Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review
Abstract
1. Introduction
1.1. Systematic Review Process
1.2. Protocol and Manuscript Selection
1.3. Search, Data Abstraction, Synthesis, and Scope
2. Vitamin D—Innate and Acquired Immunity
2.1. Vitamin D Activates Immune Cells
2.2. Modes of Stimulating the Immune System by Vitamin D
2.3. Vitamin D and Immune System
2.4. Vitamin D Is Fundamental to the Defense against Microbes and Preventing Autoimmunity
3. Mechanisms of How Vitamin D Controls Infections and Autoimmunity
3.1. The Importance of Intracellular Generation of Calcitriol for Immune Cell Signaling
3.2. Intracellular Calcitriol Signaling
3.3. The Importance of Autocrine and Paracrine Signaling for Immune Cell Functions
3.4. Mechanisms of Decreasing Inflammation with Vitamin D Adequacy
3.5. Anti-Microbial Activities of Vitamin D
3.6. Vitamin D Enhances the Expression of Bactericidal Proteins
3.7. Multiple Sclerosis and Autoimmune Encephalomyelitis
3.8. Autoimmunity—Rheumatoid Arthritis and Lupus
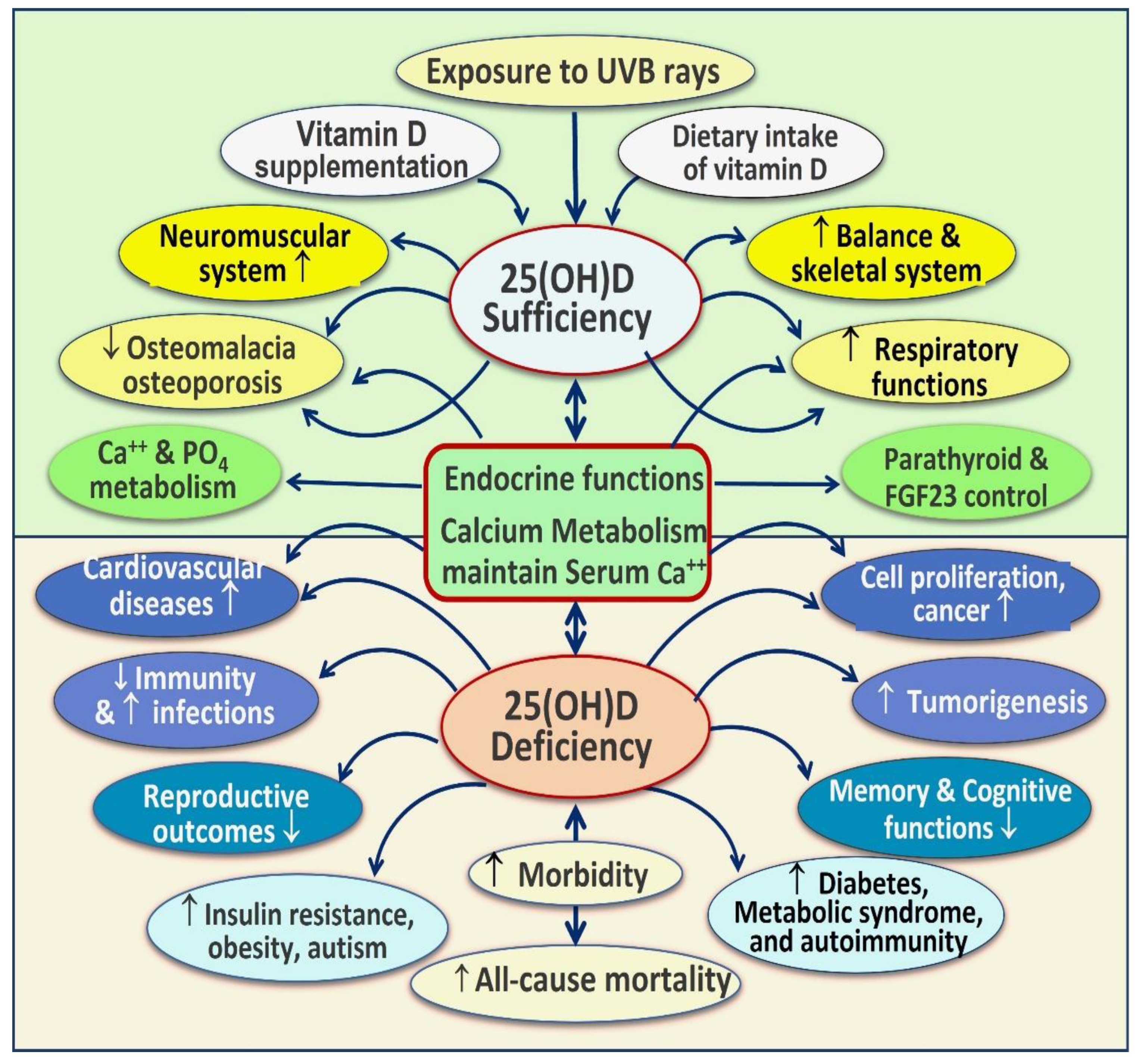
4. Improving Clinical Outcomes via Vitamin D Sufficiency
4.1. Importance of cofactors and micronutrients for the full functions of vitamin D
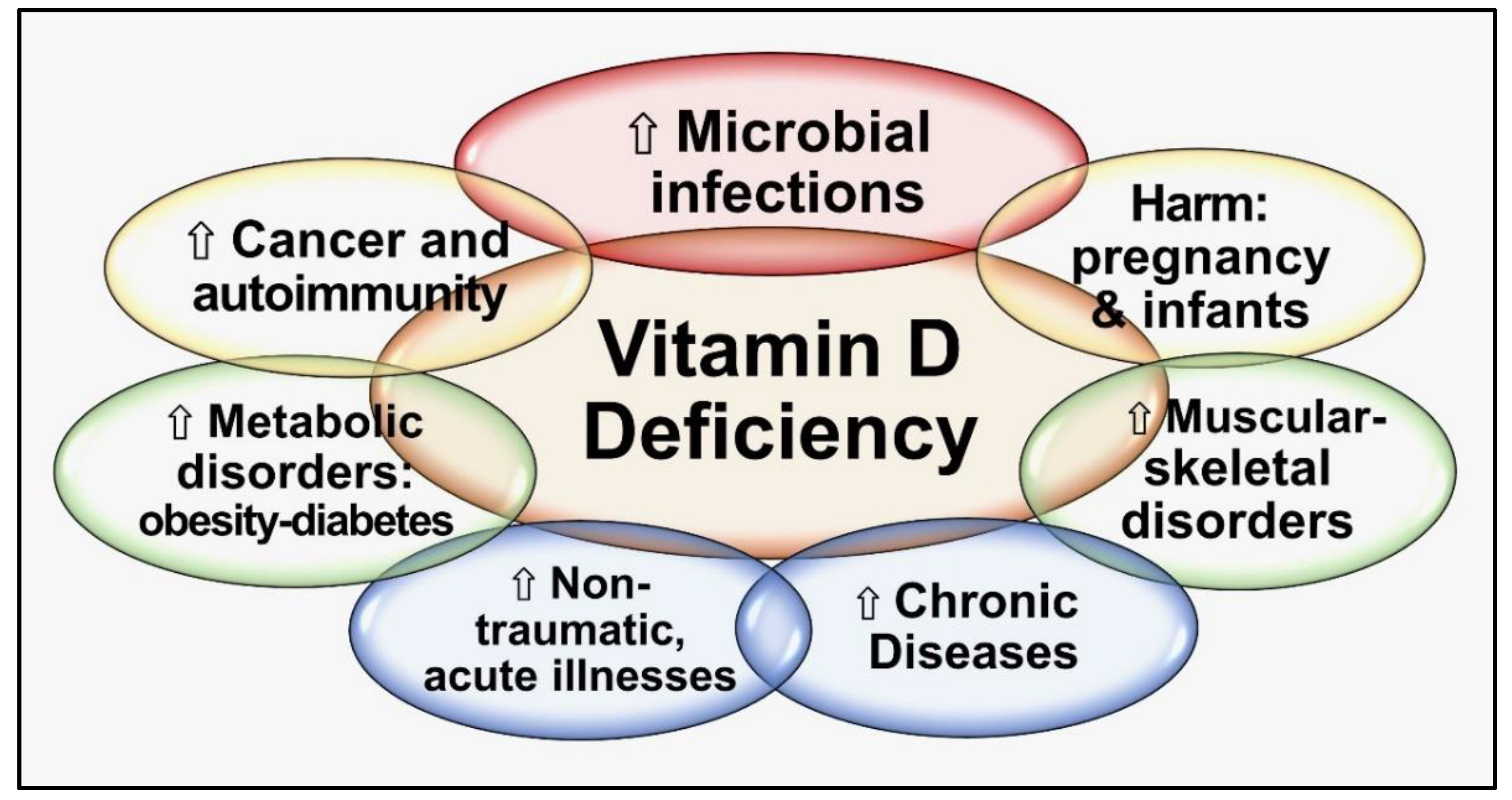
4.2. Consequences of Hypovitaminosis D
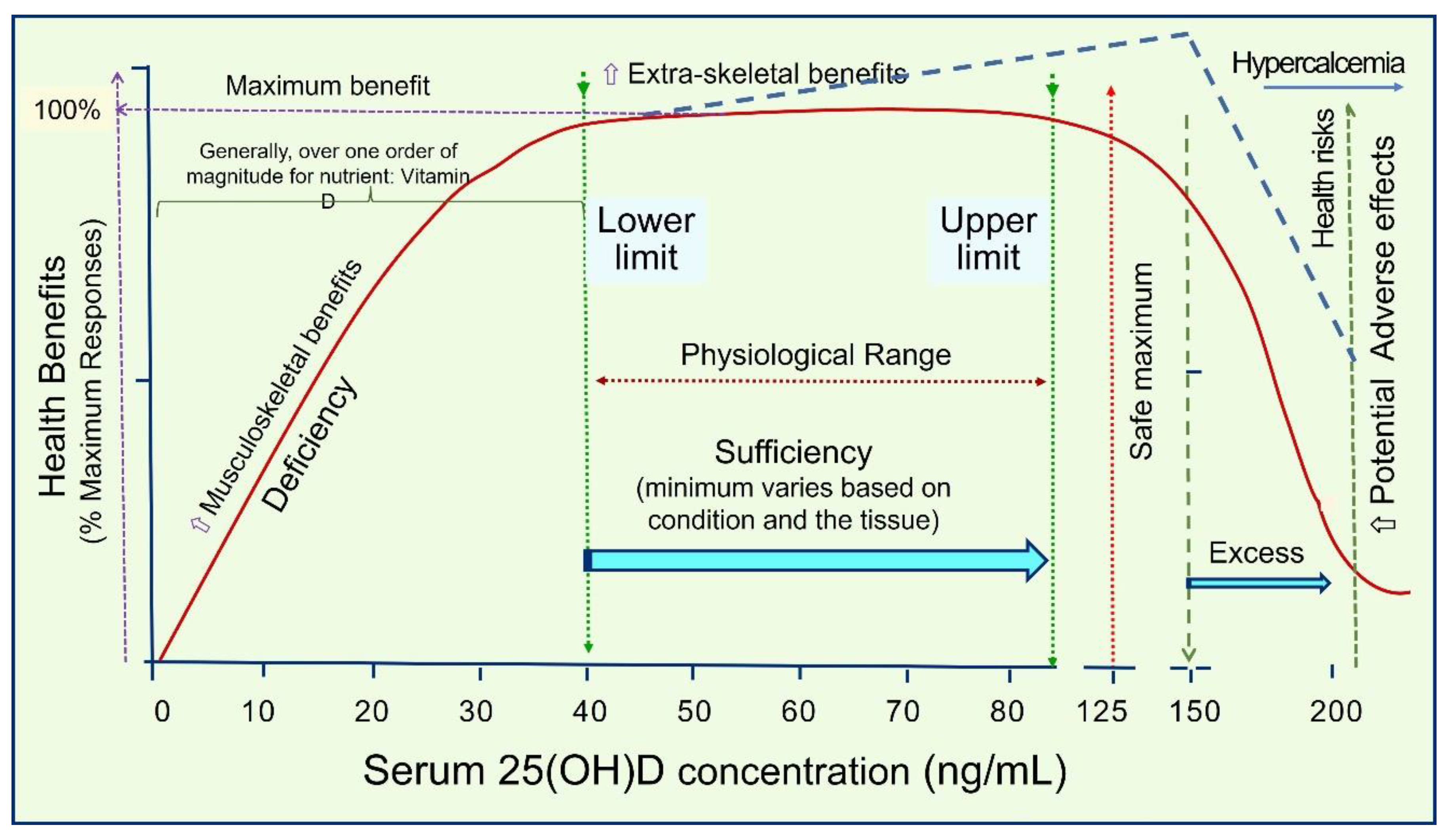
4.3. One Serum 25(OH)D Concentration Would Not Control All Diseases
4.4. Minimum and the Range of Serum 25(OH)D (ng/mL) Necessary to Minimize Diseases and Obtain Maximum Benefits
4.5. Vitamin D Intakes and Optimum Circulating 25(OH)D Concentrations Needed to Overcome Diseases
4.6. Vitamin D Dose-Responses
5. Other Considerations with Vitamin D
5.1. Adverse Effects of Vitamin D Are Rare
5.2. Personal Vitamin D Response and Targeted Serum 25(OH)D Concentrations
5.3. Vitamin D Is a Threshold Nutrient
5.4. Vitamin D Deficiency Increases Vulnerability to SARS-CoV-2 Infections
5.5. Issues with Published RCTs and Limitations of Data and Interpretation
5.6. New Vitamin D Recommendations
- I.
- Not obese (average wt.: BMI, <29): 70–90 IU/kg BW
- II.
- Moderately obese (BMI, 30–39): 100–130 IU/kg BW
- III.
- Morbid obesity (BMI, over 40): 140–180 IU/kg BW
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1:25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxy vitamin D |
| BMI | Body mass index |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| D3 | Cholecalciferol |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PCR | Polymerase chain reaction |
| PTH | Parathyroid hormone |
| RAS | Renin–angiotensin system |
| RCTs | Randomized controlled clinical trials |
| ROS | Reactive oxygen species |
| SR | Systematic Review |
| T1D | Type 1 diabetes mellitus |
| T2D | Type 2 diabetes mellitus |
| UVB | Ultraviolet B |
| VDR/CTR | Vitamin D (Calcitriol) receptor |
| VDBP | Vitamin D binding protein |
| VDR | Vitamin D receptor |
References
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Commonsense Approaches to Minimizing Risks from COVID-19. Open J. Pulmonol. Respir. Med. 2020, 2, 28–37. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Reducing Risks from COVID-19: Cost-Effective Ways of Strengthening Individual’s and the Population Immunity with Vitamin D. J. Endocrinol. Sci. 2020, 2, 5–13. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D in the New Millennium. Curr. Osteoporos. Rep. 2012, 10, 4–15. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F. Vitamin D supplements and reasonable solar UVB should be recommended to prevent escalating incidence of chronic diseases. BMJ 2015, 350, h321. [Google Scholar] [CrossRef]
- Holick, M.F. High Prevalence of Vitamin D Inadequacy and Implications for Health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef]
- Haq, A.; Wimalawansa, S.J.; Carlberg, C. Highlights from the 5th International Conference on Vitamin D Deficiency, Nutrition and Human Health, Abu Dhabi, United Arab Emirates, March 24–25, 2016. J. Steroid Biochem. Mol. Biol. 2018, 175, 1–3. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2017, 175, 125–135. [Google Scholar] [CrossRef]
- Ganmaa, D.; Enkhmaa, D.; Nasantogtokh, E.; Sukhbaatar, S.; Tumur-Ochir, K.; Manson, J. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern. Med. 2021, 291, 141–164. [Google Scholar] [CrossRef]
- Vintilescu, B.; E Niculescu, C.; Stepan, M.D.; Ioniță, E. Involvement of Vitamin D in Chronic Infections of the Waldeyer`s Ring in the School Aged Child. Curr. Health Sci. J. 2019, 45, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, A.B.; Kupczak, M.; Konecki, T. Does Vitamin Supplementation Play a Role in Chronic Kidney Disease? Nutrients 2023, 15, 2847. [Google Scholar] [CrossRef]
- Özdemir, B.; Köksal, B.T.; Karakaş, N.M.; Tekindal, M.A.; Özbek, Y. Serum Vitamin D Levels in Children with Recurrent Respiratory Infections and Chronic Cough. Indian. J. Pediatr. 2016, 83, 777–782. [Google Scholar] [CrossRef]
- Pérez-López, F.R. Vitamin D: The secosteroid hormone and human reproduction. Gynecol. Endocrinol. 2007, 23, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Razzaque, M.S.; Al-Daghri, N.M. Calcium and vitamin D in human health: Hype or real? J. Steroid Biochem. Mol. Biol. 2018, 180, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Boland, R.; de Boland, A.R.; Marinissen, M.J.; Santillan, G.; Vazquez, G.; Zanello, S. Avian muscle cells as targets for the secosteroid hormone 1,25-dihydroxy-vitamin D3. Mol. Cell. Endocrinol. 1995, 114, 1–8. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Welch, V.; Petticrew, M.; Tugwell, P.; Moher, D.; O’Neill, J.; Waters, E.; White, H. The PRISMA-Equity Bellagio group PRISMA-Equity 2012 Extension: Reporting Guidelines for Systematic Reviews with a Focus on Health Equity. PLOS Med. 2012, 9, e1001333. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Grant, W.B. The Institute of Medicine did not find the vitamin D–cancer link because it ignored UV-B dose studies. Public. Health Nutr. 2011, 14, 745–746. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Overcoming Infections Including COVID-19, by Maintaining Circulating 25(OH)D Concentrations Above 50 ng/mL. Pathol. Lab. Med. Int. 2022, ume 14, 37–60. [Google Scholar] [CrossRef]
- Grant, W.B. Vitamin D Acceptance Delayed by Big Pharma Following the Disinformation Playbook. Available online: http://orthomolecular.org/resources/omns/v14n22.shtml (accessed on 22 March 2022).
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Cancela, L.; Nemere, I.; Norman, A.W. 1α,25 (OH)2 vitamin D3: A steroid hormone capable of producing pleiotropic receptor-mediated biological responses by both genomic and nongenomic mechanisms. J. Steroid Biochem. 1988, 30, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know; Karunaratne & Sons: Homagama, Sri Lanka, 2012; Volume 1.0, ISBN 978-955-9098-94-2. [Google Scholar]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef]
- Gotelli, E.; Soldano, S.; Hysa, E.; Paolino, S.; Campitiello, R.; Pizzorni, C.; Sulli, A.; Smith, V.; Cutolo, M. Vitamin D and COVID-19: Narrative Review after 3 Years of Pandemic. Nutrients 2022, 14, 4907. [Google Scholar] [CrossRef]
- Hanel, A.; Bendik, I.; Carlberg, C. Transcriptome-Wide Profile of 25-Hydroxyvitamin D3 in Primary Immune Cells from Human Peripheral Blood. Nutrients 2021, 13, 4100. [Google Scholar] [CrossRef]
- Aygun, H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020, 393, 1157–1160. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- McGregor, E.; Kazemian, M.; Afzali, B.; Freiwald, T.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Zhang, Z.; Teague, H.; West, E.E.; et al. An autocrine Vitamin D-driven Th1 shutdown program can be exploited for COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Biology of vitamin D. J. Steroids Horm. Sci. 2019, 10, 198. [Google Scholar]
- Grant, W.B. Variations in Vitamin D Production Could Possibly Explain the Seasonality of Childhood Respiratory Infections in Hawaii. Pediatr. Infect. Dis. J. 2008, 27, 853. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutr. Food Sci. J. 2020, 3, 126. [Google Scholar]
- Chetty, V.V.; Chetty, M. Potential benefit of vitamin D supplementation in people with respiratory illnesses, during the COVID-19 pandemic. Clin. Transl. Sci. 2021, 14, 2111–2116. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity,” Perspectives of Global Relevance Based on Research, Experience and Successes in Combating COVID-19 in Sri Lanka, Colombo, Sri Lanka, 27 January 2021; pp. 171–198. [Google Scholar]
- D’avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’keefe, J.H. Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in COVID-19 patients. Mo. Med. 2021, 118, 68–73. [Google Scholar]
- Cicero, A.F.G.; Fogacci, F.; Borghi, C. Vitamin D Supplementation and COVID-19 Outcomes: Mounting Evidence and Fewer Doubts. Nutrients 2022, 14, 3584. [Google Scholar] [CrossRef]
- Xu, Y.; Baylink, D.J.; Chen, C.-S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef]
- Argano, C.; Bocchio, R.M.; Natoli, G.; Scibetta, S.; Monaco, M.L.; Corrao, S. Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals 2023, 16, 130. [Google Scholar] [CrossRef]
- Davies, G.; Mazess, R.B.; Benskin, L.L. Letter to the editor in response to the article: “Vitamin D concentrations and COVID-19 infection in UK biobank” (Hastie et al.). Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 643–644. [Google Scholar] [CrossRef]
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2020, 60, 545–548. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; McCracken, C.; Bethell, M.S.; Cooper, J.; Cooper, C.; Caulfield, M.J.; Munroe, P.B.; Harvey, N.C.; E Petersen, S. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health 2020, 42, 451–460. [Google Scholar] [CrossRef]
- Annweiler, C.; Hanotte, B.; de L’eprevier, C.G.; Sabatier, J.-M.; Lafaie, L.; Célarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Deficiency and Treatment with COVID-19 Incidence. medRxiv 2020. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Abbeele, K.V.D.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2020, 97, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed]
- Gönen, M.S.; Alaylıoğlu, M.; Durcan, E.; Özdemir, Y.; Şahin, S.; Konukoğlu, D.; Nohut, O.K.; Ürkmez, S.; Küçükece, B.; Balkan, I.I.; et al. Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Cutolo, M.; Pizzorni, C.; Sulli, A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun. Rev. 2011, 11, 84–87. [Google Scholar] [CrossRef]
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892. [Google Scholar] [CrossRef]
- Delvin, E.; Souberbielle, J.-C.; Viard, J.-P.; Salle, B. Role of vitamin D in acquired immune and autoimmune diseases. Crit. Rev. Clin. Lab. Sci. 2014, 51, 232–247. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 17, 9784. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How Does Vitamin D Affect Immune Cells Crosstalk in Autoimmune Diseases? Int. J. Mol. Sci. 2023, 24, 4689. [Google Scholar] [CrossRef]
- Székely, J.I.; Pataki, Á. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: A short review. Expert Rev. Respir. Med. 2012, 6, 683–704. [Google Scholar] [CrossRef]
- Hassan, V.; Hassan, S.; Seyed-Javad, P.; Ahmad, K.; Asieh, H.; Maryam, S.; Farid, F.; Siavash, A. Association between Serum 25 (OH) Vitamin D Concentrations and Inflammatory Bowel Diseases (IBDs) Activity. Med. J. Malays. 2013, 68, 34–38. [Google Scholar]
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a High Loading Dose of Cholecalciferol to Correct Hypovitaminosis D in Patients with Inflammatory/Autoimmune Rheumatic Diseases. J. Rheumatol. 2013, 40, 166–172. [Google Scholar] [CrossRef]
- Tuohimaa, P.; Keisala, T.; Minasyan, A.; Cachat, J.; Kalueff, A. Vitamin D, nervous system and aging. Psychoneuroendocrinology 2009, 34, S278–S286. [Google Scholar] [CrossRef] [PubMed]
- Pani, M.; Regulla, K.; Segni, M.; Krause, M.; Hofmann, S.; Hufner, M.; Herwig, J.; Pasquino, A.; Usadel, K.; Badenhoop, K. Vitamin D 1alpha-hydroxylase (CYP1alpha) polymorphism in Graves' disease, Hashimoto's thyroiditis and type 1 diabetes mellitus. Eur. J. Endocrinol. 2002, 146, 777–781. [Google Scholar] [CrossRef]
- Song, L.; Papaioannou, G.; Zhao, H.; Luderer, H.F.; Miller, C.; Dall’osso, C.; Nazarian, R.M.; Wagers, A.J.; Demay, M.B. The Vitamin D Receptor Regulates Tissue Resident Macrophage Response to Injury. Endocrinology 2016, 157, 4066–4075. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the immune system: Role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008, 17, 348–352. [Google Scholar] [CrossRef]
- Lin, R.; White, J.H. The pleiotropic actions of vitamin D. BioEssays 2004, 26, 21–28. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, S.; Zhang, J.; Zhon, L.; Pen, Z.; Xing, T. 1,25(OH) 2 D 3 induces regulatory T cell differentiation by influencing the VDR/PLC-γ1/TGF-β1/pathway. Mol. Immunol. 2017, 91, 156–164. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, S.S.; Nouri, M.; Sakhinia, E.; Babaloo, Z.; Mohammadzaeh, A.; Alipour, S.; Jadideslam, G.; Khabbazi, A. The molecular and clinical evidence of vitamin D signaling as a modulator of the immune system: Role in Behçet’s disease. Immunol. Lett. 2019, 210, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tong, Y.; Quan, Y.; Wang, G.; Cheng, H.; Gu, S.; Jiang, J.X. Protein kinase A activation alleviates cataract formation via increased gap junction intercellular communication. iScience 2023, 26, 106114. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; De Saint-Georges, L.; Bowman, B.M.; Jee, W.S. Bone lining cells: Structure and Function. Scanning Microsc. 1989, 3, 953–960. Available online: https://europepmc.org/article/med/2694361 (accessed on 16 January 2021).
- Valiunas, V. Biophysical Properties of Connexin-45 Gap Junction Hemichannels Studied in Vertebrate Cells. J. Gen. Physiol. 2002, 119, 147–164. [Google Scholar] [CrossRef]
- Tikellis, C.; Thomas, M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. ACE inhibitors and angiotensin receptor blockers reduce the complications associated with COVID-19 infection. World J. Pharma Res. 2021, 10, 2579–2600. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.K.A.A.; Shurbaji, S. Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms. Int. J. Mol. Sci. 2021, 22, 6703. [Google Scholar] [CrossRef]
- Bradding, P.; Richardson, M.; Hinks, T.S.; Howarth, P.H.; Choy, D.F.; Arron, J.R.; Wenzel, S.E.; Siddiqui, S. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma—Implications for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Kunutsor, S.; Franco, O.H.; Chowdhury, R. Vitamin D, type 2 diabetes and other metabolic outcomes: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013, 72, 89–97. [Google Scholar] [CrossRef]
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and Diabetes. Rheum. Dis. Clin. N. Am. 2012, 38, 179–206. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Rosella, G.; Wu, Y.; Lubel, J.S.; Gibson, P.R. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin. Nutr. 2017, 37, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Wasse, H.; Cardarelli, F.; De Staercke, C.; Hooper, C.; Veledar, E.; Guessous, I. 25-hydroxyvitamin D concentration is inversely associated with serum MMP-9 in a cross-sectional study of African American ESRD patients. BMC Nephrol. 2011, 12, 24. [Google Scholar] [CrossRef]
- WöBke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Mascitelli, L.; Pezzetta, F.; Goldstein, M.R. Inflammatory bowel disease and the vitamin D endocrine system. Intern. Med. J. 2011, 41, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Milestone, A.N.; Walters, J.R.; Hart, A.L.; Ghosh, S. Vitamin D and gastrointestinal diseases: Inflammatory bowel disease and colorectal cancer. Ther. Adv. Gastroenterol. 2011, 4, 49–62. [Google Scholar] [CrossRef]
- Ardizzone, S.; Cassinotti, A.; Bevilacqua, M.; Clerici, M.; Porro, G.B. Vitamin D and Inflammatory Bowel Disease. Vitam. Horm. 2011, 86, 367–377. [Google Scholar] [CrossRef]
- Attar, S.M. Vitamin D deficiency in rheumatoid arthritis. Prevalence and association with disease activity in Western Saudi Arabia. Saudi Med. J. 2012, 33, 520–525. [Google Scholar]
- Furuya, T.; Hosoi, T.; Tanaka, E.; Nakajima, A.; Taniguchi, A.; Momohara, S.; Yamanaka, H. Prevalence of and factors associated with vitamin D deficiency in 4,793 Japanese patients with rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 1081–1087. [Google Scholar] [CrossRef]
- Rossini, M.; Bongi, S.M.; La Montagna, G.; Minisola, G.; Malavolta, N.; Bernini, L.; Cacace, E.; Sinigaglia, L.; Di Munno, O.; Adami, S. Vitamin D deficiency in rheumatoid arthritis: Prevalence, determinants and associations with disease activity and disability. Arthritis Res. Ther. 2010, 12, R216. [Google Scholar] [CrossRef]
- Ishikawa, L.L.W.; Colavite, P.M.; Fraga-Silva, T.F.d.C.; Mimura, L.A.N.; França, T.G.D.; Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Marcolino, L.D.; Penitenti, M.; Ikoma, M.R.V.; et al. Vitamin D Deficiency and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2017, 52, 373–388. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W. The Roles of Vitamin D and Its Analogs in Inflammatory Diseases. Curr. Top. Med. Chem. 2016, 16, 1242–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, M.-B.; Zhang, R.-H.; Chen, Z.-J.; Hua, C.; Lin, J.-P.; Yang, L.-R. Advances in Computational Structure-Based Drug Design and Application in Drug Discovery. Curr. Top. Med. Chem. 2016, 16, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Niesner, U.; Chang, H.-D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D3promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D regulation of immune function during covid-19. Rev. Endocr. Metab. Disord. 2022, 23, 279–285. [Google Scholar] [CrossRef]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- E Nnoaham, K.; Clarke, A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Leuk. Res. 2008, 37, 113–119. [Google Scholar] [CrossRef]
- Grant, W.B.; Goldstein, M.; Mascitelli, L. Ample evidence exists from human studies that vitamin D reduces the risk of selected bacterial and viral infections. Exp. Biol. Med. 2010, 235, 1395–1396. [Google Scholar] [CrossRef]
- Liu, N.; Kaplan, A.; Low, J.; Nguyen, L.; Liu, G.; Equils, O.; Hewison, M. Vitamin D Induces Innate Antibacterial Responses in Human Trophoblasts via an Intracrine Pathway1. Biol. Reprod. 2009, 80, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Vasilyevna Belyaeva, I.; Pavlovitch Churilov, L.; Robertovnsmalla, C.M.L.; Vladimirovitch Nikolaev, A.; Andreevna Starshinova, A.; Kazimirovitch Yablonsky, P. Vitamin D, Cathelicidin, Prolactin, Autoantibodies, and Cytokines in Different Forms of Pulmonary Tuberculosis versus Sarcoidosis. Isr. Med. Assoc. J. 2017, 19, 499–505. [Google Scholar]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef]
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Anty, R.; Anstee, Q.M.; Gual, P.; Tran, A. Prophylaxis of bacterial infections in cirrhosis: Is an optimal 25-OH vitamin D level required? J. Hepatol. 2014, 61, 965–966. [Google Scholar] [CrossRef][Green Version]
- Grant, W.B.; Boucher, B.J.; Pludowski, P.; Wimalawansa, S.J. The emerging evidence for non-skeletal health benefits of vitamin D supplementation in adults. Nat. Rev. Endocrinol. 2022, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef]
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A., Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014, 5, 2365–2370. [Google Scholar] [CrossRef]
- Adams, J.S.; Chen, H.; Chun, R.; Ren, S.; Wu, S.; Gacad, M.; Nguyen, L.; Ride, J.; Liu, P.; Modlin, R.; et al. Substrate and Enzyme Trafficking as a Means of Regulating 1,25-Dihydroxyvitamin D Synthesis and Action: The Human Innate Immune Response. J. Bone Miner. Res. 2007, 22, V20–V24. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef]
- Carlberg, C. Genomic signaling of vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal Expression of 25-Hydroxyvitamin D3-1α-Hydroxylase1. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef]
- White, J.H. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch. Biochem. Biophys. 2012, 523, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-J.; Wang, L.-F.; Xu, X.-Y.; Zhou, Y.; Jin, W.-F.; Wang, H.-F.; Gao, J. Autocrine/Paracrine Action of Vitamin D on FGF23 Expression in Cultured Rat Osteoblasts. Calcif. Tissue Int. 2010, 86, 404–410. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Whittle, R. Vitamin D: A single initial dose is not bogus if followed by an appropriate maintenance intake. JBMR Plus 2022, 6, e10606. [Google Scholar] [CrossRef]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini-Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef]
- Bravo, S.; Paredes, R.; Izaurieta, P.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Hinrichs, M.V.; Olate, J.; Aguayo, L.G.; Montecino, M. The classic receptor for 1α,25-dihydroxy vitamin D3 is required for non-genomic actions of 1α,25-dihydroxy vitamin D3 in osteosarcoma cells. J. Cell. Biochem. 2006, 99, 995–1000. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Stio, M.; Retico, L.; Annese, V.; Bonanomi, A.G. Vitamin D regulates the tight-junction protein expression in active ulcerative colitis. Scand. J. Gastroenterol. 2016, 51, 1193–1199. [Google Scholar] [CrossRef]
- Valdés-López, J.F.; Velilla, P.; Urcuqui-Inchima, S. Vitamin D modulates the expression of Toll-like receptors and pro-inflammatory cytokines without affecting Chikungunya virus replication, in monocytes and macrophages. Acta Trop. 2022, 232, 106497. [Google Scholar] [CrossRef]
- Ellfolk, M.; Norlin, M.; Wikvall, K. Isolation and properties of the CYP2D25 promoter: Transcriptional regulation by vitamin D3 metabolites. Biochem. Biophys. Res. Commun. 2006, 345, 568–572. [Google Scholar] [CrossRef]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Chen, Q.; Hu, C.; Zhu, C.; He, G. Effects of calcitriol (1, 25-dihydroxy-vitamin D3) on the inflammatory response induced by H9N2 influenza virus infection in human lung A549 epithelial cells and in mice. Virol. J. 2017, 14, 10. [Google Scholar] [CrossRef]
- Elamir, Y.M.; Amir, H.; Lim, S.; Rana, Y.P.; Lopez, C.G.; Feliciano, N.V.; Omar, A.; Grist, W.P.; Via, M.A. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone 2022, 154, 116175. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Domínguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafián, P.; Grau, M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines 2021, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Arababadi, M.K.; Nosratabadi, R.; Asadikaram, G. Vitamin D and toll like receptors. Life Sci. 2018, 203, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 92–102. [Google Scholar]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.H.; Chakraverty, R. Differential Regulation of Vitamin D Receptor and Its Ligand in Human Monocyte-Derived Dendritic Cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Ramírez-Mejía, J.M.; Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D modulates expression of antimicrobial peptides and proinflammatory cytokines to restrict Zika virus infection in macrophages. Int. Immunopharmacol. 2023, 119, 110232. [Google Scholar] [CrossRef]
- Amado, C.A.; García-Unzueta, M.T.; Fariñas, M.C.; Santos, F.; Ortiz, M.; Muñoz-Cacho, P.; Amado, J.A. Vitamin D nutritional status and vitamin D regulated antimicrobial peptides in serum and pleural fluid of patients with infectious and noninfectious pleural effusions. BMC Pulm. Med. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Zierold, C.; Nehring, J.A.; Deluca, H.F. Nuclear receptor 4A2 and C/EBPβ regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D3-1α-hydroxylase. Arch. Biochem. Biophys. 2007, 460, 233–239. [Google Scholar] [CrossRef]
- Bai, X.; Dinghong, Q.; Miao, D.; Goltzman, D.; Karaplis, A.C. Klotho ablation converts the biochemical and skeletal alterations in FGF23 (R176Q) transgenic mice to a Klotho-deficient phenotype. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E79–E88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Miao, J.; Guo, R.; Guo, J.; Fan, Z.; Kong, X.; Gao, R.; Yang, L. Mechanism of COVID-19 Causing ARDS: Exploring the Possibility of Preventing and Treating SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022, 12, 931061. [Google Scholar] [CrossRef] [PubMed]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9, 20503121211014073. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Qayyum, R. Relation Between Serum 25-Hydroxyvitamin D and C-Reactive Protein in Asymptomatic Adults (From the Continuous National Health and Nutrition Examination Survey 2001 to 2006). Am. J. Cardiol. 2012, 109, 226–230. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, J.; Sun, T.; Li, G.; Szeto, F.L.; Liu, W.; Deb, D.K.; Wang, Y.; Zhao, Q.; Thadhani, R.; et al. 1,25-Dihydroxyvitamin D3 suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch. Biochem. Biophys. 2011, 507, 241–247. [Google Scholar] [CrossRef]
- Venkatesh, B.; Nair, P. Hypovitaminosis D and morbidity in critical illness: Is there proof beyond reasonable doubt? Crit. Care 2014, 18, 138. [Google Scholar] [CrossRef]
- Chiellini, G.; DeLuca, H.F. The importance of stereochemistry on the actions of vitamin D. Curr. Top. Med. Chem. 2011, 11, 840–859. [Google Scholar] [CrossRef]
- Afarideh, M.; Ghanbari, P.; Noshad, S.; Ghajar, A.; Nakhjavani, M.; Esteghamati, A. Raised serum 25-hydroxyvitamin D levels in patients with active diabetic foot ulcers. Br. J. Nutr. 2016, 115, 1938–1946. [Google Scholar] [CrossRef]
- Willershausen, B.; Ross, A.; Försch, M.; Willershausen, I.; Mohaupt, P.; Callaway, A. The influence of micronutrients on oral and general health. Eur. J. Med. Res. 2011, 16, 514–518. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Aloia, J.F.; Li-Ng, M. Re: Epidemic influenza and vitamin D. Epidemiol. Infect. 2007, 135, 1095–1096; author reply 1097–1098. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Lindh, A.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [PubMed]
- Banajeh, S.M. Nutritional rickets and vitamin D deficiency-association with the outcomes of childhood very severe pneumonia: A prospective cohort study. Pediatr. Pulmonol. 2009, 44, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yao, J.; Chen, G.; Lin, C. A severe H7N9 pneumonia with syndrome of inappropriate antidiuresis and vitamin D deficiency. Respir. Med. Case Rep. 2014, 12, 37–38. [Google Scholar] [CrossRef][Green Version]
- Pletz, M.W.; For the CAPNETZ-Study Group; Terkamp, C.; Schumacher, U.; Rohde, G.; Schütte, H.; Welte, T.; Bals, R. Vitamin D deficiency in community-acquired pneumonia: Low levels of 1,25(OH)2 D are associated with disease severity. Respir. Res. 2014, 15, 53. [Google Scholar] [CrossRef]
- Niederstrasser, J.; Herr, C.; Wolf, L.; Lehr, C.M.; Beisswenger, C.; Bals, R. Vitamin D Deficiency Does Not Result in a Breach of Host Defense in Murine Models of Pneumonia. Infect. Immun. 2016, 84, 3097–3104. [Google Scholar] [CrossRef]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology1. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Höck, A.D. Review: Vitamin D3 deficiency results in dysfunctions of immunity with severe fatigue and depression in a variety of diseases. In Vivo 2014, 28, 133–145. [Google Scholar] [PubMed]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S.; Hou, T.Z.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D.M. Availability of 25-Hydroxyvitamin D3 to APCs Controls the Balance between Regulatory and Inflammatory T Cell Responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef]
- Vuichard Gysin, D.; Dao, D.; Gysin, C.M.; Lytvyn, L.; Loeb, M. Effect of Vitamin D3 Supplementation on Respiratory Tract Infections in Healthy Individuals: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0162996. [Google Scholar] [CrossRef]
- Lucas, R.M.; Ponsonby, A.L.; Dear, K.; Valery, P.C.; Pender, M.P.; Taylor, B.V.; Kilpatrick, T.J.; Dwyer, T.; Coulthard, A.; Chapman, C.; et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 2011, 76, 540–548. [Google Scholar] [CrossRef]
- Lucas, R.M.; Byrne, S.N.; Correale, J.; Ilschner, S.; Hart, P.H. Ultraviolet radiation, vitamin D and multiple sclerosis. Neurodegener. Dis. Manag. 2015, 5, 413–424. [Google Scholar] [CrossRef]
- Orton, S.M.; Wald, L.; Confavreux, C.; Vukusic, S.; Krohn, J.P.; Ramagopalan, S.V.; Herrera, B.M.; Sadovnick, A.D.; Ebers, G.C. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 2011, 76, 425–431. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2017, 7, 59–85. [Google Scholar] [CrossRef]
- Wang, Y.; Marling, S.J.; Martino, V.M.; Prahl, J.M.; Deluca, H.F. The absence of 25-hydroxyvitamin D 3 -1α-hydroxylase potentiates the suppression of EAE in mice by ultraviolet light. J. Steroid Biochem. Mol. Biol. 2016, 163, 98–102. [Google Scholar] [CrossRef]
- Wang, Y.; Marling, S.J.; McKnight, S.M.; Danielson, A.L.; Severson, K.S.; Deluca, H.F. Suppression of experimental autoimmune encephalomyelitis by 300–315 nm ultraviolet light. Arch. Biochem. Biophys. 2013, 536, 81–86. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.-P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.; Simpson, S.; van der Mei, I.; Ponsonby, A.-L.; Blizzard, L.; Dwyer, T.; Pittas, F.; Eyles, D.; Ko, P.; Taylor, B.V. Interferon- and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012, 79, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Munger, K.L.; Köchert, K.; Arnason, B.G.W.; Comi, G.; Cook, S.; Goodin, D.S.; Filippi, M.; Hartung, H.-P.; Jeffery, D.R.; et al. Association of Vitamin D Levels With Multiple Sclerosis Activity and Progression in Patients Receiving Interferon Beta-1b. JAMA Neurol. 2015, 72, 1458–1465. [Google Scholar] [CrossRef]
- Emamgholipour, S.; Eshaghi, S.M.; Hossein-Nezhad, A.; Mirzaei, K.; Maghbooli, Z.; Sahraian, M.A. Adipocytokine Profile, Cytokine Levels and Foxp3 Expression in Multiple Sclerosis: A Possible Link to Susceptibility and Clinical Course of Disease. PLoS ONE 2013, 8, e76555. [Google Scholar] [CrossRef]
- Bock, G.; Prietl, B.; Mader, J.K.; Höller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: A randomized controlled trial. Diabetes/Metab. Res. Rev. 2011, 27, 942–945. [Google Scholar] [CrossRef]
- Konijeti, G.G.; Arora, P.; Boylan, M.R.; Song, Y.; Huang, S.; Harrell, F.; Newton-Cheh, C.; O’Neill, D.; Korzenik, J.; Wang, T.J.; et al. Vitamin D Supplementation Modulates T Cell–Mediated Immunity in Humans: Results from a Randomized Control Trial. J. Clin. Endocrinol. Metab. 2016, 101, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Sotirchos, E.S.; Bhargava, P.; Eckstein, C.; Van Haren, K.; Baynes, M.; Ntranos, A.; Gocke, A.; Steinman, L.; Mowry, E.M.; Calabresi, P.A. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology 2016, 86, 382–390. [Google Scholar] [CrossRef]
- Ashtari, F.; Toghianifar, N.; Zarkesh-Esfahani, S.H.; Mansourian, M. High dose Vitamin D intake and quality of life in relapsing-remitting multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Neurol. Res. 2016, 38, 888–892. [Google Scholar] [CrossRef]
- Patel, S.; Farragher, T.; Berry, J.; Bunn, D.; Silman, A.; Symmons, D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007, 56, 2143–2149. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D in rheumatoid arthritis—Towards clinical application. Nat. Rev. Rheumatol. 2016, 12, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A.; Bruce, I.N. Vitamin D treatment for connective tissue diseases: Hope beyond the hype? Rheumatology 2017, 56, 178–186. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal–Fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef]
- Birmingham, D.; Hebert, L.; Song, H.; Noonan, W.; Rovin, B.; Nagaraja, H.; Yu, C. Evidence that abnormally large seasonal declines in vitamin D status may trigger SLE flare in non-African Americans. Lupus 2012, 21, 855–864. [Google Scholar] [CrossRef]
- Cutolo, M.; Otsa, K.; Laas, K.; Yprus, M.; Lehtme, R.; E Secchi, M.; Sulli, A.; Paolino, S.; Seriolo, B. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin. Exp. Rheumatol. 2006, 24, 702–704. [Google Scholar]
- Racovan, M.; Walitt, B.; Collins, C.E.; Pettinger, M.; Parks, C.G.; Shikany, J.M.; Wactawski-Wende, J.; Manson, J.E.; Moreland, L.; Wright, N.; et al. Calcium and vitamin D supplementation and incident rheumatoid arthritis: The Women’s Health Initiative Calcium plus Vitamin D trial. Rheumatol. Int. 2012, 32, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT 1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Sarandi, E. Micronutrient deficiencies in patients with COVID-19: How metabolomics can contribute to their prevention and replenishment. BMJ Nutr. Prev. Health 2020, 3, 419–420. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; Pilz, S.; Tomaschitz, A.; Grubler, M.R.; Verheyen, N. The synergistic interplay between vitamins D and K for bone and cardiovascular Health: A narrative review. Int. J. Endocrinol. 2017, 2017, 7454376. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2018, 38, 2098–2105. [Google Scholar] [CrossRef]
- Sims, J.T.; Krishnan, V.; Chang, C.-Y.; Engle, S.M.; Casalini, G.; Rodgers, G.H.; Bivi, N.; Nickoloff, B.J.; Konrad, R.J.; de Bono, S.; et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2020, 147, 107–111. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Iannaccone, G.; Scacciavillani, R.; Del Buono, M.G.; Camilli, M.; Ronco, C.; Lavie, C.J.; Abbate, A.; Crea, F.; Massetti, M.; Aspromonte, N. Weathering the Cytokine Storm in COVID-19: Therapeutic Implications. Cardiorenal Med. 2020, 10, 277–287. [Google Scholar] [CrossRef]
- McGregor, T.B.; Sener, A.; Yetzer, K.; Gillrie, C.; Paraskevas, S. The impact of COVID-19 on the Canadian Kidney Paired Donation program: An opportunity for universal implementation of kidney shipping. Can. J. Surg. 2020, 63, E451–E453. [Google Scholar] [CrossRef]
- Wallis, G.; Siracusa, F.; Blank, M.; Painter, H.; Sanchez, J.; Salinas, K.; Mamuyac, C.; Marudamuthu, C.; Wrigley, F.; Corrah, T.; et al. Experience of a novel community testing programme for COVID-19 in London: Lessons learnt. Clin. Med. 2020, 20, e165–e169. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.A.; McGregor, A.J. Sex- and Gender-specific Observations and Implications for COVID-19. West J. Emerg. Med. 2020, 21, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Rigante, D.; Lepri, G.; Cerinic, M.M.; Falcini, F. Severe vitamin D deficiency in patients with Kawasaki disease: A potential role in the risk to develop heart vascular abnormalities? Clin. Rheumatol. 2016, 35, 1865–1872. [Google Scholar] [CrossRef]
- Baggs, J.; Gee, J.; Lewis, E.; Fowler, G.; Benson, P.; Lieu, T.; Naleway, A.; Klein, N.P.; Baxter, R.; Belongia, E.; et al. The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety. Pediatrics 2011, 127, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K. Post-adenoviral-based COVID-19 vaccines thrombosis: A proposed mechanism. J. Thromb. Haemost. 2021, 19, 1831–1832. [Google Scholar] [CrossRef]
- Long, B.; Bridwell, R.; Gottlieb, M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021, 49, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.A.; Bracis, C.; Janes, H.; Moore, M.; Matrajt, L.; Reeves, D.B.; Burns, E.; Donnell, D.; Cohen, M.S.; Schiffer, J.T.; et al. COVID-19 vaccines that reduce symptoms but do not block infection need higher coverage and faster rollout to achieve population impact. Sci. Rep. 2021, 11, 1553. [Google Scholar] [CrossRef]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2014, 17, 8–12. [Google Scholar] [CrossRef]
- Khan, S.R.; Whiteman, D.C.; Kimlin, M.G.; Janda, M.; Clarke, M.W.; Lucas, R.M.; Neale, R.E. Effect of solar ultraviolet radiation exposure on serum 25(OH)D concentration: A pilot randomised controlled trial. Photochem. Photobiol. Sci. 2018, 17, 570–577. [Google Scholar] [CrossRef]
- Pérez-López, F.R. Vitamin D and its implications for musculoskeletal health in women: An update. Maturitas 2007, 58, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Behradmanesh, S.; Ahmadi, A.; Rafieian-Kopaei, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J. Nephropathol. 2014, 3, 29–33. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Cauley, J.A.; LaCroix, A.Z.; Wu, L.; Horwitz, M.; Danielson, M.E.; Bauer, D.C.; Lee, J.S.; Jackson, R.D.; Robbins, J.A.; Wu, C.; et al. Serum 25 HydroxyVitamin D Concentrations and the Risk of Hip Fractures: The Women's Health Initiative. Ann. Intern. Med. 2008, 149, 242–250. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; Dijck-Brouwer, D.J.; Muskiet, F.A. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; van der Veer, E.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013, 52, 1115–1125. [Google Scholar] [CrossRef]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The Importance of Body Weight for the Dose Response Relationship of Oral Vitamin D Supplementation and Serum 25-Hydroxyvitamin D in Healthy Volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.E.; Boyce, T.; Chesney, M.A.; Cohen, S.; Folkman, S.; Kahn, R.L.; Syme, S.L. Socioeconomic status and health: The challenge of the gradient. Am. Psychol. 1994, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.M. Role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder: A narrative review. Medicine 2023, 102, e33477. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur. J. Clin. Nutr. 2011, 65, 1016–1026. [Google Scholar] [CrossRef]
- Dudenkov, D.V.; Mara, K.C.; Petterson, T.M.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin D Values and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study. Mayo Clin. Proc. 2018, 93, 721–730. [Google Scholar] [CrossRef]
- Rowling, M.J.; Kemmis, C.M.; A Taffany, D.; Welsh, J. Megalin-Mediated Endocytosis of Vitamin D Binding Protein Correlates with 25-Hydroxycholecalciferol Actions in Human Mammary Cells. J. Nutr. 2006, 136, 2754–2759. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 258–267. [Google Scholar] [CrossRef]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; E Gundersen, T.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and Vitamin D: Necessary for Public Health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Vieth, R.; Hollis, B.W. Vitamin D Efficacy and Safety. Arch. Intern. Med. 2011, 171, 266. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Whiting, S.J.; Schwalfenberg, G.K.; Genuis, S.J.; Kimball, S.M. Estimated economic benefit of increasing 25-hydroxyvitamin D concentrations of Canadians to or above 100 nmol/L. Derm. Endocrinol. 2016, 8, e1248324. [Google Scholar] [CrossRef] [PubMed]
- Cangoz, S.; Chang, Y.-Y.; Chempakaseril, S.J.; Guduru, R.C.; Huynh, L.M.; John, J.S.; John, S.T.; Joseph, M.E.; Judge, R.; Kimmey, R.; et al. Vitamin D and type 2 diabetes mellitus. J. Clin. Pharm. Ther. 2013, 38, 81–84. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Hartaigh, B.; Tomaschitz, A.; März, W. Vitamin D in preventive medicine. Anticancer Res. 2015, 35, 1161–1170. [Google Scholar]
- Weinert, L.S.; Silveiro, S.P. Maternal–Fetal Impact of Vitamin D Deficiency: A Critical Review. Matern. Child Health J. 2015, 19, 94–101. [Google Scholar] [CrossRef]
- Mazahery, H.; Von Hurst, P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Sath, S.; Government Medical College; Shah, S.R.; Rafiq, S.N.; Jeelani, I. Hypervitaminosis D in Kashmiri Population: A Case Series of 11 Patients. Int. J. Med. Sci. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Haq, A.; Wimalawansa, S.J.; Pludowski, P.; Al Anouti, F. Clinical practice guidelines for vitamin D in the United Arab Emirates. J. Steroid Biochem. Mol. Biol. 2018, 175, 4–11. [Google Scholar] [CrossRef]
- Fuss, M.; Pepersack, T.; Gillet, C.; Karmali, R.; Corvilain, J. Calcium and vitamin D metabolism in granulomatous diseases. Clin. Rheumatol. 1992, 11, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.; Bansal, A.; Looke, D.; Whitby, M.; Hogan, P. Hypercalcaemia and Elevated 1,25(OH)2D3Levels Associated with Disseminated Mycobacterium avium Infection in AIDS. J. Infect. 2001, 42, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Roux, C. All the articles derived from the panel discussions incorporate the most recent data in the field, in particular those documenting the importance of the vitamin D storage form. Jt. Bone Spine 2012, 79, S85. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Dąbrowski, M.; Jankowski, P. The Association between Serum Vitamin D Concentration and New Inflammatory Biomarkers—Systemic Inflammatory Index (SII) and Systemic Inflammatory Response (SIRI)—In Patients with Ischemic Heart Disease. Nutrients 2022, 14, 4212. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular Approaches for Optimizing Vitamin D Supplementation. Vitam. Horm. 2016, 100, 255–271. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Lopez-Miranda, J.; Entrenas-Castillo, M.; Casado-Díaz, A.; Solans, X.N.Y.; Mansur, J.L.; Bouillon, R. Vitamin D Endocrine System and COVID-19: Treatment with Calcifediol. Nutrients 2022, 14, 2716. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Dueñas-Laita, A.; Brandi, M.L.; Jódar, E.; del Pino-Montes, J.; Quesada-Gómez, J.M.; Castro, F.C.; Gómez-Alonso, C.; López, L.G.; Martínez, J.M.O.; et al. Calcifediol is superior to cholecalciferol in improving vitamin D status in postmenopausal women: A randomized trial. J. Bone Miner. Res. 2021, 36, 1967–1978. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment with 25-Hydroxyvitamin D3 (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pr. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.E.; Entrenas Costa, L.M.E.; Vaquero Barrios, J.M.V.; Alcalá Díaz, J.F.A.; López Miranda, J.L.; Bouillon, R.; Quesada Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Lopez-Caleya, J.F.; Ortega-Valín, L.; Fernández-Villa, T.; Delgado-Rodríguez, M.; Martín-Sánchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case–control studies. Cancer Causes Control. 2022, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Varna, V.P.; Sharma, U.; Mavalankar, D. Does vitamin D supplementation reduce COVID-19 severity?: A systematic review. QJM 2022, 115, 665–672. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- Dror, A.A.; Morozov, N.; Daoud, A.; Namir, Y.; Yakir, O.; Shachar, Y.; Lifshitz, M.; Segal, E.; Fisher, L.; Mizrachi, M.; et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE 2022, 17, e0263069. [Google Scholar] [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C.; et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern. Child. Nutr. 2020, 16, e12987. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Kazemi, A.; Mohammadi, V.; Aghababaee, S.K.; Golzarand, M.; Clark, C.C.T.; Babajafari, S. Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: A Systematic Review and Meta-analysis. Adv. Nutr. Int. Rev. J. 2021, 12, 1636–1658. [Google Scholar] [CrossRef]
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients 2021, 13, 1714. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Lombardini, R.; Gargaro, M.; Fallarino, F.; Scarponi, A.M.; et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition 2021, 91–92, 111408. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, A.; Mohseni, S.; Narimani, B.; Ebrahimzadeh, A.; Kazemi, S.; Keshavarz, F.; Yaghoubi, M.J.; Milajerdi, A. Association between vitamin D status and risk of covid-19 in-hospital mortality: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 5033–5043. [Google Scholar] [CrossRef]
- Brown, R.; Sakar, A. Vitamin D Deficiency: A Factor in COVID-19, Progression, Severity and Mortality?- An Urgent Call for Research. Mitofit Arch. 2020. Available online: https://www.mitofit.org/images/e/ec/Brown_et_al_2020_MitoFit_Preprint_Arch_doi_10.26214_mitofit_200001.pdf (accessed on 5 March 2023).
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between Vitamin D Supplementation and Cancer Mortality: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, M.; Fan, D.; Hong, Y.; Zhao, M.; Ding, R.; Cheng, Y.; Duan, S. Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Randomized controlled trials of vitamin D and cancer incidence: A modeling study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef]
- de Souza, W.D.F.; da Fonseca, D.M.; Sartori, A. COVID-19 and Multiple Sclerosis: A Complex Relationship Possibly Aggravated by Low Vitamin D Levels. Cells 2023, 12, 684. [Google Scholar] [CrossRef]
- Akhtar, A.; Neupane, R.; Singh, A.; Khan, M. Radiological Association Between Multiple Sclerosis Lesions and Serum Vitamin D Levels. Cureus 2022, 14, e31824. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Cohen, A.G.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association Between Preoperative 25-Hydroxyvitamin D Level and Hospital-Acquired Infections Following Roux-en-Y Gastric Bypass Surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef]
- Dancer, R.C.A.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grübler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; März, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of All-Cause Mortality According to Serum 25-Hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D'Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2018, 380, 33–44. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Baidal, D.A.; Alejandro, R.; Lanzoni, G.; Sears, B.; Caprio, M.; Fabbri, A. VITAL study: An incomplete picture? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3142–3147. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. IOM recommendations vs. vitamin D guidelines applicable to the rest of the world. In Proceedings of the 5th International Conference on Vitamin D, Abu Dhabi, United Arab Emirates, 14 March 2017; p. 9. [Google Scholar]
- Grant, W.B.; Cross, H.S.; Garland, C.F.; Gorham, E.D.; Moan, J.; Peterlik, M.; Porojnicu, A.C.; Reichrath, J.; Zittermann, A. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 2009, 99, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Al Nozha, O.M. Vitamin D and Extra-Skeletal Health: Causality or Consequence. Int. J. Health Sci. 2016, 10, 443–452. [Google Scholar] [CrossRef]
- Body, J.-J.; Bergmann, P.; Boonen, S.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; Rozenberg, S.; Reginster, J.-Y. Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporos. Int. 2012, 23 (Suppl. S1), S1–S23. [Google Scholar] [CrossRef]
- Cianferotti, L.; Bertoldo, F.; Bischoff-Ferrari, H.A.; Bruyere, O.; Cooper, C.; Cutolo, M.; Kanis, J.A.; Kaufman, J.-M.; Reginster, J.-Y.; Rizzoli, R.; et al. Vitamin D supplementation in the prevention and management of major chronic diseases not related to mineral homeostasis in adults: Research for evidence and a scientific statement from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Endocrine 2017, 56, 245–261. [Google Scholar] [CrossRef]
- Chiu, K.C.; Chu, A.; Go, V.L.W.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Brashear, M.M.; Johnson, W.D. Prediabetes and Prehypertension in Healthy Adults Are Associated With Low Vitamin D Levels. Diabetes Care 2011, 34, 658–660. [Google Scholar] [CrossRef]
- A Hamed, E.; Abu Faddan, N.H.; Elhafeez, H.A.A.; Sayed, D. Parathormone-25(OH)-vitamin D axis and bone status in children and adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2011, 12, 536–546. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women's Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Willett, W.C.; A Colditz, G. Calcium, vitamin D, milk consumption, and hip fractures: A prospective study among postmenopausal women. Am. J. Clin. Nutr. 2003, 77, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Woitge, H.W.; Witte, K.; Lemmer, B.; Seibel, M.J. Supplementation With Oral Vitamin D3and Calcium During Winter Prevents Seasonal Bone Loss: A Randomized Controlled Open-Label Prospective Trial. J. Bone Miner. Res. 2004, 19, 1221–1230. [Google Scholar] [CrossRef]
- Akdere, G.; Efe, B.; Sisman, P.; Yorulmaz, G. The relationship between vitamin D level and organ-specific autoimmune disorders in newly diagnosed type I diabetes mellitus. Bratisl. Med. J. 2018, 119, 544–549. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Prindiville, S.; Weiss, D.G.; Willett, W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003, 290, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Rodriguez, C.; Jacobs, E.J.; Chao, A.; Jonas, C.; Calle, E.E.; Willett, W.C.; Thun, M.J. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control. 2003, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control. 2012, 23, 363–370. [Google Scholar] [CrossRef]
- Consiglio, M.; Destefanis, M.; Morena, D.; Foglizzo, V.; Forneris, M.; Pescarmona, G.; Silvagno, F. The Vitamin D Receptor Inhibits the Respiratory Chain, Contributing to the Metabolic Switch that Is Essential for Cancer Cell Proliferation. PLoS ONE 2014, 9, e115816. [Google Scholar] [CrossRef] [PubMed]
- Rothen, J.-P.; Rutishauser, J.; Walter, P.N.; Hersberger, K.E.; Arnet, I. Vitamin D oral intermittent treatment (DO IT) study, a randomized clinical trial with individual loading regimen. Sci. Rep. 2021, 11, 18746. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Cui, Q.Q.; Hong, Y.M.; Yao, W.G. A Meta-Analysis of High Dose, Intermittent Vitamin D Supplementation among Older Adults. PLoS ONE 2015, 10, e0115850. [Google Scholar] [CrossRef]
- Solís, F.; Salas, A.A.; Bartolomé, M.J.L.; Ballestín, S.S. The Effects of Vitamin D Supplementation in COVID-19 Patients: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12424. [Google Scholar] [CrossRef]
- Greer, F.R. 25-Hydroxyvitamin D: Functional outcomes in infants and young children. Am. J. Clin. Nutr. 2008, 88, 529S–533S. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A randomized clinical trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- van Helmond, N.; Brobyn, T.L.; LaRiccia, P.J.; Cafaro, T.; Hunter, K.; Roy, S.; Bandomer, B.; Ng, K.Q.; Goldstein, H.; Mitrev, L.V.; et al. Vitamin D3 Supplementation at 5000 IU Daily for the Prevention of Influenza-like Illness in Healthcare Workers: A Pragmatic Randomized Clinical Trial. Nutrients 2022, 15, 180. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Van Tran, K.; Chen, S.-Y.; Tam, K.-W. A Systematic Review and Meta-Analysis of Randomized Controlled Trials of the Effects of Vitamin D Supplementation on Children and Young Adults with HIV Infection. J. Nutr. 2023, 153, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low Vitamin D Status: Definition, Prevalence, Consequences, and Correction. Rheum. Dis. Clin. N. Am. 2012, 38, 45–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollis, B.W.; Wagner, C.L. Clinical review: The Role of the Parent Compound Vitamin D with Respect to Metabolism and Function: Why Clinical Dose Intervals Can Affect Clinical Outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nishimura, K.-I.; Mori, J.-I. Update on recent progress in vitamin D research. Molecular basis of epigenetic regulation by vitamin D via its nuclear receptor. Clin. Calcium 2017, 27, 1543–1550. [Google Scholar]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]


| Examples of Infections | Autoimmune Diseases and Others |
|---|---|
| Tuberculosis, leprosy, common cold (intracellular microorganisms) | Allergy/eczema |
| Influenza type A $ | Asthma |
| Coryza (common cold) | Chronic hives |
| Upper respiratory tract infections | Fibromyalgia |
| Lower urinary tract infections | Inflammatory bowel disease |
| Bacterial vaginosis in pregnant women | Multiple sclerosis |
| Periodontal gum disease and infections | Myositis and periostitis |
| Osteonecrosis of the jaw | Primary biliary cirrhosis |
| Miscellaneous fungal infections | Psoriasis |
| Yeast infection | Polyautoimmunity |
| Coxsackie A and B | Rheumatoid arthritis/ Behcet’s disease |
| SARS-CoV-2 | Type 1 diabetes mellitus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimalawansa, S.J. Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. https://doi.org/10.3390/nu15173842
Wimalawansa SJ. Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients. 2023; 15(17):3842. https://doi.org/10.3390/nu15173842
Chicago/Turabian StyleWimalawansa, Sunil J. 2023. "Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review" Nutrients 15, no. 17: 3842. https://doi.org/10.3390/nu15173842
APA StyleWimalawansa, S. J. (2023). Infections and Autoimmunity—The Immune System and Vitamin D: A Systematic Review. Nutrients, 15(17), 3842. https://doi.org/10.3390/nu15173842






