The Effects of Flavonoids on Skeletal Muscle Mass, Muscle Function, and Physical Performance in Individuals with Sarcopenia: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
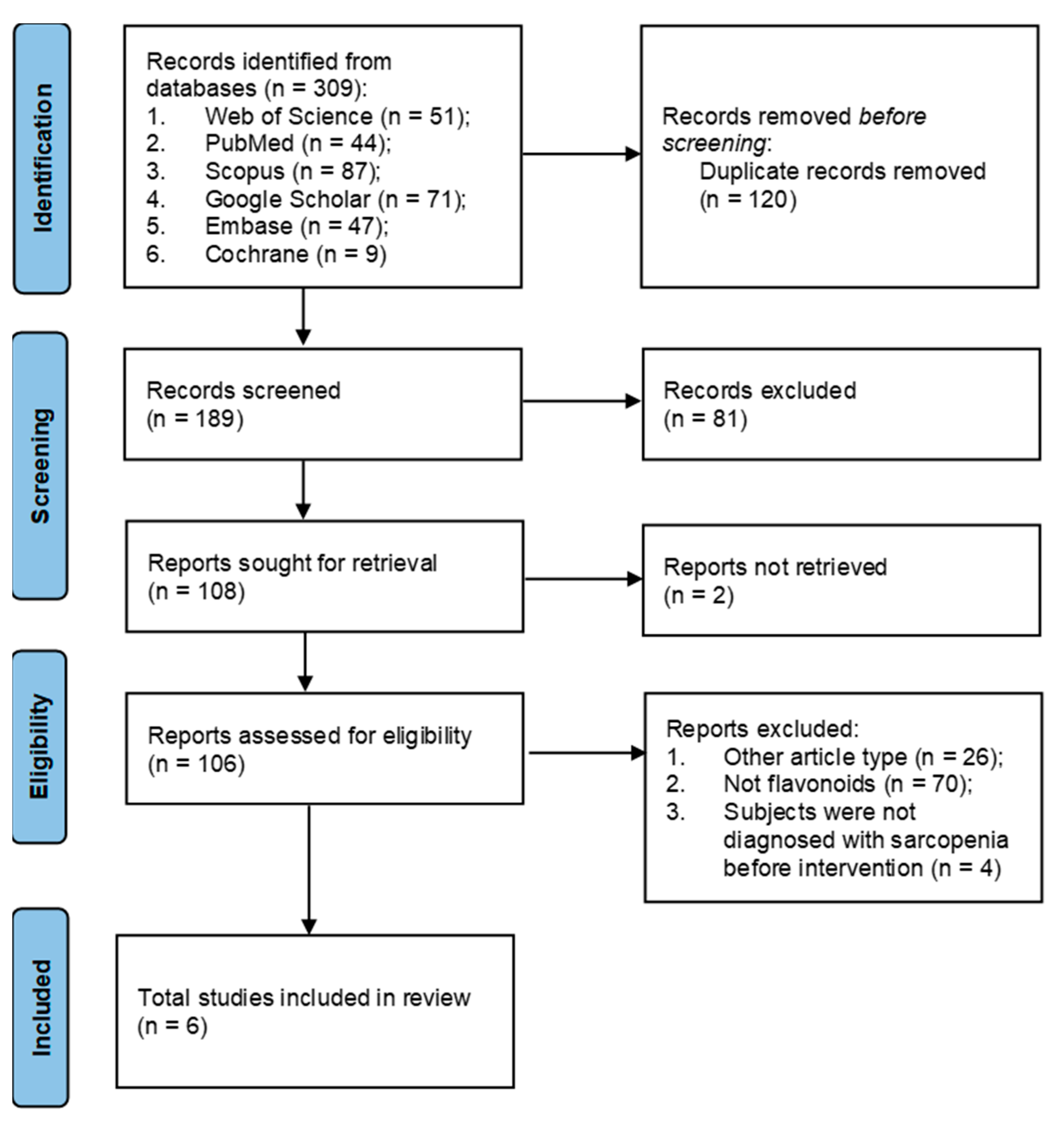
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment for Clinical Studies
3. Results
3.1. Study Characteristics
3.2. Study Outcomes
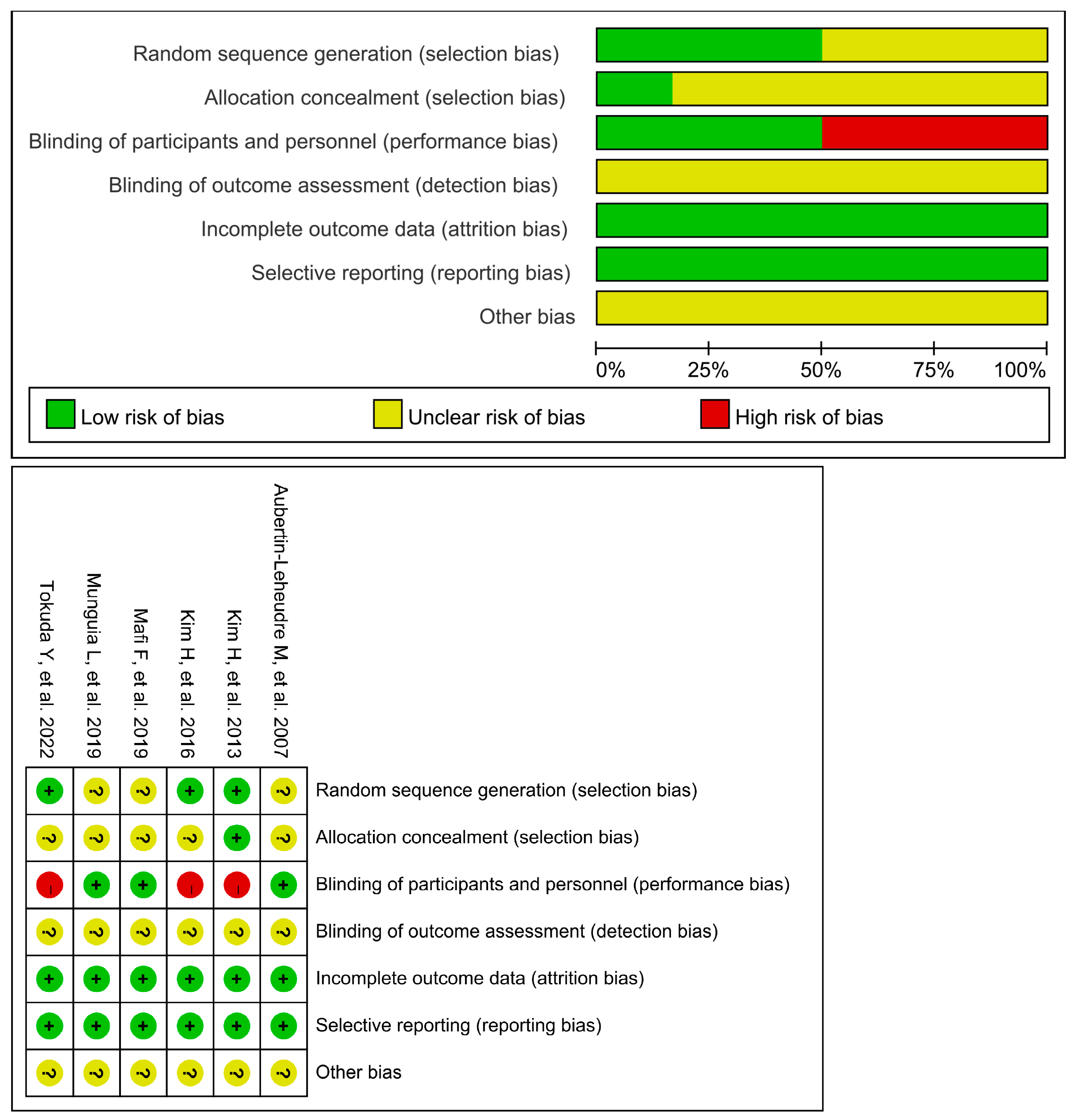
3.3. Quality Assessment
3.4. Flavonoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Database | The Detail of the Strategy of the Electronic Searching |
|---|---|
| Pubmed | (“sarcopenia”[Title/Abstract]) AND ((“2-Phenyl-Chromenes”[Title/Abstract]) OR (“2 Phenyl Chromenes”[Title/Abstract]) OR (“2-Phenyl-Benzopyran”[Title/Abstract]) OR (“2 Phenyl Benzopyran”[Title/Abstract]) OR (“2-Phenyl-Benzopyrans”[Title/Abstract]) OR (“2 Phenyl Benzopyrans”[Title/Abstract]) OR (“2-Phenyl-Chromene”[Title/Abstract]) OR (“2 Phenyl Chromene”[Title/Abstract]) OR (“Flavonoid”[Title/Abstract]) OR (“Bioflavonoids”[Title/Abstract]) OR (“Bioflavonoid”[Title/Abstract]) OR (“Flavonoids”[Title/Abstract]) OR (“Anthocyanins”[Title/Abstract]) OR (“Benzoflavones”[Title/Abstract]) OR (“beta-Naphthoflavone”[Title/Abstract]) OR (“Biflavonoids”[Title/Abstract]) OR (“Catechin”[Title/Abstract]) OR (“Chalcones”[Title/Abstract]) OR (“Flavanones”[Title/Abstract]) OR (“Hesperidin”[Title/Abstract]) OR (“Flavones”[Title/Abstract]) OR (“Apigenin”[Title/Abstract]) OR (“Diosmin”[Title/Abstract]) OR (“Flavoxate”[Title/Abstract]) OR (“Luteolin”[Title/Abstract]) OR (“Flavonolignans”[Title/Abstract]) OR (“Silymarin”[Title/Abstract]) OR (“Flavonols”[Title/Abstract]) OR (“Kaempferols”[Title/Abstract]) OR (“Quercetin”[Title/Abstract]) OR (“Rutin”[Title/Abstract]) OR (“Isoflavones”[Title/Abstract]) OR (“Coumestrol”[Title/Abstract]) OR (“Genistein”[Title/Abstract]) OR (“Pterocarpans”[Title/Abstract]) OR (“Rotenone”[Title/Abstract]) OR (“Phloretin”[Title/Abstract]) OR (“Polyphloretin Phosphate”[Title/Abstract]) OR (“Proanthocyanidins”[Title/Abstract])) |
| Scopus | TITLE-ABS-KEY ( “sarcopenia” AND ( “2-Phenyl-Chromenes” OR “2 Phenyl Chromenes” OR “2-Phenyl-Benzopyran” OR “2 Phenyl Benzopyran” OR “2-Phenyl-Benzopyrans” OR “2 Phenyl Benzopyrans” OR “2-Phenyl-Chromene” OR “2 PhenylChromene” OR “Flavonoid” OR “Bioflavonoids” OR “Bioflavonoid” OR “Flavonoids” OR “Anthocyanins” OR “Benzoflavones” OR “beta-Naphthoflavone” OR “Biflavonoids” OR “Catechin” OR “Chalcones” OR “Flavanones” OR “Hesperidin” OR “Flavones” OR “Apigenin” OR “Diosmin” OR “Flavoxate” OR “Luteolin” OR “Flavonolignans” OR “Silymarin” OR “Flavonols” OR “Kaempferols” OR “Quercetin” OR “Rutin” OR “Isoflavones” OR “Coumestrol” OR “Genistein” OR “Pterocarpans” OR “Rotenone” OR “Phloretin” OR “Polyphloretin Phosphate” OR “Proanthocyanidins” OR “Silybin” OR “Hydroxyethylrutoside”)) |
| WOS | TS=(sarcopenic AND (2-Phenyl-Chromenes OR 2 Phenyl Chromenes OR 2-Phenyl-Benzopyran OR 2 Phenyl Benzopyran OR 2-Phenyl-Benzopyrans OR 2 Phenyl Benzopyrans OR 2-Phenyl-Chromene OR 2 Phenyl Chromene OR Flavonoid OR Bioflavonoids OR Bioflavonoid OR Flavonoids OR Anthocyanins OR Benzoflavones OR beta-Naphthoflavone OR Biflavonoids OR Catechin OR Chalcones OR Flavanones OR Hesperidin OR Flavones OR Apigenin OR Diosmin OR Flavoxate OR Luteolin OR Flavonolignans OR Silymarin OR Flavonols OR Kaempferols OR Quercetin OR Rutin OR Isoflavones OR Coumestrol OR Genistein OR Pterocarpans OR Rotenone OR Phloretin OR Polyphloretin Phosphate OR Proanthocyanidins OR Silybin OR Hydroxyethylrutoside)) |
| Embase | sarcopenia:ti,ab,kw AND (‘2-phenyl-chromenes’:ti,ab,kw OR ‘2 phenyl chromenes’:ti,ab,kw OR ‘2-phenyl-benzopyran’:ti,ab,kw OR ‘2 phenyl benzopyran’:ti,ab,kw OR ‘2-phenyl-benzopyrans’:ti,ab,kw OR ‘2 phenyl benzopyrans’:ti,ab,kw OR ‘2-phenyl-chromene’:ti,ab,kw OR ‘2 phenyl chromene’:ti,ab,kw OR ‘flavonoid’:ti,ab,kw OR ‘bioflavonoids’:ti,ab,kw OR ‘bioflavonoid’:ti,ab,kw OR ‘flavonoids’:ti,ab,kw OR ‘anthocyanins’:ti,ab,kw OR ‘benzoflavones’:ti,ab,kw OR ‘beta-naphthoflavone’:ti,ab,kw OR ‘biflavonoids’:ti,ab,kw OR ‘catechin’:ti,ab,kw OR ‘chalcones’:ti,ab,kw OR ‘flavanones’:ti,ab,kw OR ‘hesperidin’:ti,ab,kw OR ‘flavones’:ti,ab,kw OR ‘apigenin’:ti,ab,kw OR ‘diosmin’:ti,ab,kw OR ‘flavoxate’:ti,ab,kw OR ‘luteolin’:ti,ab,kw OR ‘flavonolignans’:ti,ab,kw OR ‘silymarin’:ti,ab,kw OR ‘flavonols’:ti,ab,kw OR ‘kaempferols’:ti,ab,kw OR ‘quercetin’:ti,ab,kw OR ‘rutin’:ti,ab,kw OR ‘isoflavones’:ti,ab,kw OR ‘coumestrol’:ti,ab,kw OR ‘genistein’:ti,ab,kw OR ‘pterocarpans’:ti,ab,kw OR ‘rotenone’:ti,ab,kw OR ‘phloretin’:ti,ab,kw OR ‘polyphloretin phosphate’:ti,ab,kw OR ‘proanthocyanidins’:ti,ab,kw) |
| Cochrane | ((“Sarcopenia”) AND (“Flavonoid” OR “Bioflavonoids” OR “Bioflavonoid” OR “Flavonoids” OR “Anthocyanins” OR “Benzoflavones” OR “beta-Naphthoflavone” OR “Biflavonoids” OR “Catechin” OR “Chalcones” OR “Flavanones” OR “Hesperidin” OR “Flavones” OR “Apigenin” OR “Diosmin” OR “Flavoxate” OR “Luteolin” OR “Flavonolignans” OR “Silymarin” OR “Silybin” OR “Flavonols” OR “Kaempferols” OR “Quercetin” OR “Rutin” OR “Hydroxyethylrutoside” OR “Isoflavones” OR “Coumestrol” OR “Genistein” OR “Pterocarpans” OR “Rotenone” OR “Phloretin” OR “Polyphloretin Phosphate” OR “Proanthocyanidins”)):ti,ab,kw |
References
- Janssen, I. Evolution of sarcopenia research. Appl. Physiol. Nutr. Metab. 2010, 35, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.C.; Dennison, E.M.; Cooper, C. Epidemiology of Sarcopenia: Determinants Throughout the Lifecourse. Calcif. Tissue Int. 2017, 101, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.P.; Rejeski, W.J.; Espeland, M.A.; Miller, M.E.; Church, T.S.; Fielding, R.A.; Gill, T.M.; Guralnik, J.M.; Newman, A.B.; Pahor, M.; et al. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P). J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Kim, S.W.; Kim, J.W.; Park, H.Y. Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia-A Narrative Review. Life 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Borrás, C.; Viña, J. Genistein, a tool for geroscience. Mech. Ageing Dev. 2022, 204, 111665. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef] [PubMed]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.S.; Lee, W.K.; Lee, S.J.; Lee, S.Y.; Seo, J.H.; Kim, E.J.; Choe, Y.I.; Kim, S.G.; Yoo, J.I. Rutin Prevents Dexamethasone-Induced Muscle Loss in C2C12 Myotube and Mouse Model by Controlling FOXO3-Dependent Signaling. Antioxidants 2023, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Zhang, Y.; Qu, T.Q.; Sang, X.Q.; Li, Y.X.; Ren, F.Z.; Wen, P.C.; Sun, Y.N. Nobiletin Improves D-Galactose-Induced Aging Mice Skeletal Muscle Atrophy by Regulating Protein Homeostasis. Nutrients 2023, 15, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Shin, S.K.; Kwon, E.Y. Luteolin Protects Against Obese Sarcopenia in Mice with High-Fat Diet-Induced Obesity by Ameliorating Inflammation and Protein Degradation in Muscles. Mol. Nutr. Food Res. 2023, 67, e2200729. [Google Scholar] [CrossRef] [PubMed]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef] [PubMed]
- Boutry-Regard, C.; Vinyes-Parés, G.; Breuillé, D.; Moritani, T. Supplementation with Whey Protein, Omega-3 Fatty Acids and Polyphenols Combined with Electrical Muscle Stimulation Increases Muscle Strength in Elderly Adults with Limited Mobility: A Randomized Controlled Trial. Nutrients 2020, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Orsatti, F.L.; Maestá, N.; de Oliveira, E.P.; Nahas Neto, J.; Burini, R.C.; Nunes, P.R.P.; Souza, A.P.; Martins, F.M.; Nahas, E.P. Adding Soy Protein to Milk Enhances the Effect of Resistance Training on Muscle Strength in Postmenopausal Women. J. Diet. Suppl. 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Choquette, S.; Dion, T.; Brochu, M.; Dionne, I.J. Soy isoflavones and exercise to improve physical capacity in postmenopausal women. Climacteric 2013, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009, 399, b2700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Tokuda, Y.; Mori, H. Essential Amino Acid and Tea Catechin Supplementation after Resistance Exercise Improves Skeletal Muscle Mass in Older Adults with Sarcopenia: An Open-Label, Pilot, Randomized Controlled Trial. J. Am. Nutr. Assoc. 2023, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef]
- Munguia, L.; Ramirez-Sanchez, I.; Meaney, E.; Villarreal, F.; Ceballos, G.; Najera, N. Flavonoids from dark chocolate and (−)-epicatechin ameliorate high-fat diet-induced decreases in mobility and muscle damage in aging mice. Food Biosci. 2020, 37, 100710. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Suzuki, T.; Saito, K.; Yoshida, H.; Kojima, N.; Kim, M.; Sudo, M.; Yamashiro, Y.; Tokimitsu, I. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 458–465. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese-sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Maesta, N.; Nahas, E.A.; Nahas-Neto, J.; Orsatti, F.L.; Fernandes, C.E.; Traiman, P.; Burini, R.C. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas 2007, 56, 350–358. [Google Scholar] [CrossRef]
- Thomson, R.L.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D. Muscle strength gains during resistance exercise training are attenuated with soy compared with dairy or usual protein intake in older adults: A randomized controlled trial. Clin. Nutr. 2016, 35, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Maruyama, K.; Yamamoto, N.; Saito, I. The effects of dietary licorice flavonoid oil supplementation on body balance control in healthy middle-aged and older Japanese women undergoing a physical exercise intervention: A randomized, double-blind, placebo-controlled trial. Aging Clin. Exp. Res. 2021, 33, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Mori, H. Effect of ingestion of essential amino acids and tea catechins after resistance exercise on the muscle mass, physical performance, and quality of life of healthy older people: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2021, 30, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, Q.; Li, Q.; Lin, H.; Li, J.; Zhu, M.; Liu, Z.; Wang, K. Integrative analysis of transcriptome and metab-olome reveals the mechanism of foliar application of Bacillus amyloliquefaciens to improve summer tea quality (Camellia sinensis). Plant Physiol. Biochem. 2022, 185, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Miyazaki, H.; Fang, S.H.; Li, C.Y.; Suzuki, K. The Structural Characteristics of Green Tea Polyphenols on Lipopolysaccharide-Stimulated RAW Cells. J. Nutr. Biol. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Sperringer, J.; Mohamed, J.S.; Edens, N.K.; Pereira, S.L. Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats. J. Appl. Physiol. 2015, 118, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, S.; Ma, S.; Suzuki, K. Effect of Genistein Supplementation on Exercise-Induced Inflammation and Oxidative Stress in Mice Liver and Skeletal Muscle. Medicina 2021, 57, 1028. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Petroni, M.L.; Caletti, M.T.; Dalle Grave, R.; Bazzocchi, A.; Aparisi Gómez, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Quan, J.; Wang, X.; Gu, Y.; Zhang, S.; Meng, G.; Zhang, Q.; Liu, L.; Wang, X.; Sun, S.; et al. Soy Food Consumption Is Inversely Associated with Handgrip Strength: Results from the TCLSIH Cohort Study. Nutrients 2023, 15, 391. [Google Scholar] [CrossRef] [PubMed]
| PICOS Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Subjects diagnosed with sarcopenia | Subjects not diagnosed with sarcopenia previously |
| Intervention/exposure | Intake of flavonoids/flavonoids combined with other supplementation/flavonoid-rich supplementations to treat sarcopenia | Used administration other than flavonoids |
| Comparison | Effectiveness of flavonoids, flavonoids combined with other supplementation, or flavonoid-rich supplementations vs. effectiveness of placebo/control/other intervention | Randomized controlled trials that combined both exercise and flavonoids intervention except where both control and intervention groups underwent the same exercise program |
| Outcome | Skeletal muscle mass, muscle strength, physical performance | Parameters for a mixed population of adults with and without sarcopenia |
| Study design | Randomized controlled trials | In silico, in vitro, animal studies, case reports, cohort studies, and cross-sectional studies |
| Year | Author | Country | Subjects | Flavonoids Studied | Study Design | Sarcopenia Diagnose Criteria | Groups | Dosage Form | Dose | Intervention Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | Tokuda, Y. et al. [26] | Japan | 46 older people (6 males, 40 females) aged 78~79 years with sarcopenia | TCCs | RCT | AWGS 2019 criteria: low hand-grip strength (males: <28.0 kg, females: <18.0 kg) or slow gait speed (males and females: <1.0 m/s), and low SMI (SMI, males: <7.0 kg/m2, females: <5.7 kg/m2). | (1) RE alone (RE) (2) RE followed by EAA ingestion (RE + EAA) (3) RE followed by EAA and TCCs supplementation (RE + EAA + TCC) | TCCs supplement powder | TCCs (540 mg/day, twice a week after RE) | 24 weeks |
| 2019 | Mafi, F. et al. [27] | Iran | 62 sarcopenic elderly males (mean age 68.63 ± 2.86 years) with class I sarcopenia | EC | RCT | Class I sarcopenia: AppMMI < 10.75 kg/m2 | (1) RE Training (RT) (2) EC supplementation (EC) (3) RE Training + EC (RT + EC) (4) Placebo (PL) | EC capsules | EC (1 mg/kg body weight/day) | 8 weeks |
| 2019 | Munguia, L. et al. [28] | Mexico | 61 ndividuals (13 males and 48 females (mean age 75.9 ± 5.7 years) with sarcopenia | EC | RCT | SMI < 8.87 kg/m2 (men) and <6.42 kg/m2 (women) | (1) Highly alkalinized (no-flavonoid; NF) (2) Flavonoid-rich natural cocoa (F) | Flavonoid-rich natural cocoa beverage | 179 mg flavonoids/day | 8 weeks |
| 2016 | Kim, H. et al. [29] | Japan | 137 female (mean age 80.9 ± 4.2~81.1 ± 5.1 years) defined with sarcopenia obesity | TCCs | RCT | Body fat percent > 32%, combined with SMI < 5.67 kg/m2 or grip strength < 17.0 kg or walking speed < 1.0 m/s | (1) Exercise only (Ex) (2) Exercise + EAA and TCCs supplementation (Ex + N) (3) EAA and TCCs supplementation (N) (4) Contorl (C) | Tea fortified with TCCs | One bottle of tea fortified with 540 mg of TCCs per day | 3 months |
| 2013 | Kim, H. et al. [30] | Japan | 116 women (mean age 79.6 ± 4.2~81.1 ± 3.7 years) were defined as sarcopenic | TCCs | RCT | At least one of the following condition: (1) AppMMI < 6.42 kg/m2 and knee extension strength < 1.01 Nm/kg; (2) AppMMI < 6.42 kg/m2 and walking speed < 1.10 m/s; (3) BMI < 22 and knee extension strength < 1.10 Nm/kg; (4) BMI < 22 and walking speed < 1.10 m/s | (1) Exercise and TCCs supplementation (Ex + TC) (2) Exercise (Ex) (3) TCCs supplementation (TC) (4) Contorl | Tea fortified with 540 mg of TCCs | One bottle of tea fortified with 540 mg of TCCs per day | 3 months |
| 2007 | Aubertin-Leheudre, M. et al. [31] | Canada | 18 sarcopenic–obese women (58 ± 5-year-old) | Isoflavones (daidzein, glycitein and genistein) | RCT | SMI < 6.87 kg/m2 and obesity as a body fat percent > 40% | (1) Isoflavone (ISO) (2) Placebo (PLA) | Capsules | 70 mg i soflavones (44 mg of diadzein, 16 mg glycitein and 10 mg genestein)/day | 24 weeks |
| Author | Flavonoids Studied | Groups | Outcome Measurement | Main Outcome |
|---|---|---|---|---|
| Tokuda, Y. et al. [26] | TCCs | (1) RE alone (RE) (2) RE followed by EAA ingestion (RE + EAA) (3) RE followed by EAA and TCCs supplementation (RE + EAA + TCC) | (1) SMM: SMM measured by BIA (2) MS: maximum isometric hand-grip strength and knee extension strength (3) PP: usual walking speed | %Δ SMM was significantly greater in the RE + EAA + TCC group vs. RE group. |
| Mafi, F. et al. [27] | EC | (1) RE Training (RT) (2) EC supplementation (EC) (3) RE Training+EC (RT + EC) (4) Placebo (PL) | (1) SMM: SMI measured by DXA (2) MS: maximal strength in leg press and chest press (3) PP: TUG | (1) AppMMI significantly increased in RT + EC, RT, and EC vs. PL. The increases were significantly greater in RT + EC than EC. (2) Maximal MS of chest press and leg press significantly increased in RT + EC and RT, not in EC or PL. (3) TUG time significantly reduced in RT + EC, RT, and EC vs PL, also a significant difference between EC and RT + EC. |
| Munguia, L. et al. [28] | EC | (1) Highly alkalinized (no-flavonoid; NF) (2) Flavonoid-rich natural cocoa (F) | (1) SMM: SMI messured by BIA (2) MS: maximal handgrip strength (3) PP: six-minute walk test, step test, sit-up test, TUG | Flavonoids treatment significantly improved physical performance and muscle strength (F vs. NF) |
| Kim, H. et al. [29] | TCCs | (1) Exercise only (Ex) (2) Exercise + EAA and TCCs supplementation (Ex + N) (3) EAA and TCCs supplementation (N)(4) Contorl (C) | (1) SMM: SMI messured by BIA (2) MS: maximal grip strength, knee extension strength (3) PP: usual walking speed | (1) Significant increases in knee extension strength were observed in the Ex + N, Ex, and N groups (2) Usual walking increased in the Ex + N, but not in Ex, N and C group |
| Kim, H. et al. [30] | TCCs | (1) Exercise and TCCs supplementation (Ex + TC) (2) Exercise (Ex) (3) TCCs supplementation (TC) (4) Contorl (C) | (1) SMM: LBM, total and segmental muscle mass measured by BIA (2) MS: grip strength, knee extension strength (3) PP: usual and maximum walking speed, TUG | Combination of exercise and tea catechin supplementation had a beneficial effect on PP measured by walking ability and SMM vs. control but not significant in TC group. |
| Aubertin-Leheudre, M. et al. [31] | Isoflavones (daidzein, glycitein and genistein) | (1) Isoflavone (ISO) (2) Placebo (PLA) | SMM: SMI measured by DXA | Isoflavone significantly increased appendicular and leg LBM, SMI but not the placebo group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Suzuki, K. The Effects of Flavonoids on Skeletal Muscle Mass, Muscle Function, and Physical Performance in Individuals with Sarcopenia: A Systematic Review of Randomized Controlled Trials. Nutrients 2023, 15, 3897. https://doi.org/10.3390/nu15183897
Wu C, Suzuki K. The Effects of Flavonoids on Skeletal Muscle Mass, Muscle Function, and Physical Performance in Individuals with Sarcopenia: A Systematic Review of Randomized Controlled Trials. Nutrients. 2023; 15(18):3897. https://doi.org/10.3390/nu15183897
Chicago/Turabian StyleWu, Cong, and Katsuhiko Suzuki. 2023. "The Effects of Flavonoids on Skeletal Muscle Mass, Muscle Function, and Physical Performance in Individuals with Sarcopenia: A Systematic Review of Randomized Controlled Trials" Nutrients 15, no. 18: 3897. https://doi.org/10.3390/nu15183897
APA StyleWu, C., & Suzuki, K. (2023). The Effects of Flavonoids on Skeletal Muscle Mass, Muscle Function, and Physical Performance in Individuals with Sarcopenia: A Systematic Review of Randomized Controlled Trials. Nutrients, 15(18), 3897. https://doi.org/10.3390/nu15183897