP | People with ulcerative colitis (n = 24) | (n) Cytokine levels (−) endoscopic colonic inflammation |
| Wernlund et al., 2021 [91] | GMP | Healthy adults (n = 24) | (n) No significant change | |
| Hansen et al., 2023 [94] | GMP | Obese postmenopausal women (n = 13) | (n) No significant change | |
| Animal Study | Daddaoua et al., 2005 [134] | GMP | Rats with trinitrobenzenesulfonic acid-induced colitis—fed | (−) IL-1 |
| Requena et al., 2008 [135] | GMP | Rats with induced ileitis—fed | (−) IL-1β; (−) TNF-α; (−) IL-17; (n) IFN-γ; (−) IL-2; (−) IL-1Ra | |
| Requena et al., 2010 [136] | GMP | Rat splenocytes and Wistar rats—fed | (+) IL-10; (−) IFN-γ; (−) TNF-α | |
| López-Posadas et al., 2010 [137] | GMP | Rats—fed | (−) IL-1β; (−) IL-17; (−) IL-23; (−) IL-6; (−) TGF-β; (−) IL-10 | |
| Ortega-González et al., 2014 [138] | GMP | C57BL/6 mice—fed | (+) IL-6; (+) IL-10; (+) TNF-α; (+) IFN-γ | |
| Sawin et al., 2015 [139] | GMP | PKU (Pah(enu2)) and wild-type (WT) C57Bl/6 mice—fed | (+) Acetate; (+) propionate; (+) butyrate; (−) IFN-γ; (−) TNF-α; (−) IL-1β; (−) IL-2; (−) IL-10 | |
| Muñoz et al., 2017 [140] | GMP | C57BL/6 wild-type and Rag−/− mice—fed | (−) IL-4; (−) IL-5; (−) IL-13; (+) IL-10 | |
| Cervantes-García et al., 2020 [141] | GMP | Rats—fed | (−) IL-1β | |
| Reyes-Pavón et al., 2020 [142] | GMP | Rats—fed | (−) IL-1β; (−) TNF-α; (−) IL-5; (−) IL-13 | |
| Cell study | Mikkelsen et al., 2005 [143] | GMP | Murine spleen cells and dendritic cells challenged with LPS, Concanavalin-A, and PHA | (−) IL-1β; (−) TNF-α; (−) IL-6 |
| Requena et al., 2010 [136] | GMP | THP-1 cells | (+) IL-8; (+) IL-1β | |
| Cheng et al., 2015 [144] | GHP | Macrophages | (−) TNF-α; (−) IL-1β; (−) IL-6 | |
| Li et al., 2017 [145] | GHP | LPS-stimulated RAW264.7 macrophages | (−) TNF-α; (−) IL-1β; (−) IL-6 | |
| Foisy-Sauvé et al., 2020 [146] | GMP | Caco-2/15 Cells | (−) Oxidative stress; (−) malondialdehyde; (+) superoxide dismutase 2; (+) glutathione peroxidase | |
| Arbizu et al., 2020 [147] | GMP | HT29-MTX and Caco-2 cells | (+) Intestinal barrier function; (−) LPS-induced inflammation; (+) Tight junction proteins | |
| Lu et al., 2022 [148] | GMP | LPS-stimulated RAW264.7 macrophages | (+) IL-1α; (+) TNF-α; (+) IL-10 |
| Study Type | References | GMP Product | Study Model | Effects 1 |
|---|---|---|---|---|
| Animal Study | Vasilevskaia et al., 1977 [149] | GMP | Dogs—ntravenous injection | (−) Gastric juice secretion |
| Stan and Chernikov, 1979 [150] | GMP | Dogs—intravenous injection | (−) Gastric secretion | |
| Stan et al., 1983 [151] | GMP | Dogs—intravenous injection | (−) Food motility of the stomach fundus; (−) Cyclic-repetitive vomiting; (−) Gastric secretion; (−) Gastric motility | |
| Rong et al., 2015 [83] | GMP | Piglets—fed | (+) Protection against E. coli K88-induced barrier permeability damage | |
| Wu et al., 2020 [100] | GOS and GMP | Sow and piglet model—fed | (+) Tight junctions and mucins to enhance intestinal barrier functions | |
| Cell study | Kawasaki et al., 1992 [55] | GMP | CHO-K1 cells | (−) Cholera toxin binding; (−) morphological changes |
| Arbizu et al., 2020 [147] | GMP | HT29-MTX and Caco-2 cells | (+) Intestinal barrier function; (−) LPS-induced inflammation; (+) Tight junction proteins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Park, S.H.; Dallas, D.C. The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome. Nutrients 2023, 15, 3991. https://doi.org/10.3390/nu15183991
Qu Y, Park SH, Dallas DC. The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome. Nutrients. 2023; 15(18):3991. https://doi.org/10.3390/nu15183991
Chicago/Turabian StyleQu, Yunyao, Si Hong Park, and David C. Dallas. 2023. "The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome" Nutrients 15, no. 18: 3991. https://doi.org/10.3390/nu15183991
APA StyleQu, Y., Park, S. H., & Dallas, D. C. (2023). The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome. Nutrients, 15(18), 3991. https://doi.org/10.3390/nu15183991





