Relationship between Bladder Cancer, Nutritional Supply, and Treatment Strategies: A Comprehensive Review
Abstract
1. Introduction
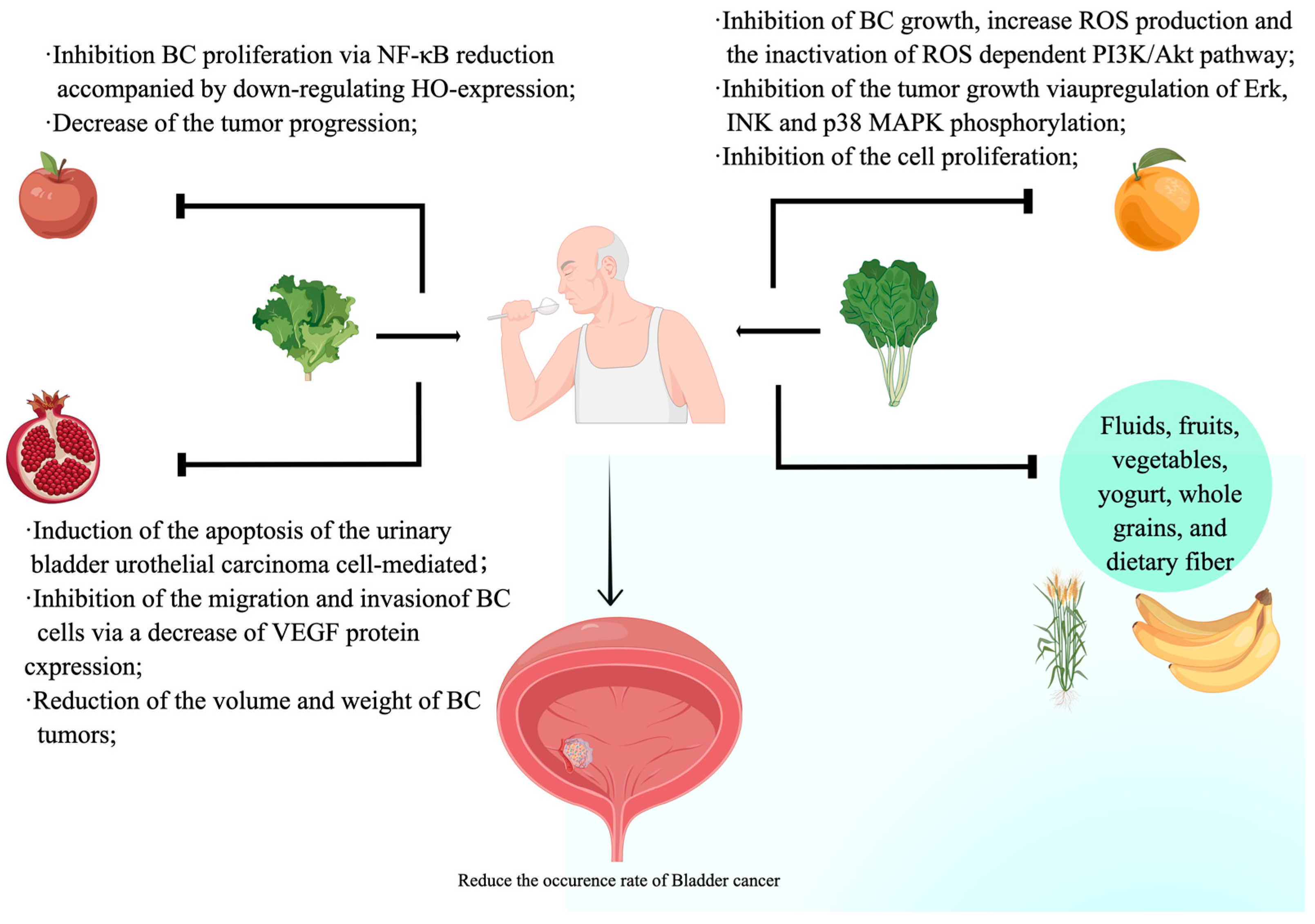
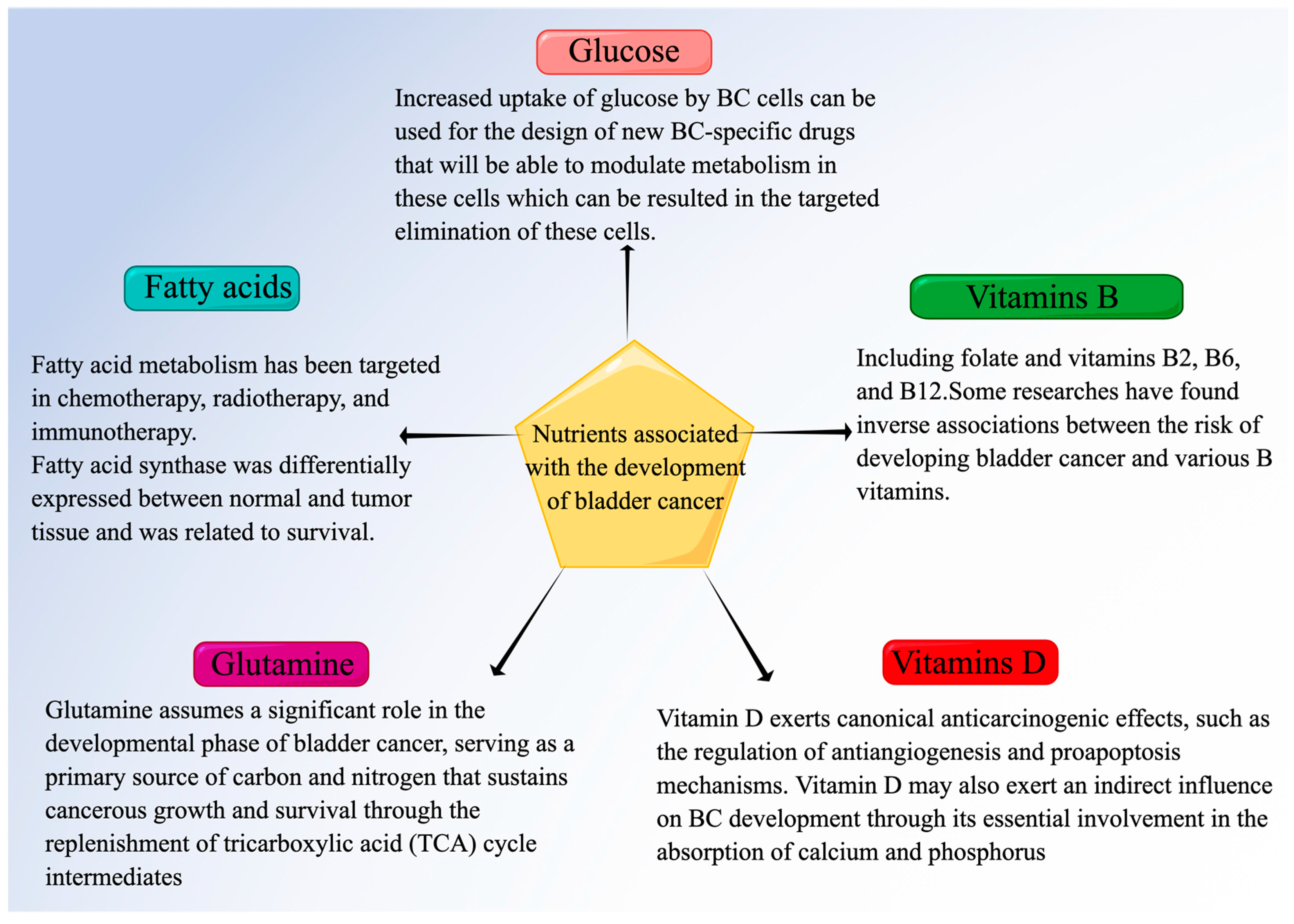
2. The Relationship between the Development of Bladder Cancer and Nutrients
3. Effects of Nutrients on Bladder Cancer Progression
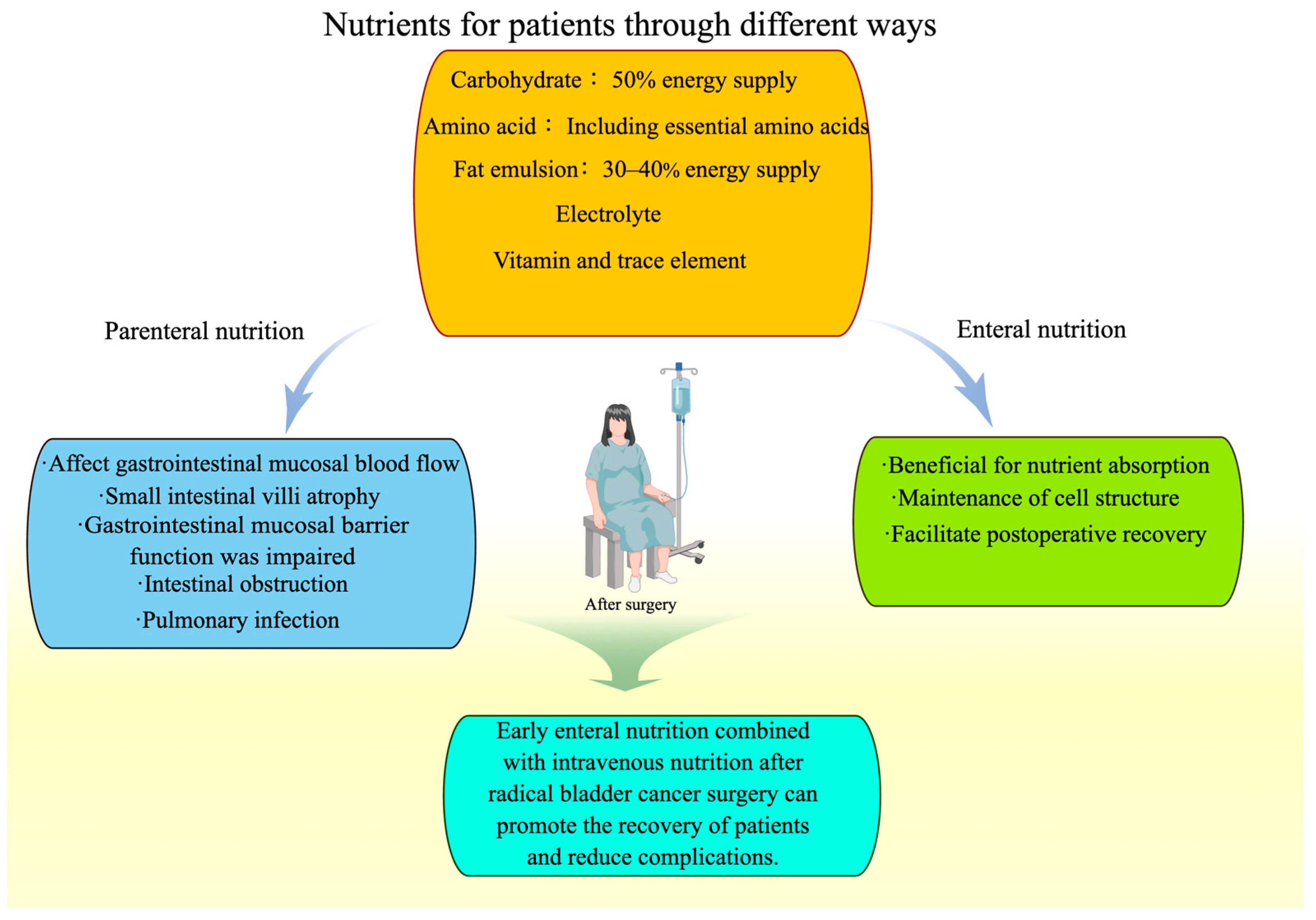
4. Perioperative Nutrition for Bladder Cancer Patients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lopez-Beltran, A. Bladder cancer: Clinical and pathological profile. Scand. J. Urol. Nephrol. Suppl. 2008, 218, 95–109. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Noon, A.P.; Albertsen, P.C.; Thomas, F.; Rosario, D.J.; Catto, J.W. Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. Br. J. Cancer 2013, 108, 1534–1540. [Google Scholar] [CrossRef]
- Westhoff, E.; Wu, X.; Kiemeney, L.A.; Lerner, S.P.; Ye, Y.; Huang, M.; Dinney, C.P.; Vrieling, A.; Tu, H. Dietary patterns and risk of recurrence and progression in non-muscle-invasive bladder cancer. Int. J. Cancer 2018, 142, 1797–1804. [Google Scholar] [CrossRef]
- De Stefani, E.; Boffetta, P.; Ronco, A.L.; Deneo-Pellegrini, H.; Acosta, G.; Mendilaharsu, M. Dietary patterns and risk of bladder cancer: A factor analysis in Uruguay. Cancer Causes Control 2008, 19, 1243–1249. [Google Scholar] [CrossRef]
- Chedgy, E.C.; Black, P.C. Radical Cystectomy and the Multidisciplinary Management of Muscle-Invasive Bladder Cancer. JAMA Oncol. 2016, 2, 855–856. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.L.; Liss, M.A.; Nseyo, U.U.; Gal, D.B.; Kane, C.J.; Kader, A.K. Weight Loss Following Radical Cystectomy for Bladder Cancer: Characterization and Effect on Survival. Clin. Genitourin. Cancer 2017, 15, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Droller, M.J. Urologic Oncology: Seminars and Original Investigations. A Twenty-Fifth Anniversary History. Urol. Oncol. 2021, 39, 506–513. [Google Scholar] [CrossRef]
- Acham, M.; Wesselius, A.; van Osch, F.H.M.; Yu, E.Y.; van den Brandt, P.A.; White, E.; Adami, H.O.; Weiderpass, E.; Brinkman, M.; Giles, G.G.; et al. Intake of milk and other dairy products and the risk of bladder cancer: A pooled analysis of 13 cohort studies. Eur. J. Clin. Nutr. 2020, 74, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ollberding, N.J.; Woolcott, C.G.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J. Nutr. 2013, 143, 1283–1292. [Google Scholar] [CrossRef]
- Yu, E.Y.W.; Wesselius, A.; Mehrkanoon, S.; Brinkman, M.; van den Brandt, P.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; Gunter, M.; Huybrechts, I.; et al. Grain and dietary fiber intake and bladder cancer risk: A pooled analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 112, 1252–1266. [Google Scholar]
- Piyathilake, C. Dietary factors associated with bladder cancer. Investig. Clin. Urol. 2016, 57 (Suppl. S1), S14–S25. [Google Scholar] [CrossRef]
- Cohen, S.M.; Johansson, S.L. Epidemiology and etiology of bladder cancer. Urol. Clin. N. Am. 1992, 19, 421–428. [Google Scholar] [CrossRef]
- Lumbreras, B.; Garte, S.; Overvad, K.; Tjonneland, A.; Clavel-Chapelon, F.; Linseisen, J.P.; Boeing, H.; Trichopoulou, A.; Palli, D.; Peluso, M.; et al. Meat intake and bladder cancer in a prospective study: A role for heterocyclic aromatic amines? Cancer Causes Control 2008, 19, 649–656. [Google Scholar] [CrossRef]
- Shao, A.; Drewnowski, A.; Willcox, D.C.; Krämer, L.; Lausted, C.; Eggersdorfer, M.; Mathers, J.; Bell, J.D.; Randolph, R.K.; Witkamp, R.; et al. Optimal nutrition and the ever-changing dietary landscape: A conference report. Eur. J. Nutr. 2017, 56 (Suppl. S1), 1–21. [Google Scholar] [CrossRef]
- Steinmaus, C.M.; Nuñez, S.; Smith, A.H. Diet and bladder cancer: A meta-analysis of six dietary variables. Am. J. Epidemiol. 2000, 151, 693–702. [Google Scholar] [CrossRef]
- Chyou, P.H.; Nomura, A.M.; Stemmermann, G.N. A prospective study of diet, smoking, and lower urinary tract cancer. Ann. Epidemiol. 1993, 3, 211–216. [Google Scholar] [CrossRef]
- Riboli, E.; González, C.A.; López-Abente, G.; Errezola, M.; Izarzugaza, I.; Escolar, A.; Nebot, M.; Hémon, B.; Agudo, A. Diet and bladder cancer in Spain: A multi-centre case-control study. Int. J. Cancer 1991, 49, 214–219. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H. Meat intake and risk of bladder cancer: A meta-analysis. Med. Oncol. 2012, 29, 848–855. [Google Scholar] [CrossRef]
- Catsburg, C.E.; Gago-Dominguez, M.; Yuan, J.M.; Castelao, J.E.; Cortessis, V.K.; Pike, M.C.; Stern, M.C. Dietary sources of N-nitroso compounds and bladder cancer risk: Findings from the Los Angeles bladder cancer study. Int. J. Cancer 2014, 134, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Narii, N.; Sobue, T.; Zha, L.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Yamaji, T.; Tsugane, S. Vegetable and fruit intake and the risk of bladder cancer: Japan Public Health Center-based prospective study. Br. J. Cancer 2022, 126, 1647–1658. [Google Scholar] [CrossRef]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. Clinical Potential of Fruit in Bladder Cancer Prevention and Treatment. Nutrients 2022, 14, 1132. [Google Scholar] [CrossRef]
- Norat, T.; Aune, D.; Chan, D.; Romaguera, D. Fruits and vegetables: Updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Adv. Nutr. Cancer 2014, 159, 35–50. [Google Scholar]
- Liu, H.; Wang, X.C.; Hu, G.H.; Guo, Z.F.; Lai, P.; Xu, L.; Huang, T.B.; Xu, Y.F. Fruit and vegetable consumption and risk of bladder cancer: An updated meta-analysis of observational studies. Eur. J. Cancer Prev. 2015, 24, 508–516. [Google Scholar] [CrossRef]
- Yao, B.; Yan, Y.; Ye, X.; Fang, H.; Xu, H.; Liu, Y.; Li, S.; Zhao, Y. Intake of fruit and vegetables and risk of bladder cancer: A dose-response meta-analysis of observational studies. Cancer Causes Control 2014, 25, 1645–1658. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, X.T.; Liu, T.Z.; Zhang, C.; Yang, Z.H.; Li, S.; Chen, X.Y. Fruits and vegetables intake and risk of bladder cancer: A PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine 2015, 94, e759. [Google Scholar] [CrossRef]
- Vieira, A.R.; Vingeliene, S.; Chan, D.S.; Aune, D.; Abar, L.; Navarro Rosenblatt, D.; Greenwood, D.C.; Norat, T. Fruits, vegetables, and bladder cancer risk: A systematic review and meta-analysis. Cancer Med. 2015, 4, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), 559s–569s. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Ding, H.; Chin, Y.W.; Kinghorn, A.D.; D’Ambrosio, S.M. Chemopreventive characteristics of avocado fruit. Semin. Cancer Biol. 2007, 17, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Arteaga, J.R.; Zhang, Q.; Huerta, S.; Go, V.L.; Heber, D. Inhibition of prostate cancer cell growth by an avocado extract: Role of lipid-soluble bioactive substances. J. Nutr. Biochem. 2005, 16, 23–30. [Google Scholar] [CrossRef]
- Chang, C.P.; Chan, Y.Y.; Li, C.F.; Chien, L.H.; Lee, S.T.; Wu, T.F. Deciphering the Molecular Mechanism Underlying the Inhibitory Efficacy of Taiwanese Local Pomegranate Peels against Urinary Bladder Urothelial Carcinoma. Nutrients 2018, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Jones, K.; Moore, R.; Barnes, S.; Leahy, M.; Roderick, R.; Juliana, M.M.; Grubbs, C.J. Effect of cranberry juice concentrate on chemically-induced urinary bladder cancers. Oncol. Rep. 2008, 19, 1565–1570. [Google Scholar] [PubMed]
- Roy, S.; Khanna, S.; Alessio, H.M.; Vider, J.; Bagchi, D.; Bagchi, M.; Sen, C.K. Anti-angiogenic property of edible berries. Free Radic. Res. 2002, 36, 1023–1031. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid Intake and Circulating Carotenoids Are Inversely Associated with the Risk of Bladder Cancer: A Dose-Response Meta-analysis. Adv. Nutr. 2020, 11, 630–643. [Google Scholar] [CrossRef]
- Dianatinasab, M.; Wesselius, A.; Salehi-Abargouei, A.; Yu, E.Y.W.; Fararouei, M.; Brinkman, M.; van den Brandt, P.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; et al. Dietary fats and their sources in association with the risk of bladder cancer: A pooled analysis of 11 prospective cohort studies. Int. J. Cancer 2022, 151, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, C.I.; Pacheco, L.S.; Romanos-Nanclares, A.; Ecsedy, E.; Giovannucci, E.L.; Eliassen, A.H.; Mucci, L.A.; Fu, B.C. Prospective Study of Avocado Consumption and Cancer Risk in U.S. Men and Women. Cancer Prev. Res. 2023, 16, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-L.; Kuo, Y.-M.; Lee, Y.-R.; Chen, W.-J.; Lee, Y.-S.; Lee, H.-J. Apple polyphenol decelerates bladder cancer growth involving apoptosis and cell cycle arrest in N-butyl-N-(4-hydroxybutyl) nitrosamine-induced experimental animal model. J. Funct. Foods 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Chatenoud, L.; Tavani, A.; La Vecchia, C.; Jacobs, D.R.; Jr Negri, E.; Levi, F.; Franceschi, S. Whole grain food intake and cancer risk. Int. J. Cancer 1998, 77, 24–28. [Google Scholar] [CrossRef]
- Yu, E.Y.W.; Wesselius, A.; Sinhart, C.; Wolk, A.; Stern, M.C.; Jiang, X.; Tang, L.; Marshall, J.; Kellen, E.; van den Brandt, P.; et al. A data mining approach to investigate food groups related to incidence of bladder cancer in the BLadder cancer Epidemiology and Nutritional Determinants International Study. Br. J. Nutr. 2020, 124, 611–619. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Taborelli, M.; Montella, M.; Libra, M.; La Vecchia, C.; Tavani, A.; Crispo, A.; Grimaldi, M.; Facchini, G.; Jenkins, D.J.A.; et al. Associations of dietary carbohydrates, glycaemic index and glycaemic load with risk of bladder cancer: A case-control study. Br. J. Nutr. 2017, 118, 722–729. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, Z.; Jia, H.; Xiu, Y.; Liu, Z.; Deng, L. Properties of flavonoids in the treatment of bladder cancer (Review). Exp. Ther. Med. 2022, 24, 676. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, Z.; Zhao, Y.; Wang, D.; Li, Y.; Ma, L.; Li, X.; Li, J.; Xiao, N.; Tian, J.; et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008, 264, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zheng, X.Y.; Ye, J.; Wu, T.T.; Wang, J.; Chen, W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr. Cancer 2012, 64, 599–606. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.C.; Kuo, C.C.; Huang, Y.C.; Yeh, C.W.; Hseu, Y.C.; Liu, J.Y.; Hsu, L.S. Naringenin inhibits migration of bladder cancer cells through downregulation of AKT and MMP-2. Mol. Med. Rep. 2014, 10, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H.; Akiyama, T.; Ishida, J.; Watanabe, S.; Suzuki, K. A specific inhibitor for tyrosine protein kinase from Pseudomonas. J. Antibiot. 1986, 39, 606–608. [Google Scholar] [CrossRef]
- Chae, H.S.; Xu, R.; Won, J.Y.; Chin, Y.W.; Yim, H. Molecular Targets of Genistein and Its Related Flavonoids to Exert Anticancer Effects. Int. J. Mol. Sci. 2019, 20, 2420. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Di Gioia, G.; Pellegrino, M.R.; Muzio, L.L. Genistein as a Potential Anticancer Agent Against Head and Neck Squamous Cell Carcinoma. Curr. Top. Med. Chem. 2018, 18, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Franke, A.A.; Blackburn, G.L.; Zhou, J.R. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006, 66, 1851–1858. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, W.; Shao, C.; Xu, P.; Shi, C.H.; Shi, J.G.; Li, Y.M.; Fu, Q.; Xue, W.; et al. Genistein sensitizes bladder cancer cells to HCPT treatment in vitro and in vivo via ATM/NF-kappaB/IKK pathway-induced apoptosis. PLoS ONE 2013, 8, e50175. [Google Scholar]
- Park, C.; Cha, H.J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.W.; Han, M.H.; Choi, S.H.; et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Kuhnle, G.G.; Williams, R.J.; Rice-Evans, C. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochem. J. 2003, 372 Pt 1, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van der Woude, H.; van Zanden, J.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M. Identification of o-quinone/quinone methide metabolites of quercetin in a cellular in vitro system. FEBS Lett. 2002, 520, 30–34. [Google Scholar] [CrossRef]
- Prasain, J.K.; Rajbhandari, R.; Keeton, A.B.; Piazza, G.A.; Barnes, S. Metabolism and growth inhibitory activity of cranberry derived flavonoids in bladder cancer cells. Food Funct. 2016, 7, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Lotan, Y.; Wittmann, B.; Neri, B.; Hansel, D.E. Metabolomics analysis reveals distinct profiles of nonmuscle-invasive and muscle-invasive bladder cancer. Cancer Med. 2017, 6, 2106–2120. [Google Scholar] [CrossRef]
- Whyard, T.; Waltzer, W.C.; Waltzer, D.; Romanov, V. Metabolic alterations in bladder cancer: Applications for cancer imaging. Exp. Cell Res. 2016, 341, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E. Nutrition and bladder cancer. Cancer Causes Control 1996, 7, 95–100. [Google Scholar] [CrossRef]
- Deng, J.; Peng, M.; Zhou, S.; Xiao, D.; Hu, X.; Xu, S.; Wu, J.; Yang, X. Metformin targets Clusterin to control lipogenesis and inhibit the growth of bladder cancer cells through SREBP-1c/FASN axis. Signal Transduct. Target. Ther. 2021, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Feng, D.; Wang, Z.; Ying, Y.; Xu, C.; Wei, Q.; Zeng, S.; Yang, L. Fatty Acid Synthase Is the Key Regulator of Fatty Acid Metabolism and Is Related to Immunotherapy in Bladder Cancer. Front. Immunol. 2022, 13, 836939. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Haidar, M.; Kooij, G.; Hendriks, J.J.A. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv. Drug Deliv. Rev. 2020, 159, 198–213. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Coloff, J.L.; Murphy, J.P.; Braun, C.R.; Harris, I.S.; Shelton, L.M.; Kami, K.; Gygi, S.P.; Selfors, L.M.; Brugge, J.S. Differential Glutamate Metabolism in Proliferating and Quiescent Mammary Epithelial Cells. Cell Metab. 2016, 23, 867–880. [Google Scholar] [CrossRef]
- Tran, T.Q.; Lowman, X.H.; Reid, M.A.; Mendez-Dorantes, C.; Pan, M.; Yang, Y.; Kong, M. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene 2017, 36, 1991–2001. [Google Scholar] [CrossRef]
- Lea, M.A.; Altayyar, M.; desBordes, C. Inhibition of Growth of Bladder Cancer Cells by 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one in Combination with Other Compounds Affecting Glucose Metabolism. Anticancer Res. 2015, 35, 5889–5899. [Google Scholar]
- Wang, L.; Xu, T.; Yang, X.; Liang, Z.; Zhang, J.; Li, D.; Chen, Y.; Ma, G.; Wang, Y.; Liang, Y.; et al. Immunosuppression Induced by Glutamine Deprivation Occurs via Activating PD-L1 Transcription in Bladder Cancer. Front. Mol. Biosci. 2021, 8, 687305. [Google Scholar] [CrossRef]
- Petrella, G.; Ciufolini, G.; Vago, R.; Cicero, D.O. The Interplay between Oxidative Phosphorylation and Glycolysis as a Potential Marker of Bladder Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8107. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Dugué, P.A.; Brinkman, M.T.; Milne, R.L.; Wong, E.M.; FitzGerald, L.M.; Bassett, J.K.; Joo, J.E.; Jung, C.H.; Makalic, E.; Schmidt, D.F.; et al. Genome-wide measures of DNA methylation in peripheral blood and the risk of urothelial cell carcinoma: A prospective nested case-control study. Br. J. Cancer 2016, 115, 664–673. [Google Scholar] [CrossRef] [PubMed]
- García-Closas, R.; García-Closas, M.; Kogevinas, M.; Malats, N.; Silverman, D.; Serra, C.; Tardón, A.; Carrato, A.; Castaño-Vinyals, G.; Dosemeci, M.; et al. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur. J. Cancer 2007, 43, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, F.; Gao, L.; Chen, C.; Wei, J.; Zheng, Y.; Mao, F. Analysis of the metastatic mechanism and progress in the treatment of breast cancer liver metastasis: A narrative review. Transl. Cancer Res. 2023, 12, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Cross, A.J.; Baris, D.; Ward, M.H.; Karagas, M.R.; Johnson, A.; Schwenn, M.; Cherala, S.; Colt, J.S.; Cantor, K.P.; et al. Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br. J. Cancer 2012, 106, 1891–1898. [Google Scholar] [CrossRef]
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Brinkman, M.T.; Karagas, M.R.; Zens, M.S.; Schned, A.; Reulen, R.C.; Zeegers, M.P. Minerals and vitamins and the risk of bladder cancer: Results from the New Hampshire Study. Cancer Causes Control 2010, 21, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar]
- Mohanty, S.; Kamolvit, W.; Hertting, O.; Brauner, A. Vitamin D strengthens the bladder epithelial barrier by inducing tight junction proteins during E. coli urinary tract infection. Cell Tissue Res. 2020, 380, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Kojs, Z.; Bednarek, W.; Markowska, J.; Huczyński, A. Role of vitamin D(3) in selected malignant neoplasms. Nutrition 2020, 79–80, 110964. [Google Scholar] [CrossRef]
- Chen, F.; Li, Q.; Yu, Y.; Yang, W.; Shi, F.; Qu, Y. Association of vitamin C, vitamin D, vitamin E and risk of bladder cancer: A dose-response meta-analysis. Sci. Rep. 2015, 5, 9599. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, U.; Neef, N.; Leiterer, M.; Kiehntopf, M.; Kratzsch, J.; Jahreis, G. Effect of calcium phosphate and vitamin D3 supplementation on bone remodelling and metabolism of calcium, phosphorus, magnesium and iron. Nutr. J. 2014, 13, 6. [Google Scholar] [CrossRef]
- Boot, I.W.A.; Wesselius, A.; Yu, E.Y.W.; White, E.; Brustad, M.; Marques, C.; Ljungberg, B.; Zeegers, M.P. Dietary vitamin D intake and the bladder cancer risk: A pooled analysis of prospective cohort studies. Clin. Nutr. 2023, 42, 1462–1474. [Google Scholar]
- Lund, L.; Jacobsen, J.; Clark, P.; Borre, M.; Nørgaard, M. Impact of comorbidity on survival of invasive bladder cancer patients, 1996–2007: A Danish population-based cohort study. Urology 2010, 75, 393–398. [Google Scholar] [CrossRef]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur. Urol. 2009, 55, 164–174. [Google Scholar] [CrossRef]
- Philpott, M.; Ferguson, L.R. Immunonutrition and cancer. Mutat. Res. 2004, 551, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Zaloga, G.P. Immunonutrition in high-risk surgical patients: A systematic review and analysis of the literature. JPEN J. Parenter. Enteral. Nutr. 2010, 34, 378–386. [Google Scholar] [CrossRef]
- Lambert, J.W.; Ingham, M.; Gibbs, B.B.; Given, R.W.; Lance, R.S.; Riggs, S.B. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology 2013, 81, 587–592. [Google Scholar] [CrossRef]
- Roth, B.; Birkhäuser, F.D.; Zehnder, P.; Thalmann, G.N.; Huwyler, M.; Burkhard, F.C.; Studer, U.E. Parenteral nutrition does not improve postoperative recovery from radical cystectomy: Results of a prospective randomised trial. Eur. Urol. 2013, 63, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Terry, W.J.; Bueschen, A.J. Complications of radical cystectomy and correlation with nutritional assessment. Urology 1986, 27, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Enig, B.; Winther, E.; Hessov, I. Nutritional status of patients with cancer of the bladder before and during radiation therapy. Influence on survival? Radiother. Oncol. 1986, 5, 277–285. [Google Scholar] [CrossRef]
- Munbauhal, G.; Drouin, S.J.; Mozer, P.; Colin, P.; Phé, V.; Cussenot, O.; Rouprêt, M. Malnourishment in bladder cancer and the role of immunonutrition at the time of cystectomy: An overview for urologists. BJU Int. 2014, 114, 177–184. [Google Scholar] [CrossRef]
- Burden, S.; Billson, H.A.; Lal, S.; Owen, K.A.; Muneer, A. Perioperative nutrition for the treatment of bladder cancer by radical cystectomy. Cochrane Database Syst. Rev. 2019, 5, Cd010127. [Google Scholar] [CrossRef] [PubMed]
- Askanazi, J.; Hensle, T.W.; Starker, P.M.; Lockhart, S.H.; LaSala, P.A.; Olsson, C.; Kinney, J.M. Effect of immediate postoperative nutritional support on length of hospitalization. Ann Surg. 1986, 203, 236–239. [Google Scholar] [CrossRef]
- Heyland, D.K.; Montalvo, M.; MacDonald, S.; Keefe, L.; Su, X.Y.; Drover, J.W. Total parenteral nutrition in the surgical patient: A meta-analysis. Can. J. Surg. 2001, 44, 102–111. [Google Scholar]
- Maffezzini, M.; Gerbi, G.; Campodonico, F.; Parodi, D. A multimodal perioperative plan for radical cystectomy and urinary intestinal diversion: Effects, limits and complications of early artificial nutrition. J. Urol. 2006, 176, 945–948; discussion 948–949. [Google Scholar] [CrossRef]
- Azhar, R.A.; Bochner, B.; Catto, J.; Goh, A.C.; Kelly, J.; Patel, H.D.; Pruthi, R.S.; Thalmann, G.N.; Desai, M. Enhanced Recovery after Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs. Eur. Urol. 2016, 70, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Brodner, G.; Van Aken, H.; Hertle, L.; Fobker, M.; Von Eckardstein, A.; Goeters, C.; Buerkle, H.; Harks, A.; Kehlet, H. Multimodal perioperative management—Combining thoracic epidural analgesia, forced mobilization, and oral nutrition—Reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth. Analg. 2001, 92, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Rattray, M.; Roberts, S.; Marshall, A.; Desbrow, B. A systematic review of feeding practices among postoperative patients: Is practice in-line with evidenced-based guidelines? J. Hum. Nutr. Diet. 2018, 31, 151–167. [Google Scholar] [CrossRef]
- Jensen, B.T.; Lauridsen, S.V.; Jensen, J.B. Prehabilitation for major abdominal urologic oncology surgery. Curr. Opin. Urol. 2018, 28, 243–250. [Google Scholar] [CrossRef]
- Carli, F.; Scheede-Bergdahl, C. Prehabilitation to enhance perioperative care. Anesthesiol. Clin. 2015, 33, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.S.; Stein, B.; Charlebois, P.; Feldman, L.S.; et al. Prehabilitation versus rehabilitation: A randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.E.; Agnihotram, R.V.; Ferri, L.E.; Carli, F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018, 153, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.; Jack, S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br. J. Anaesth. 2015, 114, 244–251. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Bousquet-Dion, G.; Ferreira, V.; Austin, B.; Audi, C.; Tanguay, S.; Aprikian, A.; Carli, F.; Kassouf, W. Multimodal Prehabilitation to Enhance Functional Capacity Following Radical Cystectomy: A Randomized Controlled Trial. Eur. Urol. Focus 2021, 7, 132–138. [Google Scholar] [CrossRef]
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Wilmore, D.W.; Kehlet, H. Management of patients in fast track surgery. BMJ 2001, 322, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, M.; Casey, R.G.; Black, P.; Koupparis, A.J. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can. Urol. Assoc. J. 2011, 5, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Tyson, M.D.; Chang, S.S. Enhanced Recovery Pathways Versus Standard Care After Cystectomy: A Meta-analysis of the Effect on Perioperative Outcomes. Eur. Urol. 2016, 70, 995–1003. [Google Scholar] [CrossRef]
- Lin, T.; Li, K.; Liu, H.; Xue, X.; Xu, N.; Wei, Y.; Chen, Z.; Zhou, X.; Qi, L.; He, W.; et al. Enhanced recovery after surgery for radical cystectomy with ileal urinary diversion: A multi-institutional, randomized, controlled trial from the Chinese bladder cancer consortium. World J. Urol. 2018, 36, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Maffezzini, M.; Gerbi, G.; Campodonico, F.; Parodi, D. Multimodal perioperative plan for radical cystectomy and intestinal urinary diversion. I. Effect on recovery of intestinal function and occurrence of complications. Urology 2007, 69, 1107–1111. [Google Scholar] [CrossRef]
- Hamilton-Reeves, J.M.; Bechtel, M.D.; Hand, L.K.; Schleper, A.; Yankee, T.M.; Chalise, P.; Lee, E.K.; Mirza, M.; Wyre, H.; Griffin, J.; et al. Effects of Immunonutrition for Cystectomy on Immune Response and Infection Rates: A Pilot Randomized Controlled Clinical Trial. Eur. Urol. 2016, 69, 389–392. [Google Scholar] [CrossRef]
- Cerantola, Y. Myths and reality about immunonutrition in uro-oncology. Rev. Medicale Suisse 2017, 13, 2079–2082. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Saito, H.; Plank, L.D.; Jamieson, G.G.; Jagannath, P.; Hwang, T.L.; Mijares, J.M.; Bihari, D. Postsurgical infections are reduced with specialized nutrition support. World J. Surg. 2006, 30, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Drover, J.W.; Dhaliwal, R.; Weitzel, L.; Wischmeyer, P.E.; Ochoa, J.B.; Heyland, D.K. Perioperative use of arginine-supplemented diets: A systematic review of the evidence. J. Am. Coll. Surg. 2011, 212, 385–399. [Google Scholar] [CrossRef]
- Abunnaja, S.; Cuviello, A.; Sanchez, J.A. Enteral and parenteral nutrition in the perioperative period: State of the art. Nutrients 2013, 5, 608–623. [Google Scholar] [CrossRef]
- Yang, H.; Söderholm, J.D.; Larsson, J.; Permert, J.; Lindgren, J.; Wirén, M. Bidirectional supply of glutamine maintains enterocyte ATP content in the in vitro using chamber model. Int. J. Colorectal. Dis. 2000, 15, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Alivizatos, V.; Athanasopoulos, P.; Makris, N.; Karageorgos, N. Early postoperative glutamine-supplemented parenteral nutrition versus enteral immunonutrition in cancer patients undergoing major gastrointestinal surgery. J. BUON 2005, 10, 119–122. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, G.; Wei, J.; Dong, Y.; Zhang, X.; Zheng, Y. Relationship between Bladder Cancer, Nutritional Supply, and Treatment Strategies: A Comprehensive Review. Nutrients 2023, 15, 3812. https://doi.org/10.3390/nu15173812
Yang F, Liu G, Wei J, Dong Y, Zhang X, Zheng Y. Relationship between Bladder Cancer, Nutritional Supply, and Treatment Strategies: A Comprehensive Review. Nutrients. 2023; 15(17):3812. https://doi.org/10.3390/nu15173812
Chicago/Turabian StyleYang, Fan, Guanmo Liu, Jiaxin Wei, Yucheng Dong, Xuebin Zhang, and Yongchang Zheng. 2023. "Relationship between Bladder Cancer, Nutritional Supply, and Treatment Strategies: A Comprehensive Review" Nutrients 15, no. 17: 3812. https://doi.org/10.3390/nu15173812
APA StyleYang, F., Liu, G., Wei, J., Dong, Y., Zhang, X., & Zheng, Y. (2023). Relationship between Bladder Cancer, Nutritional Supply, and Treatment Strategies: A Comprehensive Review. Nutrients, 15(17), 3812. https://doi.org/10.3390/nu15173812




