Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedures
2.3. Dietary Supplements
2.4. Circulating Biomarkers Assessment
2.5. Statistical Analysis
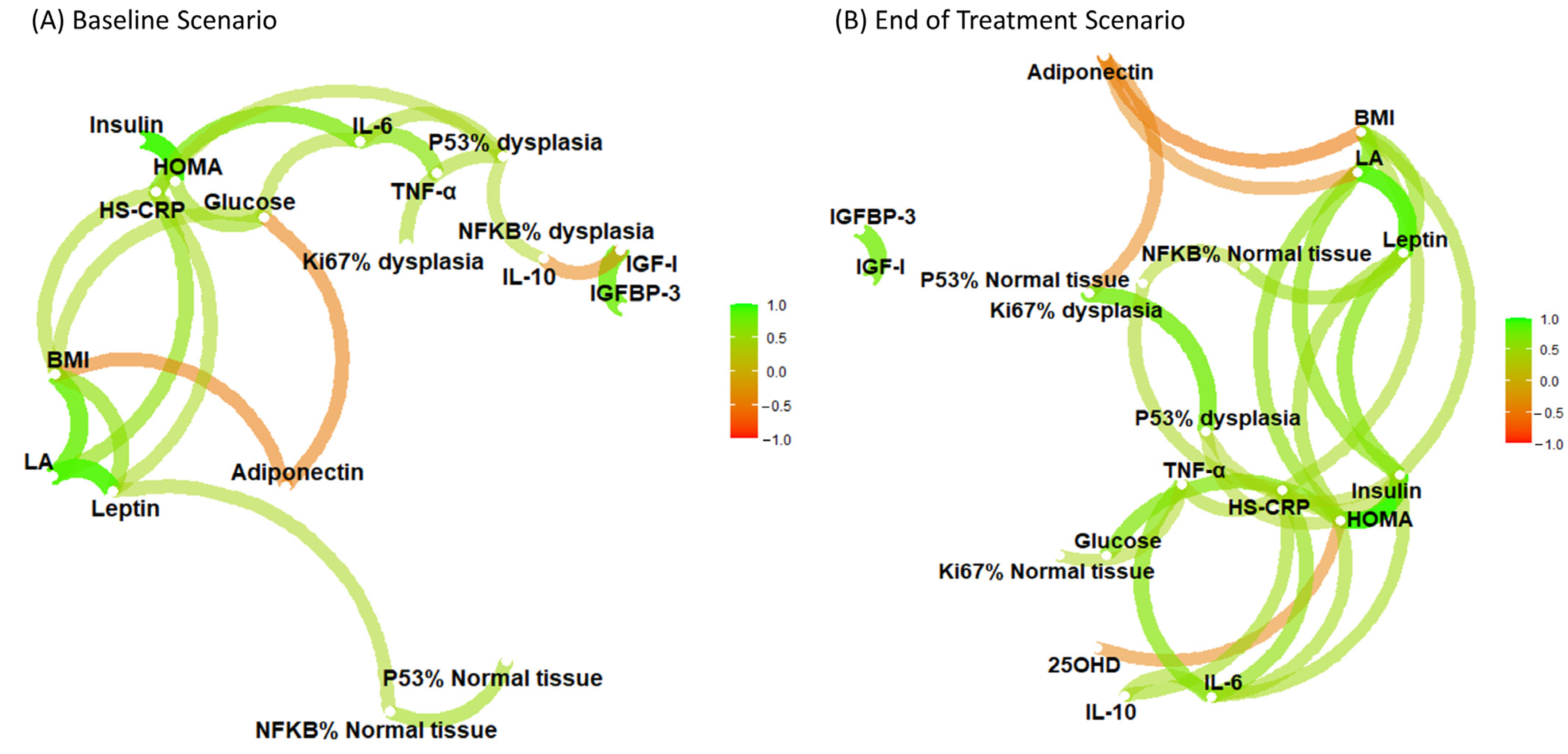
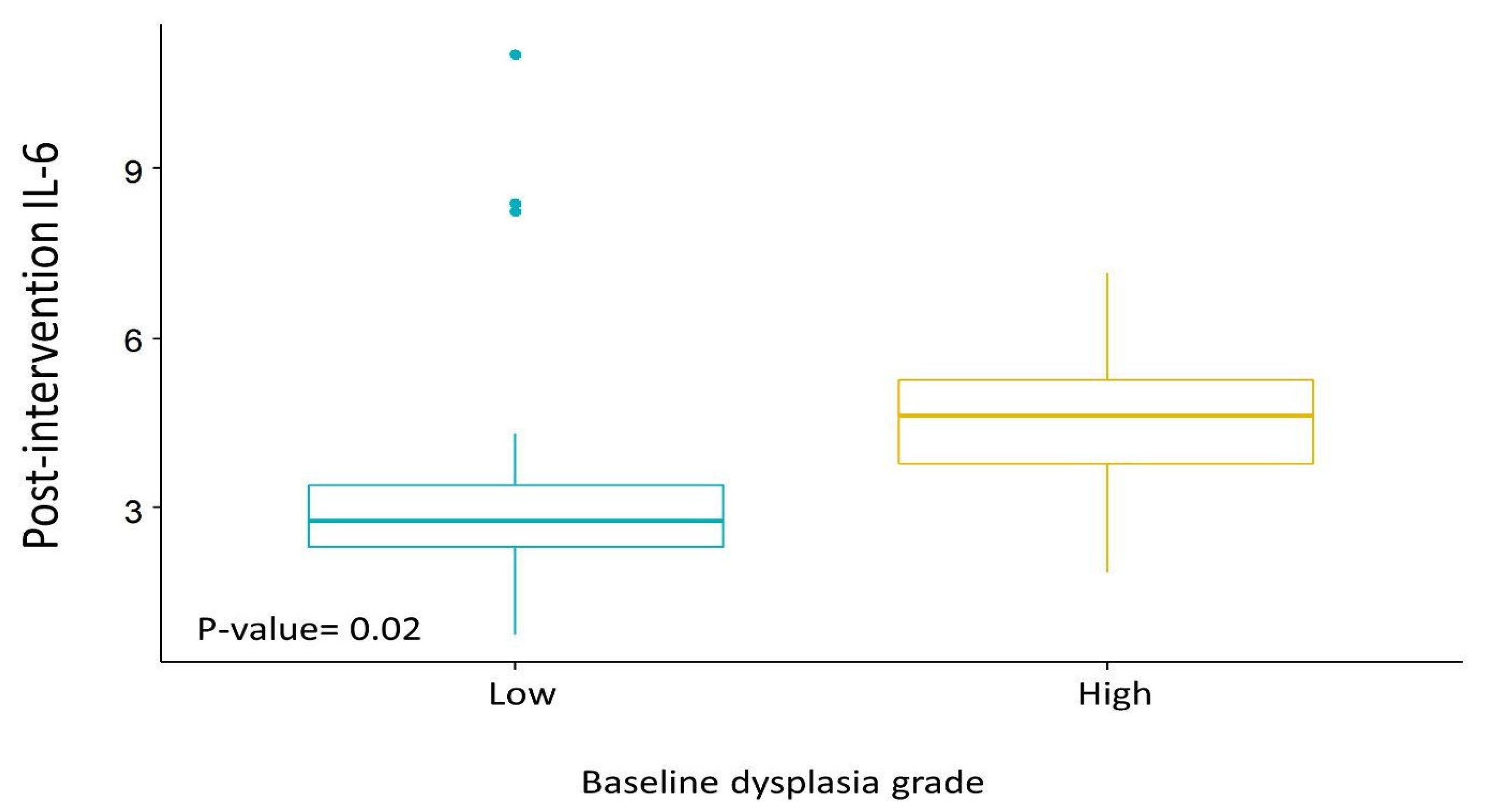
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Vilahur, N.; Bianchini, F.; Guha, N.; Straif, K. International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N. Engl. J. Med. 2018, 378, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal Cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, Y.; Wu, H.; Duerksen-Hughes, P.; Zhang, H.; Li, P.; Huang, J.; Yang, J.; Wu, Y.; Xia, D. Association between Markers of Glucose Metabolism and Risk of Colorectal Cancer. BMJ Open 2016, 6, e011430. [Google Scholar] [CrossRef]
- Kim, S.; Keku, T.O.; Martin, C.; Galanko, J.; Woosley, J.T.; Schroeder, J.C.; Satia, J.A.; Halabi, S.; Sandler, R.S. Circulating Levels of Inflammatory Cytokines and Risk of Colorectal Adenomas. Cancer Res. 2008, 68, 323–328. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Pu, X.; Chen, D. Targeting Adipokines in Obesity-Related Tumors. Front. Oncol. 2021, 11, 685923. [Google Scholar] [CrossRef]
- Peixoto, R.D.A.; de Carvalho Oliveira, L.J.; de Melo Passarini, T.; Andrade, A.C.; Diniz, P.H.; Prolla, G.; Amorim, L.C.; Gil, M.; Lino, F.; Garicochea, B.; et al. Vitamin D and Colorectal Cancer—A Practical Review of the Literature. Cancer Treat. Res. Commun. 2022, 32, 100616. [Google Scholar] [CrossRef]
- Huang, X.-M.; Yang, Z.-J.; Xie, Q.; Zhang, Z.-K.; Zhang, H.; Ma, J.-Y. Natural Products for Treating Colorectal Cancer: A Mechanistic Review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Goel, A. Curcumin and Colorectal Cancer: An Update and Current Perspective on This Natural Medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Sakhawat, A.; Piñol-Felis, C.; Motilva, M.J. Chemopreventive Effects of Anthocyanins on Colorectal and Breast Cancer: A Review. Semin. Cancer Biol. 2022, 81, 241–258. [Google Scholar] [CrossRef]
- Briata, I.M.; Paleari, L.; Rutigliani, M.; Petrera, M.; Caviglia, S.; Romagnoli, P.; Libera, M.D.; Oppezzi, M.; Puntoni, M.; Siri, G.; et al. A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps. Int. J. Mol. Sci. 2021, 22, 1024. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of Systemic Availability of Curcumin with That of Curcumin Formulated with Phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and Safety of Meriva®, a Curcumin-Phoshatidylcholine Complex, during Extended Administration in Osteoarthtitis Patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Allegri, P.; Mastromarino, A.; Neri, P. Management of Chronic Anterior Uveitis Relapses: Efficacy of Oral Phospholipidic Curcumin Treatment. Long-Term Follow-Up. Clin. Ophthalmol. 2010, 4, 1201–1206. [Google Scholar] [CrossRef]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A.; et al. Pilot Study of Oral Anthocyanins for Colorectal Cancer Chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef]
- Cai, H.; Thomasset, S.C.; Berry, D.P.; Garcea, G.; Brown, K.; Steward, W.P.; Gescher, A.J. Determination of Anthocyanins in the Urine of Patients with Colorectal Liver Metastases after Administration of Bilberry Extract. Biomed. Chromatogr. 2011, 25, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Togni, S.; Franceschi, F.; Kawada, S.; Inaba, Y.; Eggenhoffner, R.; Giacomelli, L. The Effect of a Natural, Standardized Bilberry Extract (Mirtoselect®) in Dry Eye: A Randomized, Double Blinded, Placebo-Controlled Trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2518–2525. [Google Scholar] [PubMed]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A Single Supplement of a Standardised Bilberry (Vaccinium myrtillus L.) Extract (36% Wet Weight Anthocyanins) Modifies Glycaemic Response in Individuals with Type 2 Diabetes Controlled by Diet and Lifestyle. J. Nutr. Sci. 2013, 2, E22. [Google Scholar] [CrossRef] [PubMed]
- Mazzolani, F.; Togni, S.; Franceschi, F.; Eggenhoffner, R.; Giacomelli, L. The Effect of Oral Supplementation with Standardized Bilberry Extract (Mirtoselect®) on Retino-Cortical Bioelectrical Activity in Severe Diabetic Retinopathy. Minerva Oftalmol. 2021, 59, 38–41. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Macis, D.; Aristarco, V.; Johansson, H.; Guerrieri-gonzaga, A.; Raimondi, S.; Lazzeroni, M.; Sestak, I.; Cuzick, J.; De Censi, A.; Bonanni, B.; et al. A Novel Automated Immunoassay Platform to Evaluate the Association of Adiponectin and Leptin Levels with Breast Cancer Risk. Cancers 2021, 13, 3303. [Google Scholar] [CrossRef]
- Puntoni, M.; Branchi, D.; Argusti, A.; Zanardi, S.; Crosta, C.; Meroni, E.; Munizzi, F.; Michetti, P.; Coccia, G.; De Roberto, G.; et al. A Randomized, Placebo-Controlled, Preoperative Trial of Allopurinol in Subjects with Colorectal Adenoma. Cancer Prev. Res. 2013, 6, 74–81. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and Colorectal Cancer: Role of Adipokines in Tumor Initiation and Progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef]
- Saraggi, D.; Fassan, M.; Mescoli, C.; Scarpa, M.; Valeri, N.; Michielan, A.; D’Incá, R.; Rugge, M. The Molecular Landscape of Colitis-Associated Carcinogenesis. Dig. Liver Dis. 2017, 49, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-Inflammatory Effects of Oral Supplementation with Curcumin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2021, 79, 1043–1066. [Google Scholar] [CrossRef]
- Gautron, L.; Elmquist, J.K. Sixteen Years and Counting: An Update on Leptin in Energy Balance. J. Clin. Investig. 2011, 121, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Baxter, R.C. IGF Binding Proteins in Cancer: Mechanistic and Clinical Insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Murphy, N.; Carreras-Torres, R.; Song, M.; Chan, A.T.; Martin, R.M.; Papadimitriou, N.; Dimou, N.; Tsilidis, K.K.; Banbury, B.; Bradbury, K.E.; et al. Circulating Levels of Insulin-like Growth Factor 1 and Insulin-like Growth Factor Binding Protein 3 Associate with Risk of Colorectal Cancer Based on Serologic and Mendelian Randomization Analyses. Gastroenterology 2020, 158, 1300–1312. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Effects of Curcumin/Turmeric Supplementation on Obesity Indices and Adipokines in Adults: A Grade-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2023, 37, 1703–1728. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of Dietary Anthocyanins on Systemic and Vascular Inflammation: Systematic Review and Meta-Analysis on Randomised Clinical Trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef]
| Parameter | Treatment | p-Value | ||
|---|---|---|---|---|
| Active | Placebo | |||
| n (%) | 15 (51.7) | 14 (48.3) | ||
| Gender, n (%) | F | 5 (33.3) | 7 (50.0) | 0.462 |
| M | 10 (66.7) | 7 (50.0) | ||
| Age, mean ± SD | 70.8 ± 9.8 | 67.9 ± 10.8 | 0.454 | |
| Baseline BMI, n (%) | <25 | 7 (46.7) | 9 (64.3) | 0.462 |
| ≥25 | 8 (53.3) | 5 (35.7) | ||
| Family history of CRC, n (%) | No | 10 (66.7) | 7 (50.0) | 0.462 |
| Yes | 5 (33.3) | 7 (50.0) | ||
| Foods rich in anthocyanins, (servings per day) | <2 | 7 (46.7) | 7 (50.0) | 1.000 |
| ≥2 | 7 (53.3) | 7 (50.0) | ||
| Histological type, n (%) | Tubular | 10 (66.7) | 10 (71.4) | 1.000 |
| Villous | 5 (33.3) | 4 (28.6) | ||
| Dysplasia grade, n (%) | Low-grade | 12 (80.0) | 9 (64.3) | 0.427 |
| High-grade | 3 (20.0) | 5 (35.7) | ||
| Serum Biomarkers Median (IQR) | Treatment | p-Value | ||
|---|---|---|---|---|
| Active n = 16 | Placebo n = 17 | |||
| HOMA-index | Pre | 1.52 (1.21–2.45) | 1.39 (1.03–1.66) | 0.17 |
| Post | 1.07 (0.77–1.31) | 0.66 (0.48–0.97) | ||
| %change | −28% | −53% | ||
| hs-CRP (mg/dL) | Pre | 0.15 (0.10–0.25) | 0.10 (0.10–0.10) | 0.50 |
| Post | 0.20 (0.10–0.60) | 0.15(0.10–0.30) | ||
| %change | 0% | 25% | ||
| L/A | Pre | 0.70 (0.35–1.30) | 0.80(0.40–2.30) | 0.34 |
| Post | 0.40 (0.20–0.70) | 0.30(0.20–0.90) | ||
| %change | −51% | −63% | ||
| Adiponectin (µg/mL) | Pre | 12.80 (7.60–20.80) | 12.60 (7.10–19.20) | 0.94 |
| Post | 9.70 (6.00–18.20) | 13.20 (7.60–20.30) | ||
| %change | −12% | 2% | ||
| Leptin (ng/mL) | Pre | 8.30 (3.75–11.00) | 11.00 (5.00–22.0) | 0.91 |
| Post | 2.90 (1.30–7.20) | 4.05 (2.00–12.60) | ||
| %change | −63% | −51% | ||
| IGFBP-3 (ng/mL) | Pre | 3269.15 (2860.80–3706.15) | 3150.80 (2400.20–3546.20) | 0.91 |
| Post | 3227.50 (2678.70–3545.30) | 2891.25 (2698.90–3731.90) | ||
| %change | −6% | −5% | ||
| IGF-1 (ng/mL) | Pre | 112.65 (99.65–146.15) | 109.60 (79.90–124.50) | 0.27 |
| Post | 110.20 (79.30–137.90) | 98.85 (73.70–140.70) | ||
| %change | −12% | −7% | ||
| 25OHD (ng/mL) | Pre | 16.80 (7.55–22.05) | 15.20 (8.70–19.20) | 0.92 |
| Post | 15.10 (9.20–22.90) | 16.55 (11.00–26.60) | ||
| %change | 0% | 4% | ||
| IL-10 (pg/mL) | Pre | 3.15 (2.70–4.30) | 3.20 (2.30–3.70) | 0.29 |
| Post | 4.10 (2.70–6.20) | 3.20 (2.30–3.90) | ||
| %change | 10% | 21% | ||
| IL-6 (pg/mL) | Pre | 3.50 (2.85–5.00) | 2.90 (1.90–3.60) | 0.60 |
| Post | 3.90 (2.50–6.30) | 2.80 (2.00–3.50) | ||
| %change | 14% | 6% | ||
| TNFα (pg/mL) | Pre | 11.30 (9.60–14.50) | 10.50 (9.90–12.50) | 0.42 |
| Post | 11.70 (8.80–14.20) | 11.05 (9.60–11.70) | ||
| %change | −1% | −2% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macis, D.; Briata, I.M.; D’Ecclesiis, O.; Johansson, H.; Aristarco, V.; Buttiron Webber, T.; Oppezzi, M.; Gandini, S.; Bonanni, B.; DeCensi, A. Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas. Nutrients 2023, 15, 3894. https://doi.org/10.3390/nu15183894
Macis D, Briata IM, D’Ecclesiis O, Johansson H, Aristarco V, Buttiron Webber T, Oppezzi M, Gandini S, Bonanni B, DeCensi A. Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas. Nutrients. 2023; 15(18):3894. https://doi.org/10.3390/nu15183894
Chicago/Turabian StyleMacis, Debora, Irene Maria Briata, Oriana D’Ecclesiis, Harriet Johansson, Valentina Aristarco, Tania Buttiron Webber, Massimo Oppezzi, Sara Gandini, Bernardo Bonanni, and Andrea DeCensi. 2023. "Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas" Nutrients 15, no. 18: 3894. https://doi.org/10.3390/nu15183894
APA StyleMacis, D., Briata, I. M., D’Ecclesiis, O., Johansson, H., Aristarco, V., Buttiron Webber, T., Oppezzi, M., Gandini, S., Bonanni, B., & DeCensi, A. (2023). Inflammatory and Metabolic Biomarker Assessment in a Randomized Presurgical Trial of Curcumin and Anthocyanin Supplements in Patients with Colorectal Adenomas. Nutrients, 15(18), 3894. https://doi.org/10.3390/nu15183894







