Liquorice Toxicity: A Comprehensive Narrative Review
Abstract
1. Introduction
2. Search Strategy
3. Historical Background, Source and Use of Liquorice
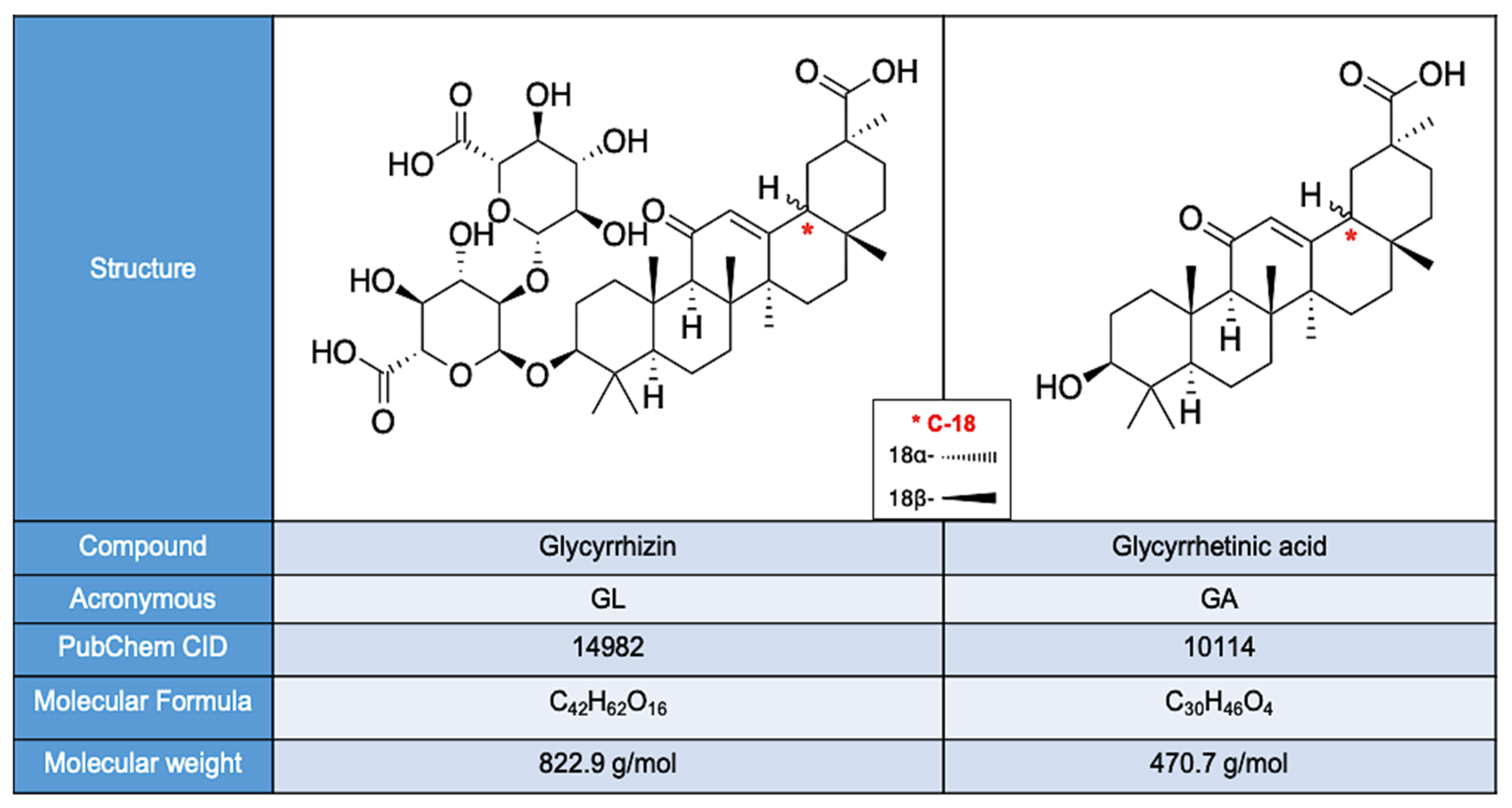
4. Chemical Composition
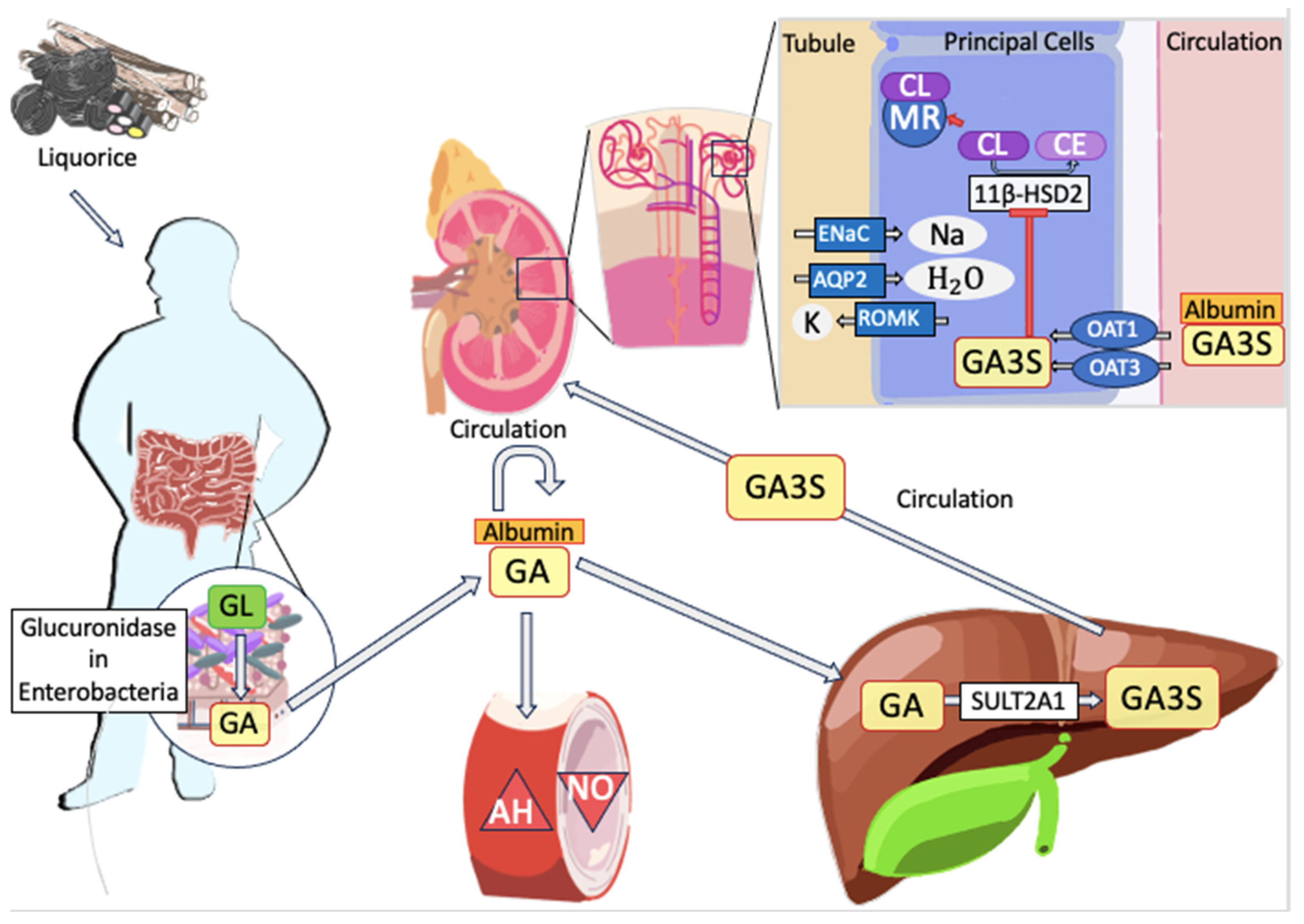
5. Pharmacokinetics
6. Biochemical Mechanism of Toxicity
Other Liquorice-Induced Side Effects
7. Clinical Risk Factors for LT
7.1. Daily Dosage
7.2. Age
7.3. Sex
7.4. Metabolism
7.5. Comorbidities and Concomitant Use of Other Medications
8. Clinical Manifestations
8.1. Cardiovascular Disorders
8.2. Muscle and Neurological Manifestations
8.3. Others
9. Diagnosis
10. Treatment and Prognosis
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Isbrucker, R.A.; Burdock, G.A. Risk and Safety Assessment on the Consumption of Licorice Root (Glycyrrhiza Sp.), Its Extract and Powder as a Food Ingredient, with Emphasis on the Pharmacology and Toxicology of Glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a Unique “Guide Drug” of Traditional Chinese Medicine: A Review of Its Role in Drug Interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Domingues, F.; Pereira, L. Metabolic Changes after Licorice Consumption: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Clinical Trials. Phytomedicine 2018, 39, 17–24. [Google Scholar] [CrossRef]
- Yoshino, T.; Shimada, S.; Homma, M.; Makino, T.; Mimura, M.; Watanabe, K. Clinical Risk Factors of Licorice-Induced Pseudoaldosteronism Based on Glycyrrhizin-Metabolite Concentrations: A Narrative Review. Front. Nutr. 2021, 8, 719197. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Komarova, I.; El-Ghonemi, M.; Fathy, A.; Rashad, R.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Helal, E.; Camporesi, E.M. Licorice Abuse: Time to Send a Warning Message. Ther. Adv. Endocrinol. Metab. 2012, 3, 125–138. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Scientific Opinion on the Safety and Efficacy of Glycyrrhizic Acid Ammoniated (Chemical Group 30, Miscellaneous Substances) When Used as a Flavouring for All Animal Species. EFSA J. 2015, 13, 3971. [CrossRef]
- Gross, E.G.; Dexter, J.D.; Roth, R.G. Hypokalemic Myopathy with Myoglobinuria Associated with Licorice Ingestion. N. Engl. J. Med. 1966, 274, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Conn, J.W.; Rovner, D.R.; Cohen, E.L. Licorice-Induced Pseudoaldosteronism. Hypertension, Hypokalemia, Aldosteronopenia, and Suppressed Plasma Renin Activity. JAMA 1968, 205, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Tourtellotte, C.R.; Hirst, A.E.; Linda, L. Hypokalemia, Muscle Weakness, and Myoglobinuria Due to Licorice Ingestion. Calif. Med. 1970, 113, 51. [Google Scholar]
- Holmes, A.M.; Young, J.; Marrott, P.K.; Prentice, E. Pseudohyperaldosteronism Induced by Habitual Ingestion of Liquorice. Postgrad. Med. J. 1970, 46, 625–629. [Google Scholar] [CrossRef][Green Version]
- Bannister, B.; Ginsburg, R.; Shneerson, J. Cardiac Arrest Due to Liquoriceinduced Hypokalaemia. Br. Med. J. 1977, 2, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Cumming, A.M.; Boddy, K.; Brown, J.J.; Fraser, R.; Lever, A.F.; Padfield, P.L.; Robertson, J.I. Severe Hypokalaemia with Paralysis Induced by Small Doses of Liquorice. Postgrad. Med. J. 1980, 56, 526–529. [Google Scholar] [CrossRef][Green Version]
- Nightingale, S.; Smith, P.E.; Turnbull, D.M. Anorexia Nervosa, Liquorice and Hypokalaemic Myopathy. Postgrad. Med. J. 1981, 57, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.B.; Swaminathan, R. Total Body Potassium Depletion and Severe Myopathy Due to Chronic Liquorice Ingestion. Postgrad. Med. J. 1981, 57, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, G.; De Mari, M.; Pozio, G.; Lamberti, P. Hypokalemic Myopathy Associated with Liquorice Ingestion. Ital. J. Neurol. Sci. 1984, 5, 463–466. [Google Scholar] [CrossRef]
- Nielsen, I.; Pedersen, R.S. Life-Threatening Hypokalaemia Caused by Liquorice Ingestion. Lancet 1984, 1, 1305. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V.; Biglieri, E.G.; Shackleton, C.H.; Irony, I.; Gomez-Fontes, R. Licorice-Induced Hypermineralocorticoidism. N. Engl. J. Med. 1991, 325, 1223–1227. [Google Scholar] [CrossRef]
- Kageyama, Y. A Case of Pseudoaldosteronism Induced by Glycyrrhizin. Jpn. J. Nephrol. 1992, 34, 99–102. [Google Scholar] [CrossRef]
- van der Zwan, A. Hypertension Encephalopathy after Liquorice Ingestion. Clin. Neurol. Neurosurg. 1993, 95, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Heikens, J.; Fliers, E.; Endert, E.; Ackermans, M.; van Montfrans, G. Liquorice-Induced Hypertension—A New Understanding of an Old Disease: Case Report and Brief Review. Neth. J. Med. 1995, 47, 230–234. [Google Scholar] [CrossRef]
- Barrella, M.; Lauria, G.; Quatrale, R.; Paolino, E. Hypokaliemic Rhabdomyolysis Associated with Liquorice Ingestion: Report of an Atypical Case. Ital. J. Neurol. Sci. 1997, 18, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.J.; Abolnik, I.Z. Pulmonary Edema Following a Licorice Binge. West. J. Med. 1997, 167, 184–185. [Google Scholar] [PubMed]
- de Klerk, G.J.; Nieuwenhuis, M.G.; Beutler, J.J. Hypokalaemia and Hypertension Associated with Use of Liquorice Flavoured Chewing Gum. BMJ 1997, 314, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Suyama, Y.; Morisawa, T. Echocardiographic Findings of the Heart Resembling Dilated Cardiomyopathy during Hypokalemic Myopathy Due to Licorice-Induced Pseudoaldosteronism. Cardiovasc. Drugs Ther. 1998, 12, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.W.; Carlberg, B.; Hillörn, V. Life-threatening Ventricular Tachycardia Due to Liquorice-induced Hypokalaemia. J. Intern. Med. 1999, 245, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Mastropasqua, M.; Mosetti, M.A.; Persegani, C.; Paggi, A. Low Doses of Liquorice Can Induce Hypertension Encephalopathy. Am. J. Nephrol. 2000, 20, 145–148. [Google Scholar] [CrossRef]
- Lozano, P.; Flores, D.; Martínez, S.; Artigues, I.; Rimbau, E.M.; Gómez, F. Upper Limb Ischemia Induced by Chronic Licorice Ingestion. J. Cardiovasc. Surg. 2000, 41, 631–632. [Google Scholar]
- Dobbins, K.R.; Saul, R.F. Transient Visual Loss after Licorice Ingestion. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2000, 20, 38–41. [Google Scholar] [CrossRef]
- Elinav, E.; Chajek-Shaul, T. Licorice Consumption Causing Severe Hypokalemic Paralysis. Mayo Clin. Proc. 2003, 78, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Yang, S.-S.; Chau, T.; Halperin, M.L. An Unusual Cause of Hypokalemic Paralysis: Chronic Licorice Ingestion. Am. J. Med. Sci. 2003, 325, 153–156. [Google Scholar] [CrossRef]
- Hall, R.C.; Clemett, R.S. Central Retinal Vein Occlusion Associated with Liquorice Ingestion. Clin. Experiment. Ophthalmol. 2004, 32, 341. [Google Scholar] [CrossRef] [PubMed]
- Janse, A.; van Iersel, M.; Hoefnagels, W.H.L.; Olde Rikker, M.G.M. The Old Lady Who Liked Liquorice: Hypertension Due to Chronic Intoxication in a Memory-Impaired Patient. Neth. J. Med. 2005, 63, 149–150. [Google Scholar] [PubMed]
- van den Bosch, A.E.; van der Klooster, J.M.; Zuidgeest, D.M.H.; Ouwendijk, R.J.T.; Dees, A. Severe Hypokalaemic Paralysis and Rhabdomyolysis Due to Ingestion of Liquorice. Neth. J. Med. 2005, 63, 146–148. [Google Scholar] [PubMed]
- Breidthardt, T.; Namdar, M.; Hess, B. A Hypertensive Urgency Induced by the Continuous Intake of a Herbal Remedy Containing Liquorice. J. Hum. Hypertens. 2006, 20, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Hamidon, B.B.; Jeyabalan, V. Exogenously-Induced Apparent Hypermineralocorticoidism Associated with Ingestion of “Asam Boi”. Singap. Med. J. 2006, 47, 156–158. [Google Scholar]
- Yasue, H.; Itoh, T.; Mizuno, Y.; Harada, E. Severe Hypokalemia, Rhabdomyolysis, Muscle Paralysis, and Respiratory Impairment in a Hypertensive Patient Taking Herbal Medicines Containing Licorice. Intern. Med. 2007, 46, 575–578. [Google Scholar] [CrossRef]
- Francini-Pesenti, F.; Puato, M.; Piccoli, A.; Brocadello, F. Liquorice-Induced Hypokalaemia and Water Retention in the Absence of Hypertension. Phytother. Res. 2008, 22, 563–565. [Google Scholar] [CrossRef]
- Mumoli, N.; Cei, M. Licorice-Induced Hypokalemia. Int. J. Cardiol. 2008, 124, e42–e44. [Google Scholar] [CrossRef] [PubMed]
- Sontia, B.; Mooney, J.; Gaudet, L.; Touyz, R.M. Pseudohyperaldosteronism, Liquorice, and Hypertension. J. Clin. Hypertens. 2008, 10, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Tancevski, I.; Eller, P.; Spiegel, M.; Kirchmair, R.; Patsch, J.R. Malicious Licorice. Circulation 2008, 117, e299. [Google Scholar] [CrossRef]
- Meltem, A.C.; Figen, C.; Nalan, M.A.; Mahir, K.; Sebnem, B.; Mehlika, I.; Kasim, K.A.; Miyase, B. A Hypokalemic Muscular Weakness after Licorice Ingestion: A Case Report. Cases J. 2009, 2, 8053. [Google Scholar] [CrossRef] [PubMed]
- Crean, A.M.; Abdel-Rahman, S.-E.-D.T.; Greenwood, J.P. A Sweet Tooth as the Root Cause of Cardiac Arrest. Can. J. Cardiol. 2009, 25, e357–e358. [Google Scholar] [CrossRef]
- Johns, C. Glycyrrhizic Acid Toxicity Caused by Consumption of Licorice Candy Cigars. CJEM 2009, 11, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.C.; Agger, S.; Rainey, P.M. Too Much of a Good Thing: A Woman with Hypertension and Hypokalemia. Clin. Chem. 2009, 55, 2093–2096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yorgun, H.; Aksoy, H.; Şendur, M.A.; Ateş, A.H.; Kaya, E.B.; Aytemir, K.; Oto, A. Brugada Syndrome with Aborted Sudden Cardiac Death Related to Liquorice-Induced Hypokalemia. Med. Princ. Pract. 2010, 19, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Katchanov, J.; Haeusler, K.G.; Strackharn, J.; Tepel, M. Lower Limb Monoparesis Due to Liquorice Consumption. Clin. Kidney J. 2010, 3, 582–583. [Google Scholar] [CrossRef]
- Velickovic-Radovanovic, R.; Mitic, B.; Kitic, D.; Kostic, S.; Cvetkovic, T.; Djordjevic, V. Acute Renal Failure after Licorice Ingestion: A Case Report. Open Med. 2011, 6, 113–116. [Google Scholar] [CrossRef]
- Støving, R.K.; Lingqvist, L.E.; Bonde, R.K.; Andries, A.; Hansen, M.H.; Andersen, M.; Hørder, K. Is Glycyrrhizin Sensitivity Increased in Anorexia Nervosa and Should Licorice Be Avoided? Case Report and Review of the Literature. Nutrition 2011, 27, 855–858. [Google Scholar] [CrossRef]
- Kasap, B.; Soylu, A.; Çetin, B.Ş.; Çamlar, S.A.; Türkmen, M.A.; Kavukçu, S. Acute Kidney Injury Following Hypokalemic Rhabdomyolysis: Complication of Chronic Heavy Cola Consumption in an Adolescent Boy. Eur. J. Pediatr. 2010, 169, 107–111. [Google Scholar] [CrossRef]
- Imtiaz, K.E. Sweet Root, Bitter Pill: Liquorice-Induced Hyperaldosteronism. QJM Mon. J. Assoc. Physicians 2011, 104, 1093–1095. [Google Scholar] [CrossRef][Green Version]
- van Beers, E.J.; Stam, J.; van den Bergh, W.M. Licorice Consumption as a Cause of Posterior Reversible Encephalopathy Syndrome: A Case Report. Crit. Care 2011, 15, R64. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Karakus, A.; Zeren, C.; Demir, M.; Bayarogullari, H.; Duru, M.; Al, M. Licorice Induced Hypokalemia, Edema, and Thrombocytopenia. Hum. Exp. Toxicol. 2012, 31, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- van Noord, C.; Zietse, R.; van den Dorpel, M.A.; Hoorn, E.J. The Case∣a 62-Year-Old Man with Severe Alkalosis. Kidney Int. 2012, 81, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Kormann, R.; Languille, E.; Amiot, H.-M.; Hertig, A. Dying for a Cup of Tea. Case Rep. 2012, 2012, bcr2012006805. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Granados, E.S.; Shouls, G.; Sainsbury, C.; Antonios, T. A Salty Cause of Severe Hypertension. BMJ Case Rep. 2012, 2012, bcr1220115336. [Google Scholar] [CrossRef] [PubMed]
- Dehours, E.; Vallé, B.; Rougé-Bugat, M.E.; Florent, B.; Bounes, V.; Franchitto, N. Suspected Hypokalaemia Following Liquorice Ingestion on Board Ship. J. Telemed. Telecare 2013, 19, 227–228. [Google Scholar] [CrossRef]
- Flores Robles, B.J.; Hurtarte Sandoval, A.R.; Penate Dardon, J.D.; Alonso Blas, C. Lethal Liquorice Lollies (Liquorice Abuse Causing Pseudohyperaldosteronism). Case Rep. 2013, 2013, bcr2013201007. [Google Scholar] [CrossRef]
- Khan, O.; Hashim, M.; Lu, T.; Raashid, S.; Uddin, S.M.M.; Shapiro, J.; Seitillari, A.; Kaur, A.; Vasudevan, S. Pseudo Hyperaldosteronism Secondary to Herbal Medicine Use. J. Community Hosp. Intern. Med. Perspect. 2022, 12, 116–118. [Google Scholar] [CrossRef]
- Panduranga, P.; Al-Rawahi, N. Licorice-Induced Severe Hypokalemia with Recurrent Torsade de Pointes. Ann. Noninvasive Electrocardiol. 2013, 18, 593–596. [Google Scholar] [CrossRef]
- Kronborg-White, S.; Roseva-Nielsen, C.N. Liquorice-Induced Hypokalaemia and Rhabdomyolysis. J. Endocrinol. Metab. 2013, 3, 124–125. [Google Scholar] [CrossRef][Green Version]
- Horwitz, H.; Woeien, V.A.; Petersen, L.W.; Jimenez-Solem, E. Hypokalemia and Rhabdomyolysis. J. Pharmacol. Pharmacother. 2015, 6, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Cortini, E.; Chiara, F.; Dolcini, G.; Lonati, D.; Crevani, M.; Locatelli, C.A. Grave Intossicazione Secondaria ad Abuso di Liquirizia: Descrizione di un Caso. Available online: https://archivio.sitox.org/congressi/2015/abs/84.pdf (accessed on 19 July 2023).
- De Putter, R.; Donck, J. Low-Dose Liquorice Ingestion Resulting in Severe Hypokalaemic Paraparesis, Rhabdomyolysis and Nephrogenic Diabetes Insipidus. Clin. Kidney J. 2014, 7, 73–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Main, A.M.; Feldt-Rasmussen, U. The Hidden Liquorice: Apparent Mineralocorticoid Excess Caused by Inadvertent Exposure to Liquorice Root Extract. AACE Clin. Case Rep. 2015, 1, e278–e281. [Google Scholar] [CrossRef]
- Ottenbacher, R.; Blehm, J. An Unusual Case of Licorice-Induced Hypertensive Crisis. South Dak. Med. 2015, 68, 346–347, 349. [Google Scholar]
- Danis, R.; Ruhi, Ç.; Berketoglu, N.; Kara, A.V.; Yilmazer, B.; Kaya, S. Licorice Ingestion; An Unusual Cause of Rhabdomyolysis And. Turk. Nephrol. Dial. Transplant. 2015, 24, 106–109. [Google Scholar] [CrossRef]
- Machalke, K.; Bramlage, P.; Bramlage, K.; Tebbe, U. Prinzmetal-Angina nach Lakritz-Konsum. DMW—Dtsch. Med. Wochenschr. 2015, 140, 590–592. [Google Scholar] [CrossRef][Green Version]
- Dai, D.W.; Singh, I.; Hershman, J.M. Lozenge-Induced Hypermineralcorticoid State—A Unique Case of Licorice Lozenges Resulting in Hypertension and Hypokalemia. J. Clin. Hypertens. 2016, 18, 159. [Google Scholar] [CrossRef]
- Hataya, Y.; Oba, A.; Yamashita, T.; Komatsu, Y. Hyponatremia in an Elderly Patient Due to Isolated Hypoaldosteronism Occurring after Licorice Withdrawal. Intern. Med. 2017, 56, 175–179. [Google Scholar] [CrossRef]
- Allcock, E.; Cowdery, J. Hypertension Induced by Liquorice Tea. BMJ Case Rep. 2015, 2015, bcr2015209926. [Google Scholar] [CrossRef]
- Caravaca-Fontan, F.; Martinez-Saez, O.; Delgado-Yague, M.; Yerovi, E.; Liaño, F. An Unexpected Cause of Severe Hypokalemia. Case Rep. Nephrol. 2015, 2015, 957583. [Google Scholar] [CrossRef]
- Tassinari, D.; Bergamaschi, R.; Corsini, I.; Landini, S.; Romanin, B.; Ballarini, E.; De Ponti, F.; Carfagnini, F.; Toni, F.; Bernardi, F. Posterior Reversible Encephalopathy Syndrome Associated with Licorice Consumption: A Case Report in a 10-Year-Old Boy. Pediatr. Neurol. 2015, 52, 457–459. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Kinsella, J.; McMahon, C.; Holian, J.; O’Riordan, S. Posterior Reversible Encephalopathy Syndrome (PRES) Associated with Liquorice Consumption. Ir. J. Med. Sci. 2016, 185, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Erkus, M.E. Atrial Fibrillation Due to Licorice Root Syrup. Turk. Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2016, 44, 237–239. [Google Scholar] [CrossRef]
- Foster, C.A.; Church, K.S.; Poddar, M.; Van Uum, S.H.M.; Spaic, T. Licorice-Induced Hypertension: A Case of Pseudohyperaldosteronism Due to Jelly Bean Ingestion. Postgrad. Med. 2017, 129, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Mehrtash, B.; Siahpoosh, M.B.; Hajikarimi, M. Licorice and Arrhythmia: A Case Report. Integr. Med. 2017, 2, 78–82. [Google Scholar]
- Sayiner, Z.A.; Abiyev, A.; Eraydin, A.; Ozkaya, M. A Rare Cause of Thyrotoxic Periodic Paralysis: Liquorice Consumption. Postgrad. Med. J. 2017, 93, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Ross, C.N. Liquorice: A Root Cause of Secondary Hypertension. JRSM Open 2017, 8, 2054270416685208. [Google Scholar] [CrossRef]
- Liew, Z.H.; Lee, K.G. Liquorice-Induced Severe Hypokalaemic Rhabdomyolysis with Acute Kidney Injury. Ann. Acad. Med. Singap. 2017, 46, 354–355. [Google Scholar] [CrossRef]
- Gallacher, S.D.; Tsokolas, G.; Dimitropoulos, I. Liquorice-Induced Apparent Mineralocorticoid Excess Presenting in the Emergency Department. Clin. Med. 2017, 17, 43–45. [Google Scholar] [CrossRef]
- Li, J.; Fan, X.; Wang, Q. Hypertensive Crisis with 2 Target Organ Impairment Induced by Glycyrrhizin: A Case Report. Medicine 2018, 97, e0073. [Google Scholar] [CrossRef]
- Ramchandran, R.; Verma, S.; Dasgupta, R.; Thomas, N. Bitter Experience with Liquorice Sweetening Agent Resulting in Apparent Mineralocorticoid Excess with Periodic Paralysis. BMJ Case Rep. 2018, 2018, bcr-2018-225686. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Chung, M.; Rose, D.Z. Licorice Root Associated With Intracranial Hemorrhagic Stroke and Cerebral Microbleeds. Neurohospitalist 2019, 9, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Smedegaard, S.B.; Svart, M.V. Licorice Induced Pseudohyperaldosteronism, Severe Hypertension, and Long QT. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0109. [Google Scholar] [CrossRef] [PubMed]
- Falet, J.-P.; Elkrief, A.; Green, L. Hypertensive Emergency Induced by Licorice Tea. Can. Med. Assoc. J. 2019, 191, E581–E583. [Google Scholar] [CrossRef]
- Attou, R.; Redant, S.; Honore, P.M.; Preseau, T.; Hantson, P.; De Bels, D. Liquorice Intoxication Can Lead to Cardiac Arrest! Case Rep. Emerg. Med. 2020, 2020, 3727682. [Google Scholar] [CrossRef]
- Benge, E.; Shah, P.; Yamaguchi, L.; Josef, V. Trick or Treat? Licorice-Induced Hypokalemia: A Case Report. Cureus 2020, 12, e11656. [Google Scholar] [CrossRef]
- Edelman, E.R.; Butala, N.M.; Avery, L.L.; Lundquist, A.L.; Dighe, A.S. Case 30-2020: A 54-Year-Old Man with Sudden Cardiac Arrest. N. Engl. J. Med. 2020, 383, 1263–1275. [Google Scholar] [CrossRef]
- Kwon, Y.E.; Oh, D.-J.; Choi, H.M. Severe Asymptomatic Hypokalemia Associated with Prolonged Licorice Ingestion: A Case Report. Medicine 2020, 99, e21094. [Google Scholar] [CrossRef]
- Awad, N. Licorice-Induced Apparent Mineralocorticoid Excess Causing Persistent Hypertension and Hypokalemia. Acta Endocrinol. Buchar. 2020, 16, 508–510. [Google Scholar] [CrossRef]
- Petersen, T.; Fraissinet, F.; Ziegler, F.; Brunel, V. An Unusual Cause of Hypokalemia. Clin. Chem. 2020, 66, 1575–1576. [Google Scholar] [CrossRef]
- Jing, L.; Yun-Yin, Z.; Qi, Z. Pseudo-Hyperaldosteronism Caused by Compound Glycyrrhizin Tablets: A Case Report. Asian Toxicol. Res. 2020, 2, 81–84. [Google Scholar] [CrossRef]
- Patel, P.; Aknouk, M.; Dawson, A.; Aya, A.; Kanukuntla, A.; Kata, P.; De Dona, A. How Much Is Too Much? Exploring Pseudohyperaldosteronism in Glycyrrhizic Acid Toxicity From Chronic Licorice Root Consumption. Cureus 2021, 13, e16454. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J.; Nagabathula, R.; Kyithar, M.P. A Life-Threatening Case of Pseudo-Aldosteronism Secondary to Excessive Liquorice Ingestion. BMC Endocr. Disord. 2021, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Yoshinuma, H.; Sugiyama, D.; Kobayashi, O. Severe Hypokalemia and Metabolic Alkalosis Caused by Licorice Discovered during the Treatment of Intraoperative Hypoxemia. Cureus 2022, 14, e25432. [Google Scholar] [CrossRef] [PubMed]
- Bettini, L.; Parent, T.; Serratrice, J. Licorice Consumption Causing Severe Hypokalemia-Induced Paresis: A Case Report. Med. Case Rep. Study Protoc. 2022, 3, e0177. [Google Scholar] [CrossRef]
- Yoshida, C.; Yamamoto, H.; Inoue, T.; Itoh, M.; Shimane, A.; Kawai, H.; Takaya, T. Torsade de Pointes in an Older Patient with Takotsubo Cardiomyopathy Caused by Licorice-induced Pseudoaldosteronism: A Case Report. Clin. Case Rep. 2022, 10, e6104. [Google Scholar] [CrossRef]
- Blanpain, J.-S. A Licorice-Flavored Edema: A Case Report of Glycyrrhizic Acid Toxicity From Chronic Licorice Root Consumption. Cureus 2023, 15, e34425. [Google Scholar] [CrossRef]
- Han, E.J.; Park, J.-S. Lethal Arrhythmia Induced by Licorice. J. Korean Med. Sci. 2023, 38, e107. [Google Scholar] [CrossRef]
- Molina-Lopez, V.; Engel-Rodriguez, A.; Escabi-Mendoza, J. Close Call from a Sweet Twist: A Case of Licorice-Induced Torsades de Pointes. Cureus 2023, 15, e34126. [Google Scholar] [CrossRef]
- Ceccuzzi, G.; Rapino, A.; Perna, B.; De Giorgio, R.; Guarino, M. A Fistful of Candies for Hypertension. Intern. Emerg. Med. 2023. [Google Scholar] [CrossRef]
- Keys, J.D. Chinese Herbs; Tuttle Publishing: North Clarendon, VT, USA, 2011; ISBN 978-1-4629-0129-6. [Google Scholar]
- Thompson, R.C. Assyrian Prescriptions for Treating Bruises or Swellings. Am. J. Semit. Lang. Lit. 1930, 47, 1–25. [Google Scholar] [CrossRef]
- Hort, A. Theophrastus: Enquiry into Plants, and Minor Works on Odours and Weather Signs. Nature 1917, 99, 282. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A History of the Therapeutic Use of Liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, N.A.; Egamberdieva, D. Phytochemical Constituents and Pharmacological Effects of Licorice: A Review. In Plant and Human Health, Volume 3; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–21. ISBN 978-3-030-04407-7. [Google Scholar]
- Cosa, S.; Chaudhary, S.K.; Chen, W.; Combrinck, S.; Viljoen, A. Exploring Common Culinary Herbs and Spices as Potential Anti-Quorum Sensing Agents. Nutrients 2019, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, M.; Mehrabani, D.; Japoni, S.; Edjtehadi, M.; Saberi Firoozi, M. The Healing Effect of Licorice (Glycyrrhiza glabra) on Helicobacter Pylori Infected Peptic Ulcers. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 532–533. [Google Scholar]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Bisht, D.; Rashid, M.; Arya, R.K.K.; Kumar, D.; Chaudhary, S.K.; Rana, V.S.; Sethiya, N.K. Revisiting Liquorice (Glycyrrhiza glabra L.) as Anti-Inflammatory, Antivirals and Immunomodulators: Potential Pharmacological Applications with Mechanistic Insight. Phytomed. Plus 2022, 2, 100206. [Google Scholar] [CrossRef]
- Chigurupati, H.; Auddy, B.; Biyani, M.; Stohs, S.J. Hepatoprotective Effects of a Proprietary Glycyrrhizin Product during Alcohol Consumption: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study: Glycyrrhizin Hepatoprotection with Alcohol Consumption. Phytother. Res. 2016, 30, 1943–1953. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Belén, L.H.; Kaur, R.; Kregiel, D.; Uprety, Y.; Beyatli, A.; Yeskaliyeva, B.; Kırkın, C.; et al. Glycyrrhiza Genus: Enlightening Phytochemical Components for Pharmacological and Health-Promoting Abilities. Oxidative Med. Cell. Longev. 2021, 2021, 7571132. [Google Scholar] [CrossRef]
- Siracusa, L.; Saija, A.; Cristani, M.; Cimino, F.; D’Arrigo, M.; Trombetta, D.; Rao, F.; Ruberto, G. Phytocomplexes from Liquorice (Glycyrrhiza glabra L.) Leaves—Chemical Characterization and Evaluation of Their Antioxidant, Anti-Genotoxic and Anti-Inflammatory Activity. Fitoterapia 2011, 82, 546–556. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, C.; Qiao, X.; Ye, M. Chemical Analysis of the Chinese Herbal Medicine Licorice (Gan-Cao): An Update Review. J. Ethnopharmacol. 2022, 299, 115686. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Brockhoff, A.; Shoshan-Galeczki, Y.B.; Kranz, M.; Stark, T.D.; Erkaya, R.; Meyerhof, W.; Niv, M.Y.; Dawid, C.; Hofmann, T. Comprehensive Structure-Activity-Relationship Studies of Sensory Active Compounds in Licorice (Glycyrrhiza glabra). Food Chem. 2021, 364, 130420. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Liu, P.; Cheng, G.; Sun, M. Glycyrrhizic Acid in the Treatment of Liver Diseases: Literature Review. BioMed Res. Int. 2014, 2014, 872139. [Google Scholar] [CrossRef] [PubMed]
- Graebin, C.S. The Pharmacological Activities of Glycyrrhizinic Acid (“Glycyrrhizin”) and Glycyrrhetinic Acid. Sweeteners 2017, 245–261. [Google Scholar] [CrossRef]
- Ihlenfeldt, W.D.; Bolton, E.E.; Bryant, S.H. The PubChem Chemical Structure Sketcher. J. Cheminform. 2009, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, B.; Mensinga, T.; Sips, A.; Seinen, W.; Meulenbelt, J.; DeJongh, J. The Pharmacokinetics of Glycyrrhizic Acid Evaluated by Physiologically Based Pharmacokinetic Modeling. Drug Metab. Rev. 2001, 33, 125–147. [Google Scholar] [CrossRef]
- Akao, T.; Akao, T.; Hattori, M.; Namba, T.; Kobashi, K. Enzymes Involved in the Formation of 3 Beta, 7 Beta-Dihydroxy-12-Oxo-5 Beta-Cholanic Acid from Dehydrocholic Acid by Ruminococcus Sp. Obtained from Human Intestine. Biochim. Biophys. Acta 1987, 921, 275–280. [Google Scholar]
- Hattori, M.; Sakamoto, T.; Yamagishi, T.; Sakamoto, K.; Konishi, K.; Kobashi, K.; Namba, T. Metabolism of Glycyrrhizin by Human Intestinal Flora. II. Isolation and Characterization of Human Intestinal Bacteria Capable of Metabolizing Glycyrrhizin and Related Compounds. Chem. Pharm. Bull. 1985, 33, 210–217. [Google Scholar] [CrossRef]
- Yamamura, Y.; Kawakami, J.; Santa, T.; Kotaki, H.; Uchino, K.; Sawada, Y.; Tanaka, N.; Iga, T. Pharmacokinetic Profile of Glycyrrhizin in Healthy Volunteers by a New High-Performance Liquid Chromatographic Method. J. Pharm. Sci. 1992, 81, 1042–1046. [Google Scholar] [CrossRef]
- Ploeger, B.; Mensinga, T.; Meulenbelt, J.; DeJongh, J. A Human Physiologically-Based Model for Glycyrrhzic Acid, A Compound Subject to Presystemic Metabolism and Enterohepatic Cycling. Pharm. Res. 2000, 17, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Deutch, M.R.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. Bioactive Candy: Effects of Licorice on the Cardiovascular System. Foods 2019, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.H.; Menouar, M.A.; Dunn, R.J. Physiology, Aldosterone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Latif, S.A.; Conca, T.J.; Morris, D.J. The Effects of the Licorice Derivative, Glycyrrhetinic Acid, on Hepatic 3 Alpha- and 3 Beta-Hydroxysteroid Dehydrogenases and 5 Alpha- and 5 Beta-Reductase Pathways of Metabolism of Aldosterone in Male Rats. Steroids 1990, 55, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mattarello, M.J.; Benedini, S.; Fiore, C.; Camozzi, V.; Sartorato, P.; Luisetto, G.; Armanini, D. Effect of Licorice on PTH Levels in Healthy Women. Steroids 2006, 71, 403–408. [Google Scholar] [CrossRef]
- Al-Snafi, D.A.E. Glycyrrhiza Glabra: A Phytochemical and Pharmacological Review. IOSR J. Pharm. 2018, 8, 1–17. [Google Scholar]
- Sigurjónsdóttir, H.A.; Franzson, L.; Manhem, K.; Ragnarsson, J.; Sigurdsson, G.; Wallerstedt, S. Liquorice-Induced Rise in Blood Pressure: A Linear Dose-Response Relationship. J. Hum. Hypertens. 2001, 15, 549–552. [Google Scholar] [CrossRef]
- Penninkilampi, R.; Eslick, E.M.; Eslick, G.D. The Association between Consistent Licorice Ingestion, Hypertension and Hypokalaemia: A Systematic Review and Meta-Analysis. J. Hum. Hypertens. 2017, 31, 699–707. [Google Scholar] [CrossRef]
- Sigurjonsdottir, H.A.; Axelson, M.; Johannsson, G.; Manhem, K.; Nyström, E.; Wallerstedt, S. The Liquorice Effect on the RAAS Differs between the Genders. Blood Press. 2006, 15, 169–172. [Google Scholar] [CrossRef]
- Pharmacokinetic Profiles of Glycyrrhizin in Patients with Chronic Hepatitis—Tanaka—1993—Biopharmaceutics & Drug Disposition—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/bdd.2510140707 (accessed on 19 July 2023).
- Robinson, H.J.; Harrison, F.S.; Nicholson, J.T. Cardiac Abnormalities Due to Licorice Intoxication. Pa. Med. 1971, 74, 51–54. [Google Scholar]
- Fardella, C.E.; Mosso, L.; Gómez-Sánchez, C.; Cortés, P.; Soto, J.; Gómez, L.; Pinto, M.; Huete, A.; Oestreicher, E.; Foradori, A.; et al. Primary Hyperaldosteronism in Essential Hypertensives: Prevalence, Biochemical Profile, and Molecular Biology. J. Clin. Endocrinol. Metab. 2000, 85, 1863–1867. [Google Scholar] [CrossRef]
- Rossi, G.P. Primary Aldosteronism: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 2799–2811. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.; Muppidi, V.; Gupta, S. Hyperaldosteronism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rizzolo, K.; Beck, N.M.; Ambruso, S.L. Syndromes of Pseudo-Hyperaldosteronism. Clin. J. Am. Soc. Nephrol. CJASN 2022, 17, 581–584. [Google Scholar] [CrossRef] [PubMed]
| Sex | Age | Electrolyte Abnormalities | ECG Alterations | Clinical Presentation | Aldosterone (pmol/L) | Cortisol (nmol/L) | Serum GA Levels (ng/mL) | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyper-natremia | Hypokalemia | Metabolic Alkalosis | Muscle Disorders | Neurological | MACEs | GI Disorders | Hypertension | Hospital Admission | Death | |||||||
| Gross et al., 1966 [7] | F | 45 | NO | YES | YES | YES | YES | NO | NO | NO | NO | N/A | N/A | N/A | YES | NO |
| Conn et al., 1968 [8] | M | 58 | YES | YES | YES | N/A | N/A | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Tourtelotte et al., 1970 [9] | F | 63 | NO | YES | N/A | YES | YES | NO | NO | YES | YES | N/A | N/A | N/A | YES | NO |
| Holmes et al., 1970 [10] | M | 63 | YES | YES | N/A | YES | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Bannister et al., 1977 [11] | F | 58 | NO | YES | N/A | YES | YES | NO | YES | NO | YES | N/A | N/A | N/A | YES | NO |
| Cumming et al., 1980 [12] | F | 70 | NO | YES | NO | N/A | YES | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Nightingale et al., 1981 [13] | F | 25 | NO | YES | YES | YES | YES | NO | NO | YES | NO | N/A | N/A | N/A | YES | NO |
| Sundaram et al., 1981 [14] | F | 33 | NO | YES | N/A | NO | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Cibelli et al., 1984 [15] | M | 48 | NO | YES | N/A | YES | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Nielsen et al., 1984 [16] | F | 20 | NO | YES | YES | YES | YES | NO | NO | NO | NO | N/A | N/A | N/A | YES | NO |
| Farese et al., 1991 [17] | M | 70 | NO | YES | N/A | N/A | YES | NO | NO | NO | NO | N/A | N/A | N/A | YES | NO |
| Kageyama 1992 [18] | M | 54 | NO | YES | N/A | N/A | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Van Der Zwan 1993 [19] | M | 15 | NO | NO | NO | YES | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Heikens et al., 1995 [20] | F | 40 | YES | YES | YES | N/A | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Barella et al., 1997 [21] | M | 61 | N/A | YES | N/A | YES | YES | NO | NO | NO | YES | 95 | N/A | N/A | YES | NO |
| Chamberlain et al., 1997 [22] | M | 64 | NO | YES | N/A | NO | NO | NO | NO | NO | N/A | N/A | N/A | N/A | YES | NO |
| De Klerk et al., 1997 [23] | F | 21 | NO | YES | N/A | N/A | NO | NO | NO | NO | YES | 160 | N/A | N/A | NO | NO |
| De Klerk et al., 1997 [23] | F | 35 | NO | YES | N/A | N/A | NO | NO | NO | NO | YES | 80 | N/A | N/A | NO | NO |
| Hasegawa et al., 1998 [24] | M | 65 | NO | YES | YES | YES | YES | NO | YES | NO | NO | <25 | N/A | N/A | YES | NO |
| Erikson et al., 1999 [25] | F | 44 | NO | YES | N/A | YES | YES | NO | NO | NO | YES | 4200 | N/A | N/A | NO | NO |
| Russo et al., 1999 [26] | M | 42 | NO | YES | N/A | N/A | NO | YES | NO | NO | YES | 180 | N/A | N/A | YES | NO |
| Russo et al., 1999 [26] | M | 46 | NO | YES | YES | YES | NO | YES | NO | NO | YES | N/A | N/A | N/A | NO | NO |
| Lozano et al., 2000 [27] | F | 34 | NO | YES | N/A | YES | NO | NO | NO | NO | NO | 68 | 14.5 | N/A | YES | NO |
| Dobbins et al., 2000 [28] | M | 62 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | NO | NO |
| Dobbins et al., 2001 [28] | M | 76 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | NO | NO |
| Dobbins et al., 2002 [28] | F | 26 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | NO | NO |
| Dobbins et al., 2003 [28] | M | 65 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | NO | NO |
| Dobbins et al., 2004 [28] | M | 39 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | NO | NO |
| Elinav et al., 2003 [29] | M | 36 | N/A | YES | N/A | YES | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Lin S. et al., 2003 [30] | M | 76 | YES | YES | YES | N/A | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Hall et al., 2004 [31] | F | 62 | N/A | N/A | N/A | N/A | NO | NO | NO | NO | YES | N/A | N/A | N/A | NO | NO |
| Janse et al., 2005 [32] | F | 85 | NO | YES | N/A | N/A | NO | YES | NO | NO | YES | 30 | N/A | N/A | YES | NO |
| Van Den Bosch et al., 2005 [33] | M | 59 | YES | YES | YES | N/A | YES | YES | NO | NO | YES | 1.48 | N/A | 257 | YES | NO |
| Breidthardt et al., 2006 [34] | N/A | 67 | NO | YES | N/A | N/A | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Hamidon et al., 2006 [35] | M | 31 | YES | NO | YES | N/A | YES | YES | NO | NO | YES | N/A | 420 | N/A | YES | NO |
| Yasue et al., 2007 [36] | F | 93 | NO | YES | YES | YES | YES | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Francini-Pesenti et al., 2008 [37] | F | 39 | NO | YES | N/A | N/A | YES | YES | NO | YES | NO | 15.08 | N/A | N/A | YES | NO |
| Mumoli et al., 2008 [38] | M | 55 | N/A | YES | YES | YES | YES | NO | NO | NO | NO | 60 | N/A | N/A | YES | NO |
| Sontia et al., 2008 [39] | F | 55 | YES | YES | N/A | N/A | NO | NO | NO | NO | YES | 31 | N/A | N/A | NO | NO |
| Tancevsky et al., 2008 [40] | F | 54 | N/A | YES | N/A | YES | NO | YES | NO | NO | N/A | N/A | N/A | N/A | YES | NO |
| Meltem et al., 2009 [41] | M | 21 | NO | YES | NO | NO | YES | NO | NO | NO | NO | N/A | N/A | N/A | YES | NO |
| Crean et al., 2009 [42] | F | 71 | NO | YES | N/A | YES | NO | NO | YES | NO | NO | N/A | N/A | N/A | YES | NO |
| Johns 2009 [43] | F | 49 | NO | NO | NO | N/A | YES | NO | YES | NO | NO | N/A | N/A | N/A | NO | NO |
| Murphy et al., 2009 [44] | F | 64 | NO | YES | N/A | NO | NO | NO | NO | NO | YES | 60 | N/A | N/A | NO | NO |
| Yorgun et al., 2010 [45] | F | 50 | NO | YES | NO | YES | YES | YES | YES | NO | YES | N/A | N/A | N/A | YES | NO |
| Katchanov et al., 2010 [46] | M | 70 | NO | YES | YES | N/A | YES | NO | NO | NO | YES | 75 | N/A | N/A | YES | NO |
| Velickovic-Radovanovic et al., 2010 [47] | F | 39 | NO | YES | NO | YES | YES | NO | NO | YES | YES | 14.28 | N/A | N/A | YES | NO |
| Støving et al., 2010 [48] | F | 18 | NO | YES | NO | NO | NO | NO | NO | NO | NO | <25 | N/A | N/A | YES | NO |
| Kasap et al., 2010 [49] | M | 16 | NO | YES | YES | YES | YES | NO | NO | YES | NO | 1.50 | N/A | N/A | YES | NO |
| Imtiaz 2010 [50] | M | 49 | NO | YES | N/A | NO | NO | NO | NO | NO | YES | <70 | 584 | N/A | YES | NO |
| van Beers et al., 2011 [51] | M | 49 | NO | YES | YES | YES | NO | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Celik 2012 [52] | M | 45 | N/A | YES | YES | N/A | NO | NO | NO | NO | NO | 70 | N/A | N/A | NO | NO |
| van Noord et al., 2012 [53] | M | 62 | N/A | YES | NO | NO | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Kormann et al., 2012 [54] | F | 70 | N/A | YES | N/A | YES | NO | NO | YES | NO | N/A | N/A | N/A | 10000 | YES | NO |
| Ruiz-Granados et al. [55] | F | 51 | NO | YES | YES | NO | NO | NO | NO | NO | YES | 113 | N/A | N/A | YES | NO |
| Dehours et al., 2013 [56] | M | 35 | N/A | N/A | N/A | YES | NO | NO | NO | NO | NO | N/A | N/A | N/A | NO | NO |
| Flores-Robles et al., 2013 [57] | F | 47 | NO | YES | NO | YES | NO | NO | NO | NO | YES | 40 | N/A | N/A | YES | NO |
| Khan et al., 2013 [58] | F | 69 | YES | YES | YES | N/A | NO | NO | NO | NO | YES | 70 | N/A | N/A | NO | NO |
| Panduranga et al., 2013 [59] | F | 38 | NO | YES | YES | YES | NO | NO | YES | YES | NO | N/A | N/A | N/A | YES | NO |
| Kronborg-white 2013 [60] | M | 60 | NO | YES | YES | N/A | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Horwitz et al., 2014 [61] | M | 65 | N/A | YES | YES | N/A | YES | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Cortini E. et al., 2014 [62] | F | 55 | NO | YES | NO | YES | NO | YES | NO | YES | YES | N/A | N/A | 63 | YES | NO |
| De Putter et al., 2014 [63] | M | 52 | NO | YES | YES | N/A | YES | NO | NO | NO | YES | 28 | 535 | N/A | YES | NO |
| Main et al., 2015 [64] | N/A | 21 | YES | YES | N/A | N/A | YES | NO | NO | NO | NO | 38 | N/A | N/A | NO | NO |
| koku et al., 2015 [65] | M | 57 | NO | YES | N/A | YES | NO | NO | NO | NO | NO | 30.88 | N/A | N/A | YES | NO |
| Danis et al., 2015 [66] | M | 49 | NO | YES | YES | N/A | YES | NO | NO | NO | NO | N/A | N/A | N/A | YES | NO |
| Machalke et al., 2015 [67] | M | 57 | NO | YES | N/A | N/A | NO | NO | NO | NO | YES | 11,000 | 4.25 | N/A | YES | NO |
| Dai et al., 2015 [68] | M | 66 | n/a | YES | YES | N/A | NO | NO | NO | NO | YES | 12.87 | N/A | N/A | YES | NO |
| Hataya et al., 2015 [69] | M | 81 | NO | YES | YES | NO | YES | NO | NO | NO | YES | 61.8 | 357 | N/A | YES | NO |
| Allcock et al., 2015 [70] | F | 45 | NO | N/A | N/A | NO | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Caravaca-Fontan et al., 2015 [71] | M | 15 | N/A | YES | YES | YES | YES | NO | NO | YES | NO | 43.8 | 236 | N/A | YES | NO |
| Tassinari et al., 2015 [72] | M | 10 | NO | NO | N/A | N/A | NO | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| O’Connell et al., 2016 [73] | F | 56 | N/A | YES | N/A | N/A | NO | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Erkus et al., 2016 [74] | M | 57 | N/A | YES | N/A | YES | NO | NO | NO | NO | NO | 171.36 | N/A | N/A | YES | NO |
| Foster et al., 2017 [75] | F | 48 | NO | YES | N/A | NO | NO | NO | NO | NO | YES | <100 | N/A | N/A | YES | NO |
| Foster et al., 2017 [75] | M | 51 | YES | YES | N/A | N/A | YES | NO | NO | YES | YES | 83 | N/A | N/A | YES | NO |
| Mehrtash et al., 2017 [76] | M | 22 | NO | YES | N/A | YES | NO | NO | NO | NO | NO | N/A | N/A | N/A | NO | NO |
| Sayiner et al., 2017 [77] | M | 43 | NO | YES | YES | N/A | YES | YES | NO | NO | NO | N/A | N/A | N/A | YES | NO |
| Varma et al., 2017 [78] | F | 70 | NO | YES | N/A | NO | NO | NO | NO | NO | YES | N/A | N/A | N/A | NO | NO |
| Zhong et al., 2017 [79] | M | 71 | NO | YES | N/A | N/A | YES | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Gallacher et al., 2017 [80] | M | 65 | N/A | YES | N/A | NO | YES | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Jing et al., 2018 [81] | F | 47 | N/A | YES | N/A | N/A | YES | NO | NO | NO | YES | N/A | N/A | N/A | NO | NO |
| Ramchandran et al., 2018 [82] | M | 45 | YES | YES | YES | NO | YES | NO | NO | NO | YES | 43.88 | 441.44 | N/A | YES | NO |
| Shin et al., 2019 [83] | F | 68 | N/A | N/A | N/A | N/A | N/A | YES | N/A | N/A | YES | N/A | N/A | N/A | YES | NO |
| Smedegaard et al., 2019 [84] | F | 43 | NO | YES | N/A | YES | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Falet et al., 2019 [85] | M | 84 | N/A | YES | N/A | NO | NO | NO | NO | NO | YES | 71 | N/A | N/A | YES | NO |
| Attou et al., 2020 [86] | M | 45 | NO | YES | NO | YES | YES | NO | NO | NO | YES | 106 | N/A | N/A | YES | NO |
| Attou et al., 2020 [86] | F | 30 | NO | YES | YES | N/A | YES | YES | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Benge et al., 2020 [87] | F | 74 | NO | YES | N/A | NO | NO | YES | NO | YES | YES | 12.87 | N/A | N/A | YES | NO |
| Edelman et al., 2020 [88] | M | 54 | YES | NO | NO | YES | YES | YES | YES | NO | YES | 51.48 | N/A | N/A | YES | YES |
| Kwon et al., 2020 [89] | M | 79 | NO | YES | YES | YES | NO | NO | NO | NO | YES | 24.58 | N/A | N/A | YES | NO |
| Awad et al., 2020 [90] | M | 62 | NO | YES | NO | N/A | NO | NO | NO | NO | YES | 12.87 | N/A | N/A | YES | NO |
| Petersen et al., 2020 [91] | F | 77 | YES | YES | N/A | YES | NO | NO | NO | NO | YES | 39 | N/A | N/A | YES | NO |
| Jing et al., 2020 [92] | M | 59 | YES | YES | NO | N/A | NO | NO | NO | NO | YES | N/A | N/A | N/A | NO | NO |
| Patel et al., 2021 [93] | M | 61 | NO | YES | N/A | YES | YES | NO | NO | YES | YES | 38.61 | 606.98 | N/A | YES | NO |
| McHugh J. et al., 2021 [94] | F | 79 | NO | YES | YES | YES | YES | NO | YES | NO | YES | 2600 | N/A | N/A | YES | NO |
| Khan et al., 2022 [58] | M | 56 | NO | YES | YES | N/A | YES | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Shibata et al., 2022 [95] | F | 87 | NO | YES | YES | YES | NO | NO | NO | NO | YES | 92.5 | N/A | N/A | YES | NO |
| Bettini et al., 2022 [96] | F | 84 | YES | YES | N/A | YES | YES | YES | NO | NO | YES | 66.07 | N/A | N/A | YES | NO |
| Yoshida et al., 2022 [97] | F | 84 | NO | YES | YES | YES | YES | NO | YES | NO | YES | N/A | N/A | N/A | YES | YES |
| Blanpain 2023 [98] | M | 49 | N/A | YES | N/A | NO | NO | NO | NO | NO | YES | N/A | N/A | N/A | YES | NO |
| Han et al., 2023 [99] | F | 89 | N/A | YES | N/A | YES | N/A | N/A | YES | NO | N/A | 706 | N/A | N/A | YES | NO |
| Molina-Lopez et al., 2023 [100] | M | 95 | YES | YES | N/A | YES | NO | NO | YES | YES | YES | 12.87 | N/A | N/A | YES | NO |
| Ceccuzzi et al., 2023 [101] | F | 38 | NO | YES | YES | YES | YES | YES | NO | YES | YES | 24.45 | 817.26 | 115 | YES | NO |
| System | Clinical Manifestations |
|---|---|
| Cardiovascular disorder | Hypertension |
| Cardiac arrest | |
| Heart failure and pulmonary edema | |
| Cardiac arrhythmias and death due to QT prolongation | |
| Hypertensive encephalopathy | |
| Embolic ischemia | |
| Hypertensive retinopathy | |
| Neurological disorders | Liquorice induced myoclonus |
| Cerebral microhemorrhages | |
| Paralysis | |
| Visual impairment due to retinic and occipital vasospasm | |
| Epileptiform manifestations | |
| Glasgow Coma Scale < 15 | |
| Electrolyte and renal abnormalities | Hypokalemia |
| Hypernatremia | |
| Metabolic alkalosis | |
| Acute kidney injury | |
| Muscular disorders | Spasm |
| Rhabdomyolysis | |
| Elevated creatinine phospho-kinase | |
| Gastrointestinal disorders | Abdominal tenderness |
| Nausea | |
| Vomiting | |
| Diarrhoea |
| HYPERALDOSTERONISM |
|---|
| Low Renin (Angiotensin II Independent) |
High Aldosterone
|
Low Aldosterone
|
| High Renin (Angiotensin II dependent) |
Secondary Hyperaldosteronism
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccuzzi, G.; Rapino, A.; Perna, B.; Costanzini, A.; Farinelli, A.; Fiorica, I.; Marziani, B.; Cianci, A.; Rossin, F.; Cesaro, A.E.; et al. Liquorice Toxicity: A Comprehensive Narrative Review. Nutrients 2023, 15, 3866. https://doi.org/10.3390/nu15183866
Ceccuzzi G, Rapino A, Perna B, Costanzini A, Farinelli A, Fiorica I, Marziani B, Cianci A, Rossin F, Cesaro AE, et al. Liquorice Toxicity: A Comprehensive Narrative Review. Nutrients. 2023; 15(18):3866. https://doi.org/10.3390/nu15183866
Chicago/Turabian StyleCeccuzzi, Giovanna, Alessandro Rapino, Benedetta Perna, Anna Costanzini, Andrea Farinelli, Ilaria Fiorica, Beatrice Marziani, Antonella Cianci, Federica Rossin, Alice Eleonora Cesaro, and et al. 2023. "Liquorice Toxicity: A Comprehensive Narrative Review" Nutrients 15, no. 18: 3866. https://doi.org/10.3390/nu15183866
APA StyleCeccuzzi, G., Rapino, A., Perna, B., Costanzini, A., Farinelli, A., Fiorica, I., Marziani, B., Cianci, A., Rossin, F., Cesaro, A. E., Spampinato, M. D., De Giorgio, R., & Guarino, M. (2023). Liquorice Toxicity: A Comprehensive Narrative Review. Nutrients, 15(18), 3866. https://doi.org/10.3390/nu15183866









