Inverse J-Shaped Relationship of Dietary Carbohydrate Intake with Serum Klotho in NHANES 2007–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Dietary Carbohydrate Intake
2.3. Determination of Serum Klotho Levels
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of All Participants
3.2. Association between Dietary Carbohydrate Intake and Serum Klotho Levels
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuro-O, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [PubMed]
- Espuch-Oliver, A.; Vázquez-Lorente, H.; Jurado-Fasoli, L.; de Haro-Muñoz, T.; Díaz-Alberola, I.; López-Velez, M.d.S.; de Haro-Romero, T.; Castillo, M.J.; Amaro-Gahete, F.J. References Values of Soluble α-Klotho Serum Levels Using an Enzyme-Linked Immunosorbent Assay in Healthy Adults Aged 18–85 Years. J. Clin. Med. 2022, 11, 2415. [Google Scholar] [PubMed]
- Li, J.M.; Chen, F.F.; Li, G.H.; Zhu, J.L.; Zhou, Y.; Wei, X.Y.; Zheng, F.; Wang, L.L.; Zhang, W.; Zhong, M.; et al. Soluble Klotho-integrin β1/ERK1/2 pathway ameliorates myocardial fibrosis in diabetic cardiomyopathy. FASEB J. 2021, 35, e21960. [Google Scholar] [PubMed]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [PubMed]
- Yamazaki, Y.; Imura, A.; Urakawa, I.; Shimada, T.; Murakami, J.; Aono, Y.; Hasegawa, H.; Yamashita, T.; Nakatani, K.; Saito, Y.; et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010, 398, 513–518. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, C.Y.; Li, X.H.; Yang, T.T.; Kuang, X.; Du, J.R. Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13239. [Google Scholar]
- Wang, Y.; Sun, Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 2009, 54, 810–817. [Google Scholar]
- Wang, K.; Mao, Y.; Lu, M.; Liu, X.; Sun, Y.; Li, Z.; Li, Y.; Ding, Y.; Zhang, J.; Hong, J.; et al. Association between serum Klotho levels and the prevalence of diabetes among adults in the United States. Front. Endocrinol. 2022, 13, 1005553. [Google Scholar]
- Donate-Correa, J.; Martín-Carro, B.; Cannata-Andía, J.B.; Mora-Fernández, C.; Navarro-González, J.F. Klotho, Oxidative Stress, and Mitochondrial Damage in Kidney Disease. Antioxidants 2023, 12, 239. [Google Scholar]
- Izquierdo, M.C.; Perez-Gomez, M.V.; Sanchez-Niño, M.D.; Sanz, A.B.; Ruiz-Andres, O.; Poveda, J.; Moreno, J.A.; Egido, J.; Ortiz, A. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27 (Suppl. S4), iv6–iv10. [Google Scholar]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; Arias-Tellez, M.J.; Gil, A.; Labayen, I.; Ruiz, J.R. Relationship between dietary factors and S-Klotho plasma levels in young sedentary healthy adults. Mech. Ageing Dev. 2021, 194, 111435. [Google Scholar]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-La-O, A.; Martinez-Tellez, B.; Ruiz, J.R.; Gutiérrez, Á.; Castillo, M.J. Adherence to the Mediterranean diet, dietary factors, and S-Klotho plasma levels in sedentary middle-aged adults. Exp. Gerontol. 2019, 119, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Edmonston, D.; Grabner, A.; Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 2023. [Google Scholar] [CrossRef]
- Kuro-O, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [PubMed]
- Kuro-O, M. Klotho and endocrine fibroblast growth factors: Markers of chronic kidney disease progression and cardiovascular complications? Nephrol. Dial. Transplant. 2019, 34, 15–21. [Google Scholar] [PubMed]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017, 26, 539–546.e5. [Google Scholar] [CrossRef]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Hillesund, E.R.; Øverby, N.C.; Vik, F.N.; Medin, A.C. Individual nutrients and serum klotho levels in adults aged 40–79 years. Food Sci. Nutr. 2023, 11, 3279–3286. [Google Scholar] [CrossRef]
- Maekawa, R.; Seino, Y.; Ogata, H.; Murase, M.; Iida, A.; Hosokawa, K.; Joo, E.; Harada, N.; Tsunekawa, S.; Hamada, Y.; et al. Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice. J. Nutr. Biochem. 2017, 49, 71–79. [Google Scholar]
- De-La-O, A.; Jurado-Fasoli, L.; Gracia-Marco, L.; Henriksson, P.; Castillo, M.J.; Amaro-Gahete, F.J. Association of Energy and Macronutrients Intake with S-Klotho Plasma Levels in Middle-Aged Sedentary Adults: A Cross-Sectional Study. J. Nutr. Health Aging 2022, 26, 360–366. [Google Scholar] [CrossRef]
- Li, L.; Shan, Z.; Wan, Z.; Li, R.; Geng, T.; Lu, Q.; Zhu, K.; Qiu, Z.; Zhang, X.; Liu, Y.; et al. Associations of lower-carbohydrate and lower-fat diets with mortality among people with prediabetes. Am. J. Clin. Nutr. 2022, 116, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Fulgoni, V.L.; Agarwal, S.; Pasiakos, S.M.; Berryman, C.E. Protein intake is more stable than carbohydrate or fat intake across various US demographic groups and international populations. Am. J. Clin. Nutr. 2020, 112, 180–186. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Choi, J.-Y.; Lee, J.; Lee, H.-J.; Min, J.-Y.; Min, K.-B. Association of α-klotho and lead and cadmium: A cross-sectional study. Sci. Total Environ. 2022, 843, 156938. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Li, J.; Deng, L.; Geng, J.; Jin, K.; Zheng, X.; Qiu, S.; Dong, B. Association between Dietary Inflammatory Index and serum Klotho concentration among adults in the United States. BMC Geriatr. 2022, 22, 528. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Ning, Z.; Xiao, M.; Li, L.; Liu, M.; Zhang, Y. Dietary inflammatory potential and biological aging among US adults: A population-based study. Aging Clin. Exp. Res. 2023, 35, 1273–1281. [Google Scholar] [CrossRef]
- Liu, S.; Wu, M.; Wang, Y.; Xiang, L.; Luo, G.; Lin, Q.; Xiao, L. The Association between Dietary Fiber Intake and Serum Klotho Levels in Americans: A Cross-Sectional Study from the National Health and Nutrition Examination Survey. Nutrients 2023, 15, 3147. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Hu, S.; Qi, C.; Yin, J.; Xu, S.; Hou, F.F.; Li, A. Serum Anti-Aging Protein α-Klotho Mediates the Association between Diet Quality and Kidney Function. Nutrients 2023, 15, 2744. [Google Scholar] [CrossRef]
- Wu, M.; Shu, Y.; Wang, L.; Song, L.; Chen, S.; Liu, Y.; Bi, J.; Li, D.; Yang, Y.; Hu, Y.; et al. Metabolic syndrome severity score and the progression of CKD. Eur. J. Clin. Investig. 2022, 52, e13646. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Huang, S.; Chen, Y.; Fang, Q.; Cao, Y. Sex differences in the association between serum α-Klotho and depression in middle-aged and elderly individuals: A cross-sectional study from NHANES 2007–2016. J. Affect. Disord. 2023, 337, 186–194. [Google Scholar] [CrossRef]
- Landry, T.; Li, P.; Shookster, D.; Jiang, Z.; Li, H.; Laing, B.T.; Bunner, W.; Langton, T.; Tong, Q.; Huang, H. Centrally circulating α-klotho inversely correlates with human obesity and modulates arcuate cell populations in mice. Mol. Metab. 2021, 44, 101136. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-E.; Chen, Y.-J.; Chen, W.-L. Adherence to Mediterranean Diet and Soluble Klotho Level: The Value of Food Synergy in Aging. Nutrients 2022, 14, 3910. [Google Scholar] [CrossRef] [PubMed]
- Jaturakan, O.; Buranakarl, C.; Dissayabutra, T.; Chaiyabutr, N.; Kijtawornrat, A.; Rungsipipat, A. Changes of Klotho protein and Klotho mRNA expression in a hydroxy-L-proline induced hyperoxaluric rat model. J. Veter. Med. Sci. 2017, 79, 1861–1869. [Google Scholar] [CrossRef][Green Version]
- Razzaque, M.S. FGF23, klotho and vitamin D interactions: What have we learned from in vivo mouse genetics studies? Adv. Exp. Med. Biol. 2012, 728, 84–91. [Google Scholar] [PubMed]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodríguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; de Oca, A.M.; Vergara, N.; Steppan, S.; et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef]
- Sievenpiper, J.L. Low-carbohydrate diets and cardiometabolic health: The importance of carbohydrate quality over quantity. Nutr. Rev. 2020, 78 (Suppl. S1), 69–77. [Google Scholar] [CrossRef]
- He, C.; Wu, Q.; Hayashi, N.; Nakano, F.; Nakatsukasa, E.; Tsuduki, T. Carbohydrate-restricted diet alters the gut microbiota, promotes senescence and shortens the life span in senescence-accelerated prone mice. J. Nutr. Biochem. 2020, 78, 108326. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhou, J.; Li, T.; Wu, J.; Xie, S.H.; Liu, H.-F.; Liu, Z.; Park, T.S.; Wang, Y.; Liu, W.J. Sulodexide Protects Renal Tubular Epithelial Cells from Oxidative Stress-Induced Injury via Upregulating Klotho Expression at an Early Stage of Diabetic Kidney Disease. J. Diabetes Res. 2017, 2017, 4989847. [Google Scholar] [CrossRef]
- Kan, W.-C.; Hwang, J.-Y.; Chuang, L.-Y.; Guh, J.-Y.; Ye, Y.-L.; Yang, Y.-L.; Huang, J.-S. Effect of osthole on advanced glycation end products-induced renal tubular hypertrophy and role of klotho in its mechanism of action. Phytomedicine 2019, 53, 205–212. [Google Scholar] [CrossRef]
- Paniagua, J.; de la Sacristana, A.G.; Romero, I.; Vidal-Puig, A.; Latre, J.; Sanchez, E.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007, 30, 1717–1723. [Google Scholar] [CrossRef]
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Dugan, B.; Conway, J.; Duggal, N.A. Inflammaging as a target for healthy ageing. Age Ageing 2023, 52, afac328. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martínez-Reig, M.; Mas-Bargues, C.; Inglés, M. Oxidative stress and exceptional human longevity: Systematic review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar]
- Hsu, S.-C.; Huang, S.-M.; Lin, S.-H.; Ka, S.-M.; Chen, A.; Shih, M.-F.; Hsu, Y.-J. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem. J. 2014, 464, 221–229. [Google Scholar] [CrossRef]
- Dote-Montero, M.; Amaro-Gahete, F.J.; De-La-O, A.; Jurado-Fasoli, L.; Gutierrez, A.; Castillo, M.J. Study of the association of DHEAS, testosterone and cortisol with S-Klotho plasma levels in healthy sedentary middle-aged adults. Exp. Gerontol. 2019, 121, 55–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, S.; Huang, X.; Jin, K.; Zhou, X.; Lin, T.; Zou, X.; Yang, Q.; Yang, L.; Wei, Q. Association between testosterone and serum soluble α-klotho in U.S. males: A cross-sectional study. BMC Geriatr. 2022, 22, 570. [Google Scholar] [CrossRef]
- Fountoulakis, N.; Maltese, G.; Gnudi, L.; Karalliedde, J. Reduced Levels of Anti-Ageing Hormone Klotho Predict Renal Function Decline in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2026–2032. [Google Scholar] [CrossRef]
- Gong, Z.; Banchs, P.A.P.; Liu, Y.; Fu, H.; Arena, V.C.; Forno, E.; Libman, I.; Ho, J.; Muzumdar, R. Serum α-KL, a potential early marker of diabetes complications in youth with T1D, is regulated by miRNA 192. Front. Endocrinol. 2022, 13, 937093. [Google Scholar] [CrossRef]
- Zubkiewicz-Kucharska, A.; Wikiera, B.; Noczyńska, A. Soluble Klotho Is Decreased in Children With Type 1 Diabetes and Correlated With Metabolic Control. Front. Endocrinol. 2021, 12, 709564. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.; Wahl, D.; Cogger, V.C.; Willcox, B.J.; Willcox, D.C.; Raubenheimer, D.; Simpson, S.J. New Horizons: Dietary protein, ageing and the Okinawan ratio. Age Ageing 2016, 45, 443–447. [Google Scholar] [CrossRef]
- Willcox, B.J.; Willcox, D.C.; Todoriki, H.; Fujiyoshi, A.; Yano, K.; He, Q.; Curb, J.D.; Suzuki, M. Caloric restriction, the traditional Okinawan diet, and healthy aging: The diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann. N. Y. Acad. Sci. 2007, 1114, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Solon-Biet, S.M.; Wang, Q.-P.; Wali, J.A.; Pulpitel, T.; Clark, X.; Raubenheimer, D.; Senior, A.M.; Sinclair, D.A.; Cooney, G.J.; et al. Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Rep. 2018, 25, 2234–2243.e6. [Google Scholar] [CrossRef]
- Shafie, A.; Rahimi, A.M.; Ahmadi, I.; Nabavizadeh, F.; Ranjbaran, M.; Ashabi, G. High-protein and low-calorie diets improved the anti-aging Klotho protein in the rats’ brain: The toxic role of high-fat diet. Nutr. Metab. 2020, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.; Pineda, C.; Lopez, I.; Muñoz-Castañeda, J.; Rodriguez, M.; Aguilera-Tejero, E.; Raya, A.I. Phosphorus restriction does not prevent the increase in fibroblast growth factor 23 elicited by high fat diet. PLoS ONE 2018, 13, e0198481. [Google Scholar] [CrossRef]
- Gu, L.Y.; Tang, H.T.; Xu, Z.X. Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway. J. Ethnopharmacol. 2021, 281, 113548. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All | Quartiles of Carbohydrate Intake, % of kcal | ||||
|---|---|---|---|---|---|---|
| Q1 (<41.46) | Q2 (41.46 to 48.92) | Q3 (48.92 to 56.20) | Q4 (≥56.20) | p Value | ||
| N | 10,669 | 2667 | 2667 | 2667 | 2668 | |
| Age, years | 57.90 ± 10.83 | 57.54 ± 10.52 | 57.48 ± 10.69 | 58.21 ± 10.99 | 58.38 ± 11.10 | 0.002 |
| Sex, n (%) | <0.001 | |||||
| male | 5161 (48.4) | 1445 (54.2) | 1357 (50.9) | 1227 (46.0) | 1132 (42.4) | |
| female | 5508 (51.6) | 1222 (45.8) | 1310 (49.1) | 1440 (54.0) | 1536 (57.6) | |
| BMI, kg/m2 | 0.013 | |||||
| <30 kg/m2 | 6164 (57.8) | 1499 (56.2) | 1504 (56.4) | 1556 (58.3) | 1605 (60.2) | |
| ≥30 kg/m2 | 4505 (42.2) | 1168 (43.8) | 1163 (43.6) | 1111 (41.7) | 1063 (39.8) | |
| Race, n (%) | <0.001 | |||||
| Non-Hispanic White | 4947 (46.4) | 1407 (52.8) | 1301 (48.7) | 1179 (44.2) | 1060 (39.7) | |
| Non-Hispanic Black | 2076 (19.4) | 542 (20.3) | 511 (19.2) | 525 (19.7) | 498 (18.7) | |
| Other Hispanic | 1141 (10.7) | 211 (7.9) | 263 (9.9) | 326 (12.2) | 341 (12.8) | |
| Mexican American or Other | 2505 (23.5) | 507 (19.0) | 592 (22.2) | 637 (23.9) | 769 (28.8) | |
| Education, n (%) | <0.001 | |||||
| <High school | 2752 (25.8) | 575 (21.6) | 632 (23.7) | 698 (26.2) | 847 (31.7) | |
| High school | 2379 (22.3) | 600 (22.5) | 617 (23.1) | 607 (22.8) | 555 (20.8) | |
| College or above | 5538 (51.9) | 1492 (55.9) | 1418 (53.2) | 1362 (51.0) | 1266 (47.5) | |
| PIR | 2.67 ± 1.65 | 2.90 ± 1.67 | 2.79 ± 1.65 | 2.59 ± 1.63 | 2.40 ± 1.61 | <0.001 |
| Serum cotinine, ng/mL | 0.03 (0.01, 1.49) | 0.04 (0.01, 46.25) | 0.03 (0.01, 1.31) | 0.03 (0.01, 0.56) | 0.03 (0.01, 0.44) | <0.001 |
| Alcohol drinking, n (%) | <0.001 | |||||
| More than 12 drinks/year | 3041 (28.5) | 470 (17.6) | 704 (26.4) | 840 (31.5) | 1027 (38.5) | |
| Less than 12 drinks/year | 7628 (71.5) | 2197 (82.4) | 1963 (73.6) | 1827 (68.5) | 1641 (61.5) | |
| Diabetes, n (%) | <0.001 | |||||
| No | 8110 (76.0) | 1961 (73.5) | 2010 (75.4) | 2046 (76.7) | 2093 (78.4) | |
| Yes | 2559 (24.0) | 706 (26.5) | 657 (24.6) | 621 (23.3) | 575 (21.6) | |
| Hypertension, n (%) | 0.053 | |||||
| No | 4855 (45.5) | 1158 (43.4) | 1209 (45.3) | 1235 (46.3) | 1253 (47.0) | |
| Yes | 5814 (54.5) | 1509 (56.6) | 1458 (54.7) | 1432 (53.7) | 1415 (53.0) | |
| eGFR, mL/min/1.73 m2 | 83.95 ± 19.61 | 83.30 ± 19.58 | 83.89 ± 19.24 | 84.09 ± 19.44 | 84.53 ± 20.19 | 0.148 |
| Dietary intake | ||||||
| Carbohydrate, g/day | 236.21 ± 96.82 | 178.72 ± 72.19 | 233.52 ± 85.10 | 255.14 ± 92.93 | 277.43 ± 105.11 | <0.001 |
| Energy, kcal/day | 1961.27 ± 732.44 | 2065.11 ± 756.50 | 2059.55 ± 746.67 | 1945.99 ± 706.36 | 1774.51 ± 679.81 | <0.001 |
| Carbohydrate intake, % of kcal | 48.83 ± 11.24 | 34.53 ± 5.73 | 45.38 ± 2.14 | 52.47 ± 2.09 | 62.94 ± 5.93 | <0.001 |
| Fat intake, % of kcal | 33.77 ± 9.20 | 41.11 ± 9.58 | 36.55 ± 6.50 | 32.46 ± 5.40 | 24.96 ± 5.91 | <0.001 |
| Protein intake, % of kcal | 15.97 ± 5.00 | 18.55 ± 5.92 | 16.47 ± 4.45 | 15.28 ± 4.11 | 13.57 ± 3.89 | <0.001 |
| Fiber, g/day | 16.66 ± 9.78 | 13.85 ± 8.21 | 16.52 ± 8.88 | 17.72 ± 9.84 | 18.54 ± 11.28 | <0.001 |
| Serum Klotho, pg/mL | 801.30 (654.50, 992.00) | 769.70 (631.35, 956.55) | 799.30 (657.80, 991.40) | 820.70 (666.95, 1014.25) | 814.55 (656.85, 1007.32) | <0.001 |
| Carbohydrate Intake, % of kcal | N | Crude Model | Adjusted Model * | ||
|---|---|---|---|---|---|
| Percent Changes (%) and 95% CI | p Value | Percent Changes (%) and 95% CI | p Value | ||
| Per 10% increases | 2.17 (1.37, 2.98) | <0.001 | 1.59 (0.77, 2.42) | <0.001 | |
| Q1 (<41.46) | 2667 | −6.63 (−8.66, −4.55) | <0.001 | −5.37 (−7.43, −3.26) | <0.001 |
| Q2 (41.46 to 48.92) | 2667 | −3.33 (−5.15, −1.47) | <0.001 | −2.70 (−4.51, −0.86) | 0.006 |
| Q3 (48.92 to 56.20) | 2667 | Ref | Ref | Ref | Ref |
| Q4 (≥56.20) | 2668 | −2.33 (−4.46, −0.16) | 0.039 | −2.76 (−4.86, −0.62) | 0.014 |
| Carbohydrate Intake, % of kcal | Crude Model | Adjusted Model * | ||
|---|---|---|---|---|
| Percent Changes (%) and 95% CI | p Value | Percent Changes (%) and 95% CI | p Value | |
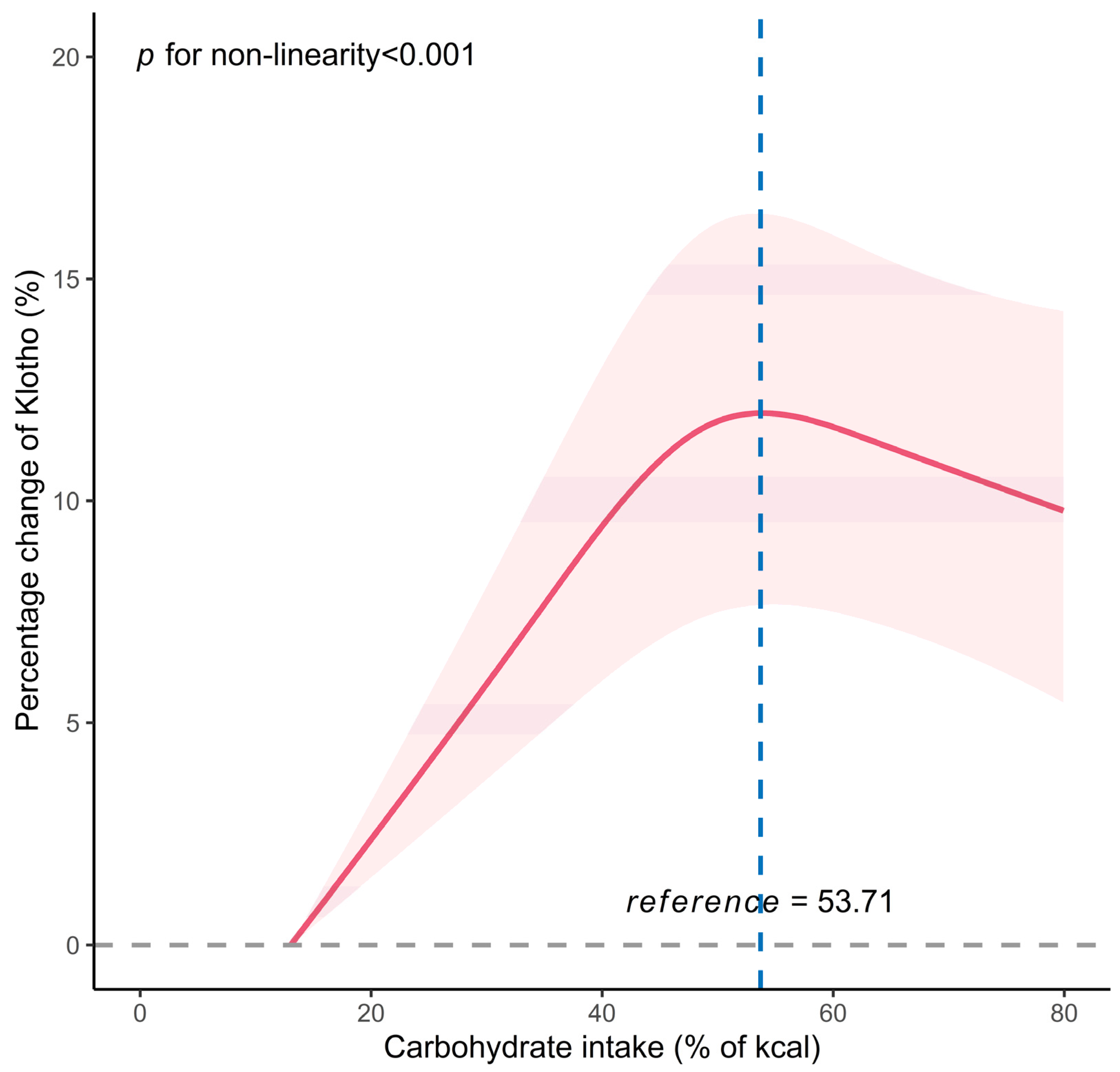
| Fitting by two-piecewise linear model | ||||
| <53.71 | 3.67 (2.64, 4.71) | <0.001 | 3.00 (1.92, 4.09) | <0.001 |
| ≥53.71 | −1.34 (−3.19, 0.55) | 0.167 | −1.70 (−3.53, 0.16) | 0.078 |
| Participants | Carbohydrate Intake, % of kcal | Percent Changes (%) and 95% CI | p Value | * p for Interaction |
|---|---|---|---|---|
| Age, years | 0.517 | |||
| <60 | Per 10% increases | 2.07 (0.99, 3.17) | <0.001 | |
| Q1 (<41.46) | −7.21 (−9.81, −4.54) | <0.001 | ||
| Q2 (41.46 to 48.92) | −3.49(−5.97, −0.94) | 0.01 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −3.53 (−6.51, −0.46) | 0.028 | ||
| ≥60 | Per 10% increases | 0.92 (−0.47, 2.33) | 0.198 | |
| Q1 (<41.46) | −2.47 (−6.01, 1.19) | 0.189 | ||
| Q2 (41.46 to 48.92) | −1.84 (−5.22, 1.66) | 0.302 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −1.38 (−5.22, 2.60) | 0.493 | ||
| Sex | 0.619 | |||
| male | Per 10% increases | 1.86 (0.79, 2.94) | 0.001 | |
| Q1 (<41.46) | −6.65 (−9.60, −3.60) | <0.001 | ||
| Q2 (41.46 to 48.92) | −5.09 (−7.57, −2.54) | <0.001 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −3.56 (−7.45, 0.48) | 0.089 | ||
| female | Per 10% increases | 1.49 (0.46, 2.53) | 0.006 | |
| Q1 (<41.46) | −4.68(−7.72, −1.54) | 0.005 | ||
| Q2 (41.46 to 48.92) | −0.74 (−3.77, 2.39) | 0.642 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −2.20 (−5.39, 1.10) | 0.194 | ||
| BMI, kg/m2 | 0.079 | |||
| <30 | Per 10% increases | 2.00 (0.84, 3.17) | 0.001 | |
| Q1 (<41.46) | −7.64 (−10.45, −4.75) | <0.001 | ||
| Q2 (41.46 to 48.92) | −4.01 (−6.52, −1.43) | 0.004 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −3.90 (−6.66, −1.05) | 0.01 | ||
| ≥30 | Per 10% increases | 1.04 (−0.02, 2.11) | 0.059 | |
| Q1 (<41.46) | −2.01 (−5.62, 1.74) | 0.295 | ||
| Q2 (41.46 to 48.92) | −0.54 (−3.96, 3.00) | 0.762 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −0.89 (−4.39, 2.73) | 0.625 | ||
| Diabetes | 0.336 | |||
| No | Per 10% increases | 1.64 (0.72, 2.58) | <0.001 | |
| Q1 (<41.46) | −6.06 (−8.18, −3.89) | <0.001 | ||
| Q2 (41.46 to 48.92) | −2.80 (−4.88, −0.66) | 0.013 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −2.91 (−5.33, −0.43) | 0.025 | ||
| Yes | Per 10% increases | 1.26 (−0.45, 3.01) | 0.154 | |
| Q1 (<41.46) | −2.42 (−7.17, 2.57) | 0.339 | ||
| Q2 (41.46 to 48.92) | −1.99 (−6.53, 2.76) | 0.408 | ||
| Q3 (48.92 to 56.20) | Ref | Ref | ||
| Q4 (≥56.20) | −2.39 (−6.85, 2.29) | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, L.; Wu, M.; Wang, Y.; Liu, S.; Lin, Q.; Luo, G.; Xiao, L. Inverse J-Shaped Relationship of Dietary Carbohydrate Intake with Serum Klotho in NHANES 2007–2016. Nutrients 2023, 15, 3956. https://doi.org/10.3390/nu15183956
Xiang L, Wu M, Wang Y, Liu S, Lin Q, Luo G, Xiao L. Inverse J-Shaped Relationship of Dietary Carbohydrate Intake with Serum Klotho in NHANES 2007–2016. Nutrients. 2023; 15(18):3956. https://doi.org/10.3390/nu15183956
Chicago/Turabian StyleXiang, Lu, Mingyang Wu, Yan Wang, Si Liu, Qian Lin, Gang Luo, and Lin Xiao. 2023. "Inverse J-Shaped Relationship of Dietary Carbohydrate Intake with Serum Klotho in NHANES 2007–2016" Nutrients 15, no. 18: 3956. https://doi.org/10.3390/nu15183956
APA StyleXiang, L., Wu, M., Wang, Y., Liu, S., Lin, Q., Luo, G., & Xiao, L. (2023). Inverse J-Shaped Relationship of Dietary Carbohydrate Intake with Serum Klotho in NHANES 2007–2016. Nutrients, 15(18), 3956. https://doi.org/10.3390/nu15183956







