Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status
Abstract
:1. Introduction
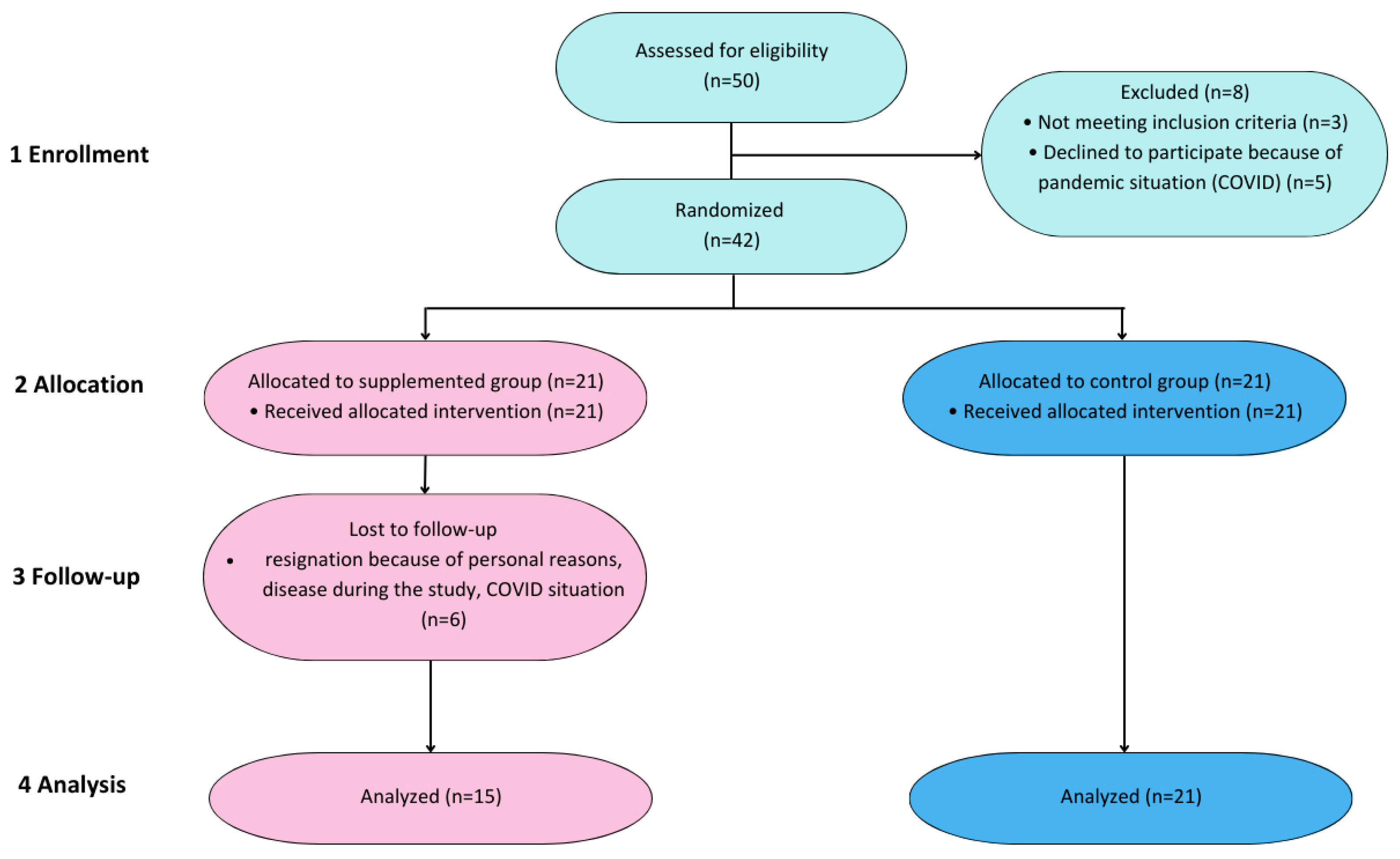
2. Materials and Methods
2.1. Design of the Study
2.2. Participants
2.3. Intervention
2.4. Visit Programme and Material Collection
2.5. Measurement of Vitamin D and Kynurenine Pathway Metabolites
2.6. Assessment of Inflammation Markers
2.7. Statistical Analysis
3. Results
3.1. Demographical Characteristics
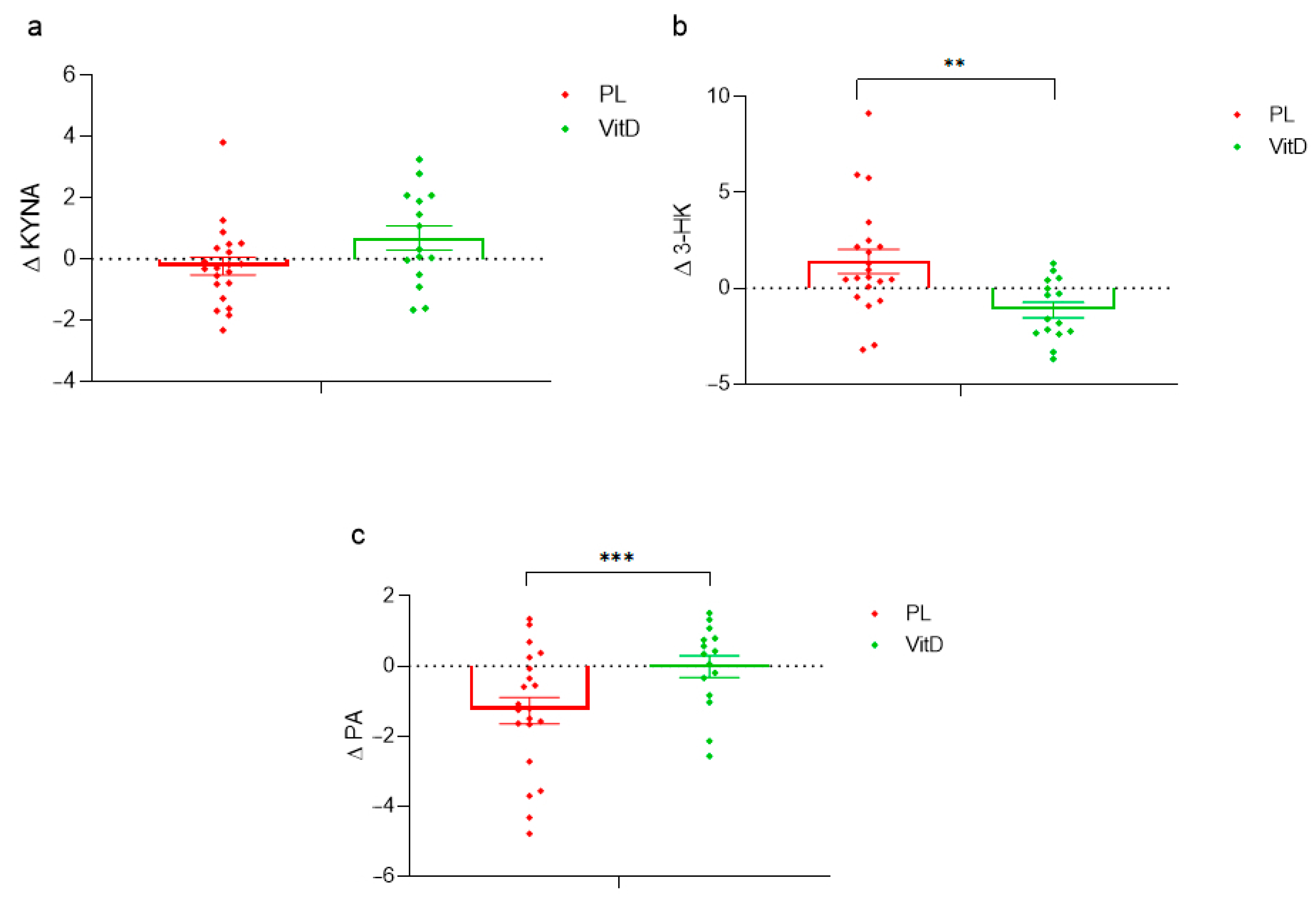
3.2. Kynurenine Pathway
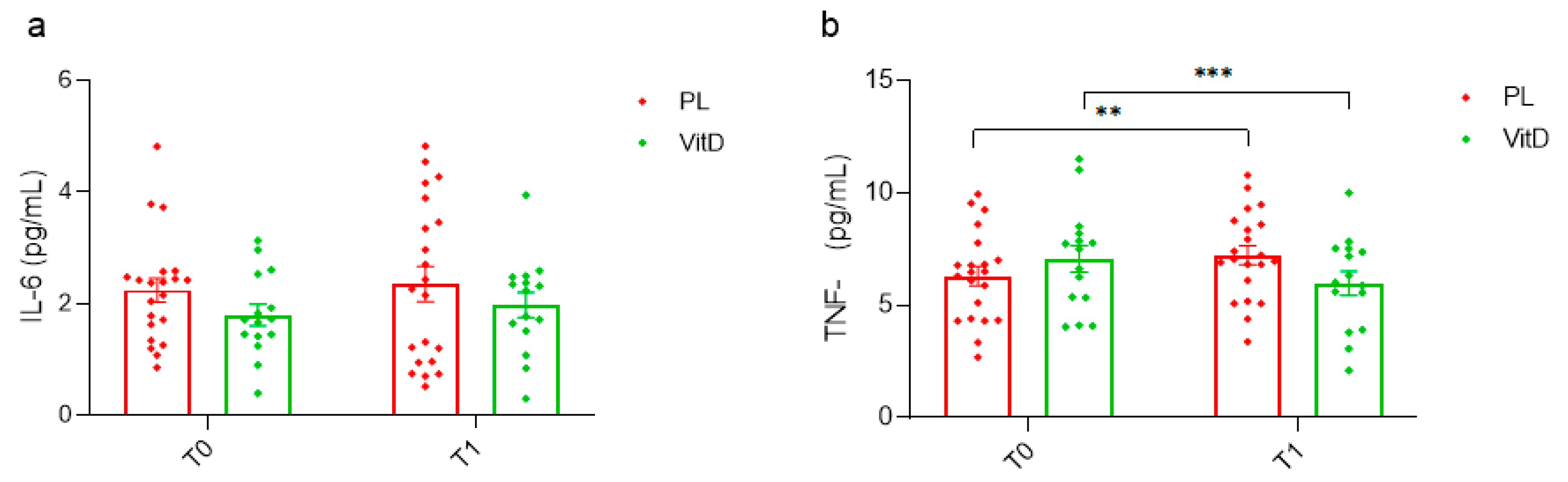
3.3. Inflammation Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Alexander, G.E. Biology of Parkinson’s Disease: Pathogenesis and Pathophysiology of a Multisystem Neurodegenerative Disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Lianeri, M.; Kozubski, W. Molecular Effects of L-Dopa Therapy in Parkinson’s Disease. Curr. Genom. 2014, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Macerollo, A.; Zrinzo, L.; Akram, H.; Foltynie, T.; Limousin, P. Subthalamic Nucleus Deep Brain Stimulation for Parkinson’s Disease: Current Trends and Future Directions. Expert Rev. Med. Devices 2020, 17, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D Signalling Pathways in Cancer: Potential for Anticancer Therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on Skeletal Muscle Function: Oxidative Stress, Energy Metabolism and Anabolic State. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Koduah, P.; Paul, F.; Dörr, J.-M. Vitamin D in the Prevention, Prediction and Treatment of Neurodegenerative and Neuroinflammatory Diseases. EPMA J. 2017, 8, 313–325. [Google Scholar] [CrossRef]
- Plantone, D.; Primiano, G.; Manco, C.; Locci, S.; Servidei, S.; De Stefano, N. Vitamin D in Neurological Diseases. Int. J. Mol. Sci. 2023, 24, 87. [Google Scholar] [CrossRef]
- Barichella, M.; Cereda, E.; Iorio, L.; Pinelli, G.; Ferri, V.; Cassani, E.; Bolliri, C.; Caronni, S.; Pusani, C.; Schiaffino, M.G.; et al. Clinical Correlates of Serum 25-Hydroxyvitamin D in Parkinson’s Disease. Nutr. Neurosci. 2022, 25, 1128–1136. [Google Scholar] [CrossRef]
- Peterson, A.L.; Murchison, C.; Zabetian, C.; Leverenz, J.B.; Watson, G.S.; Montine, T.; Carney, N.; Bowman, G.L.; Edwards, K.; Quinn, J.F. Memory, Mood, and Vitamin d in Persons with Parkinson’s Disease. J. Park. Dis. 2013, 3, 547–555. [Google Scholar] [CrossRef]
- Fullard, M.E.; Duda, J.E. A Review of the Relationship Between Vitamin D and Parkinson Disease Symptoms. Front. Neurol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin D Supplementation in Parkinson Disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef]
- Bytowska, Z.K.; Korewo-Labelle, D.; Berezka, P.; Kowalski, K.; Przewłócka, K.; Libionka, W.; Kloc, W.; Kaczor, J.J. Effect of 12-Week BMI-Based Vitamin D3 Supplementation in Parkinson’s Disease with Deep Brain Stimulation on Physical Performance, Inflammation, and Vitamin D Metabolites. IJMS 2023, 24, 10200. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Qin, W.; Shi, Y.; Chen, W.; Jia, X.; Asakawa, T. Can Kynurenine Pathway Be Considered as a Next-Generation Therapeutic Target for Parkinson’s Disease? An Update Information. BioScience Trends 2022, 16, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Lee, S.H.; Koh, J.-M. Clinical Insights into the Kynurenine Pathway in Age-Related Diseases. Exp. Gerontol. 2020, 130, 110793. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, K.; Otsuka, C.; Maeda, T.; Yamahara, K.; Kato, K.; Takahashi, K.; Takahashi, K.; Terayama, Y. Impaired Metabolism of Kynurenine and Its Metabolites in CSF of Parkinson’s Disease. Neurosci. Lett. 2020, 714, 134576. [Google Scholar] [CrossRef]
- Lim, C.K.; Fernández-Gomez, F.J.; Braidy, N.; Estrada, C.; Costa, C.; Costa, S.; Bessede, A.; Fernandez-Villalba, E.; Zinger, A.; Herrero, M.T.; et al. Involvement of the Kynurenine Pathway in the Pathogenesis of Parkinson’s Disease. Prog. Neurobiol. 2017, 155, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease. JAMA Neurol. 2016, 73, 1316. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, C.-C.; Chiang, H.-L.; Liou, J.-M.; Chang, C.-M.; Lu, T.-P.; Chuang, E.Y.; Tai, Y.-C.; Cheng, C.; Lin, H.-Y.; et al. Altered Gut Microbiota and Inflammatory Cytokine Responses in Patients with Parkinson’s Disease. J. Neuroinflammation 2019, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kim, H.J.; Kim, A.; Jang, M.; Kim, A.; Kim, Y.; Yoo, D.; Im, J.H.; Choi, J.H.; Jeon, B. Peripheral Blood Inflammatory Markers in Early Parkinson’s Disease. J. Clin. Neurosci. 2018, 58, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.B.; Silveira, A.L.M.; Teixeira, A.L. The Involvement of Kynurenine Pathway in Neurodegenerative Diseases. Curr. Neuropharmacol. 2023, 21, 260–272. [Google Scholar] [CrossRef]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Escobar Galvis, M.L.; Brundin, P.; Huang, X.; et al. Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 2020, 35, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Vermeiren, Y.; Faassen, M.; Ley, C.; Nollen, E.A.A.; Kema, I.P.; De Deyn, P.P. Age- and Disease-specific Changes of the Kynurenine Pathway in Parkinson’s and Alzheimer’s Disease. J. Neurochem. 2019, 151, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Peripheral Tryptophan—Kynurenine Metabolism Associated with Metabolic Syndrome Is Different in Parkinson’s and Alzheimer’s Diseases. Endocrinol. Diabetes Metab. J. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Chang, K.-H.; Cheng, M.-L.; Tang, H.-Y.; Huang, C.-Y.; Wu, Y.-R.; Chen, C.-M. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef]
- Ogawa, T.; Matson, W.R.; Beal, M.F.; Myers, R.H.; Bird, E.D.; Milbury, P.; Saso, S. Kynurenine Pathway Abnormalities in Parkinson’s Disease. Neurology 1992, 42, 1702. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine Metabolism in Plasma and in Red Blood Cells in Parkinson’s Disease. J. Neurol. Sci. 2005, 239, 31–35. [Google Scholar] [CrossRef]
- Lewitt, P.A.; Li, J.; Lu, M.; Beach, T.G.; Adler, C.H.; Guo, L. 3-Hydroxykynurenine and Other Parkinson’s Disease Biomarkers Discovered by Metabolomic Analysis. Mov. Disord. 2013, 28, 1653–1660. [Google Scholar] [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef]
- Burassakarn, A.; Srisathaporn, S.; Pientong, C.; Wongjampa, W.; Vatanasapt, P.; Patarapadungkit, N.; Ekalaksananan, T. Exosomes-Carrying Epstein-Barr Virus-Encoded Small RNA-1 Induces Indoleamine 2,3-Dioxygenase Expression in Tumor-Infiltrating Macrophages of Oral Squamous-Cell Carcinomas and Suppresses T-Cell Activity by Activating RIG-I/IL-6/TNF-α Pathway. Oral Oncol. 2021, 117, 105279. [Google Scholar] [CrossRef] [PubMed]
- Xiromerisiou, G.; Marogianni, C.; Lampropoulos, I.C.; Dardiotis, E.; Speletas, M.; Ntavaroukas, P.; Androutsopoulou, A.; Kalala, F.; Grigoriadis, N.; Papoutsopoulou, S. Peripheral Inflammatory Markers TNF-α and CCL2 Revisited: Association with Parkinson’s Disease Severity. Int. J. Mol. Sci. 2022, 24, 264. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Guo, P.; Zhang, Y.; Li, J.; Li, L.; Ding, D.; Li, D.; Zhang, W.; Guan, H.; Wang, X.; et al. Parkinson’s Disease With Depression: The Correlations Between Neuroinflammatory Factors and Neurotransmitters in Cerebrospinal Fluid. Front. Aging Neurosci. 2020, 12, 574776. [Google Scholar] [CrossRef] [PubMed]


| VitD Group (n = 15) | PL Group (n = 21) | |
|---|---|---|
| Age | 64 ± 9 years | 65 ± 6 years |
| Sex | 8 M, 7 W | 17 M, 4 W |
| Height | 169 ± 11 cm | 169 ± 15 cm |
| Body mass | 78 ± 12 kg | 80 ± 20 kg |
| H&y | 2.5 | 2.5 |
| Duration of the disease | 8–13 years | 8–13 years |
| Time from DBS implantation | 3–5 years | 3–5 years |
| 25(OH)D3 T0 (ng/mL) | 24.25 ± 9.23 | 18.99 ± 10.95 |
| 25(OH)D3 T1 (ng/mL) | 34.12 ± 10.75 | 18.38 ± 11.98 |
| 24,25(OH)2D3 T0 (ng/mL) | 2.05 ± 1.25 | 1.43 ± 1.11 |
| 24,25(OH)2D3 T1 (ng/mL) | 2.85 ± 1.02 | 1.31 ± 1.21 |
| BMI | ≤25→4 | ≤25→8 |
| 25–30→8 | 25–30→10 | |
| ≥30→3 | ≥30→3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bytowska, Z.K.; Korewo-Labelle, D.; Kowalski, K.; Libionka, W.; Przewłócka, K.; Kloc, W.; Kaczor, J.J. Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status. Nutrients 2023, 15, 3839. https://doi.org/10.3390/nu15173839
Bytowska ZK, Korewo-Labelle D, Kowalski K, Libionka W, Przewłócka K, Kloc W, Kaczor JJ. Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status. Nutrients. 2023; 15(17):3839. https://doi.org/10.3390/nu15173839
Chicago/Turabian StyleBytowska, Zofia Kinga, Daria Korewo-Labelle, Konrad Kowalski, Witold Libionka, Katarzyna Przewłócka, Wojciech Kloc, and Jan Jacek Kaczor. 2023. "Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status" Nutrients 15, no. 17: 3839. https://doi.org/10.3390/nu15173839
APA StyleBytowska, Z. K., Korewo-Labelle, D., Kowalski, K., Libionka, W., Przewłócka, K., Kloc, W., & Kaczor, J. J. (2023). Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status. Nutrients, 15(17), 3839. https://doi.org/10.3390/nu15173839








