Pregnancy Cholesterol Metabolism Markers and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Laboratory Determinations
2.3. Outcome Assessment
2.4. Other Variables
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Serum Biochemical Indicators and Cholesterol Metabolism Markers Concentrations of the Participants
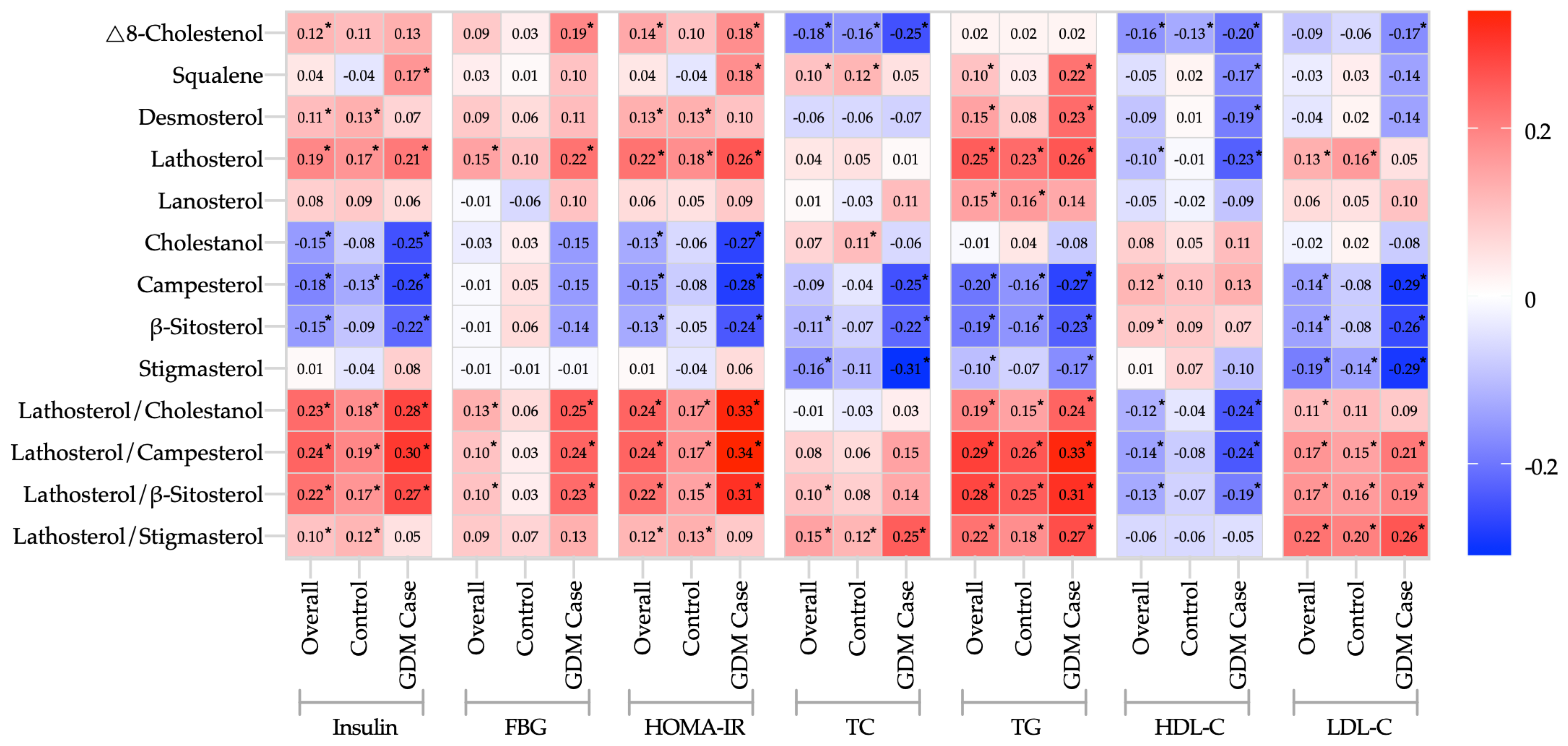
3.3. Correlations between Cholesterol Metabolism Markers and Biochemical Indicators
3.4. Association between Cholesterol Metabolism Markers and the Risk of GDM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.; Gomersall, J.C.; Tieu, J.; Han, S.; Crowther, C.A.; Middleton, P. Combined Diet and Exercise Interventions for Preventing Gestational Diabetes Mellitus. Cochrane Database Syst. Rev. 2017, 2017, CD010443. [Google Scholar] [CrossRef] [PubMed]
- Sadiya, A.; Jakapure, V.; Shaar, G.; Adnan, R.; Tesfa, Y. Lifestyle Intervention in Early Pregnancy Can Prevent Gestational Diabetes in High-Risk Pregnant Women in the UAE: A Randomized Controlled Trial. BMC Pregnancy Childbirth 2022, 22, 668. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of Liver in the Maintenance of Cholesterol and Low Density Lipoprotein Homeostasis in Different Animal Species, Including Humans. J. Lipid Res. 1993, 34, 1637–1659. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; US Department of Health and Human Services and US Department of Agriculture: Washington, DC, USA, 2015. [Google Scholar]
- Wu, Y.; Sun, G.; Zhou, X.; Zhong, C.; Chen, R.; Xiong, T.; Li, Q.; Yi, N.; Xiong, G.; Hao, L.; et al. Pregnancy Dietary Cholesterol Intake, Major Dietary Cholesterol Sources, and the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Clin. Nutr. 2020, 39, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cui, C.-Y. Dietary Cholesterol Intake and Risk of Gestational Diabetes Mellitus: A Meta-Analysis of Observational Studies. J. Am. Coll. Nutr. 2021, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Gylling, H.; Nissinen, M.J. The Role of Serum Non-Cholesterol Sterols as Surrogate Markers of Absolute Cholesterol Synthesis and Absorption. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 765–769. [Google Scholar] [CrossRef]
- Simonen, P.; Gylling, H.; Miettinen, T.A. The Validity of Serum Squalene and Non-Cholesterol Sterols as Surrogate Markers of Cholesterol Synthesis and Absorption in Type 2 Diabetes. Atherosclerosis 2008, 197, 883–888. [Google Scholar] [CrossRef]
- Simonen, P.P.; Gylling, H.K.; Miettinen, T.A. Diabetes Contributes to Cholesterol Metabolism Regardless of Obesity. Diabetes Care 2002, 25, 1511–1515. [Google Scholar] [CrossRef]
- Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. [Google Scholar] [CrossRef]
- Weingärtner, O.; Lütjohann, D.; Böhm, M.; Laufs, U. Relationship between Cholesterol Synthesis and Intestinal Absorption Is Associated with Cardiovascular Risk. Atherosclerosis 2010, 210, 362–365. [Google Scholar] [CrossRef]
- Weingärtner, O.; Lütjohann, D.; Vanmierlo, T.; Müller, S.; Günther, L.; Herrmann, W.; Böhm, M.; Laufs, U.; Herrmann, M. Markers of Enhanced Cholesterol Absorption Are a Strong Predictor for Cardiovascular Diseases in Patients without Diabetes Mellitus. Chem. Phys. Lipids 2011, 164, 451–456. [Google Scholar] [CrossRef]
- de Mello, V.D.; Lindstrom, J.; Eriksson, J.G.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Pihlajamaki, J.; Tuomilehto, J.; Uusitupa, M. Markers of Cholesterol Metabolism as Biomarkers in Predicting Diabetes in the Finnish Diabetes Prevention Study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 635–642. [Google Scholar] [CrossRef]
- Cederberg, H.; Gylling, H.; Miettinen, T.A.; Paananen, J.; Vangipurapu, J.; Pihlajamaki, J.; Kuulasmaa, T.; Stancakova, A.; Smith, U.; Kuusisto, J.; et al. Non-Cholesterol Sterol Levels Predict Hyperglycemia and Conversion to Type 2 Diabetes in Finnish Men. PLoS ONE 2013, 8, e67406. [Google Scholar] [CrossRef][Green Version]
- Miettinen, H.E.; Rono, K.; Koivusalo, S.; Stach-Lempinen, B.; Poyhonen-Alho, M.; Eriksson, J.G.; Hiltunen, T.P.; Gylling, H. Elevated Serum Squalene and Cholesterol Synthesis Markers in Pregnant Obese Women with Gestational Diabetes Mellitus. J. Lipid Res. 2014, 55, 2644–2654. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Gylling, H.; Hallikainen, M.; Pihlajamaki, J.; Simonen, P.; Kuusisto, J.; Laakso, M.; Miettinen, T.A. Insulin Sensitivity Regulates Cholesterol Metabolism to a Greater Extent than Obesity: Lessons from the METSIM Study. J. Lipid Res. 2010, 51, 2422–2427. [Google Scholar] [CrossRef]
- Simonen, P.; Gylling, H.; Howard, A.N.; Miettinen, T.A. Introducing a New Component of the Metabolic Syndrome: Low Cholesterol Absorption. Am. J. Clin. Nutr. 2000, 72, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Tuominen, J.A.; Koivisto, V.A.; Miettinen, T.A. Cholesterol Metabolism in Type 1 Diabetes. Diabetes 2004, 53, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Miettinen, T.A. Cholesterol Absorption, Synthesis, and LDL Metabolism in NIDDM. Diabetes Care 1997, 20, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Blaha, V.; Andrys, C.; Smahelova, A.; Knizek, J.; Hyspler, R.; Solichova, D.; Blaha, M.; Zadak, Z. Effect of Atorvastatin on Soluble CD14, CD40 Ligand, sE- and sP-Selectins and MCP-1 in Patients with Type 2 Diabetes Mellitus: Relationship to Cholesterol Turnover. Pharmacol. Res. 2006, 54, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Ijima, T.; Kamiyama, H.; Kamiko, K.; Terauchi, Y. Anagliptin Decreases Serum Lathosterol Level in Patients with Type 2 Diabetes: A Pilot Study. Expert Opin. Expert Opin. Pharmacother. 2015, 16, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Smahelova, A.; Hyspler, R.; Haas, T.; Ticha, A.; Blaha, V.; Zadak, Z. Effect of Atorvastatin on Non-Cholesterol Sterols in Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease. Pharmacol. Res. 2005, 51, 31–36. [Google Scholar] [CrossRef]
- Pihlajamäki, J.; Gylling, H.; Miettinen, T.A.; Laakso, M. Insulin Resistance Is Associated with Increased Cholesterol Synthesis and Decreased Cholesterol Absorption in Normoglycemic Men. J. Lipid Res. 2004, 45, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M.R.; Sellke, F.W. Insulin Resistance Is Associated with Increased Cholesterol Synthesis, Decreased Cholesterol Absorption and Enhanced Lipid Response to Statin Therapy. Atherosclerosis 2010, 211, 260–265. [Google Scholar] [CrossRef]
- Simonen, P.P.; Gylling, H.; Miettinen, T.A. Body Weight Modulates Cholesterol Metabolism in Non-Insulin Dependent Type 2 Diabetics. Obes. Res. 2002, 10, 328–335. [Google Scholar] [CrossRef] [PubMed]
- van der Wulp, M.Y.M.; Verkade, H.J.; Groen, A.K. Regulation of Cholesterol Homeostasis. Mol. Cell. Endocrinol. 2013, 368, 1–16. [Google Scholar] [CrossRef]
- Nissinen, M.J.; Gylling, H.; Miettinen, T.A. Responses of Surrogate Markers of Cholesterol Absorption and Synthesis to Changes in Cholesterol Metabolism during Various Amounts of Fat and Cholesterol Feeding among Healthy Men. Br. J. Nutr. 2008, 99, 370–378. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Gylling, H. Cholesterol Absorption Efficiency and Sterol Metabolism in Obesity. Atherosclerosis 2000, 153, 241–248. [Google Scholar] [CrossRef]
- Bosner, M.S.; Lange, L.G.; Stenson, W.F.; Ostlund, R.E. Percent Cholesterol Absorption in Normal Women and Men Quantified with Dual Stable Isotopic Tracers and Negative Ion Mass Spectrometry. J. Lipid Res. 1999, 40, 302–308. [Google Scholar] [CrossRef]
- Gylling, H.; Hallikainen, M.; Pihlajamaki, J.; Agren, J.; Laakso, M.; Rajaratnam, R.A.; Rauramaa, R.; Miettinen, T.A. Polymorphisms in the ABCG5 and ABCG8 Genes Associate with Cholesterol Absorption and Insulin Sensitivity. J. Lipid Res. 2004, 45, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Overexpression of ABCG5 and ABCG8 Promotes Biliary Cholesterol Secretion and Reduces Fractional Absorption of Dietary Cholesterol. J. Clin. Investig. 2002, 110, 671–680. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 444) | Control (n = 296) | GDM Case (n = 148) | p-Value † | |
|---|---|---|---|---|
| Characteristics | ||||
| Gestational age of serum sample (week) | 13.9 ± 2.0 | 13.9 ± 2.1 | 13.9 ± 2.0 | 0.69 |
| Gestational age at OGTT (week) | 25.3 ± 1.6 | 25.3 ± 1.3 | 25.5 ± 2.0 | 0.07 |
| Age (y) | 30.3 ± 3.3 | 30.1 ± 3.1 | 30.7 ± 3.7 | 0.22 |
| <25 | 10 (2.3) | 7 (2.4) | 3 (2.0) | 0.49 |
| 25~ | 222 (50.0) | 152 (51.4) | 70 (47.3) | |
| 30~ | 171 (38.5) | 114 (38.5) | 57 (38.5) | |
| ≥35 | 41 (9.2) | 23 (7.8) | 18 (12.2) | |
| Height (cm) | 161.4 ± 4.9 | 161.2 ± 4.9 | 161.9 ± 4.8 | 0.11 |
| Pre-pregnancy weight (kg) | 56.9 ± 8.1 | 56.4 ± 8.1 | 57.8 ± 8.1 | 0.08 |
| Pre-pregnancy BMI (kg/m2) | 21.8 ± 2.8 | 21.7 ± 2.8 | 22.0 ± 2.8 | 0.14 |
| <18.5 | 45 (10.1) | 30 (10.1) | 15 (10.1) | 0.77 |
| 18.5~ | 311 (70.0) | 210 (70.9) | 101 (68.2) | |
| 24.0~ | 70 (15.8) | 46 (15.5) | 24 (16.2) | |
| ≥28 | 18 (4.1) | 10 (3.4) | 8 (5.4) | |
| Ethnicity (Han Chinese) | 444 (100.0) | 296 (100.0) | 148 (100.0) | 1.00 |
| Education (schooling years≥ 16y) | 225 (50.7) | 150 (50.7) | 75 (50.7) | 1.00 |
| Average personal income (CNY/month) | ||||
| ≤2999 | 8 (1.8) | 6 (2.0) | 2 (1.4) | 0.13 |
| ~4999 | 76 (17.1) | 43 (14.6) | 33 (22.2) | |
| ~9999 | 236 (53.2) | 157 (53.0) | 79 (53.4) | |
| ≥10,000 | 124 (27.9) | 90 (30.4) | 34 (23.0) | |
| Gravity (times) | ||||
| 1 | 247 (55.6) | 160 (54.0) | 87 (58.8) | 0.60 |
| 2 | 120 (27.0) | 84 (28.4) | 36 (24.3) | |
| ≥3 | 77 (17.4) | 52 (17.6) | 25 (16.9) | |
| Primiparity (yes) | 333 (75.0) | 222 (75.0) | 111 (75.0) | 1.00 |
| Family history of diabetes (yes) | 49 (11.0) | 31 (10.5) | 18 (12.2) | 0.59 |
| Family history of obesity (yes) | 2 (0.5) | 2 (0.7) | 0 (0.0) | 0.56 |
| Family history of hypertension (yes) | 142 (32.0) | 90 (30.4) | 52 (35.1) | 0.31 |
| Family history of hyperlipidemia (yes) | 16 (3.6) | 9 (3.0) | 7 (4.7) | 0.37 |
| Smoking before pregnancy (yes) | 10 (2.3) | 4 (1.4) | 6 (4.1) | 0.07 |
| Drinking before pregnancy (yes) | 18 (4.1) | 12 (4.1) | 6 (4.1) | 1.00 |
| Leisure-time physical activity (yes) | 197 (44.6) | 123 (41.9) | 74 (50.0) | 0.09 |
| Outcome variables | ||||
| 0 h FBG (mmol/L) | 4.7 ± 0.4 | 4.5 ± 0.3 | 4.9 ± 0.4 | <0.001 |
| 1 h PBG (mmol/L) | 8.2 ± 1.7 | 7.6 ± 1.3 | 9.5 ± 1.6 | <0.001 |
| 2 h PBG (mmol/L) | 7.1 ± 1.4 | 6.6 ± 1.1 | 8.2 ± 1.4 | <0.001 |
| Overall (n = 444) | Control (n = 296) | GDM Case (n = 148) | p-Value † | |
|---|---|---|---|---|
| TC a (mmol/L) | 4.87 ± 1.14 | 4.87 ± 1.16 | 4.89 ± 1.08 | 0.60 |
| HDL-C (mmol/L) | 2.10 ± 0.48 | 2.13 ± 0.47 | 2.03 ± 0.48 | 0.02 |
| LDL-C (mmol/L) | 2.47 ± 0.73 | 2.45 ± 0.75 | 2.53 ± 0.67 | 0.17 |
| TG (mmol/L) | 1.68 ± 0.74 | 1.63 ± 0.70 | 1.78 ± 0.81 | 0.04 |
| FBG (mmol/L) | 4.25 ± 1.02 | 4.17 ± 0.99 | 4.42 ± 1.06 | 0.052 |
| Insulin (mU/L) | 7.52 ± 8.10 | 6.82 ± 6.44 | 8.90 ± 10.56 | <0.01 |
| HOMA-IR | 1.54 ± 2.29 | 1.36 ± 1.83 | 1.89 ± 2.99 | <0.01 |
| Absolute cholesterol metabolism markers | ||||
| Cholesterol synthesis markers (mg/L) | ||||
| Δ8-Cholestenol | 0.53 ± 0.16 | 0.53 ± 0.16 | 0.55 ± 0.18 | 0.20 |
| Squalene | 0.24 ± 0.40 | 0.23 ± 0.22 | 0.26 ± 0.61 | 0.42 |
| Desmosterol | 0.43 ± 0.27 | 0.41 ± 0.11 | 0.46 ± 0.44 | 0.18 |
| Lathosterol | 3.30 ± 1.56 | 3.24 ± 1.60 | 3.41 ± 1.49 | 0.17 |
| Lanosterol | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.66 |
| Cholesterol absorption markers (mg/L) | ||||
| Cholestanol | 2.14 ± 0.90 | 2.20 ± 0.93 | 2.02 ± 0.82 | 0.02 |
| Campesterol | 2.17 ± 1.12 | 2.23 ± 1.17 | 2.05 ± 1.01 | 0.10 |
| β-Sitosterol | 2.41 ± 1.30 | 2.49 ± 1.32 | 2.27 ± 1.24 | 0.02 |
| Stigmasterol | 0.10 ± 0.08 | 0.10 ± 0.06 | 0.10 ± 0.10 | 0.88 |
| Relative cholesterol metabolism markers | ||||
| Cholesterol synthesis markers (µmol/mmol of TC b) | ||||
| Δ8-CholestenolTC c | 0.29 ± 0.09 | 0.28 ± 0.08 | 0.30 ± 0.10 | 0.07 |
| SqualeneTC c | 0.14 ± 0.21 | 0.13 ± 0.10 | 0.15 ± 0.33 | 0.63 |
| DesmosterolTC c | 0.23 ± 0.12 | 0.22 ± 0.05 | 0.25 ± 0.19 | 0.047 |
| LathosterolTC c | 1.75 ± 0.76 | 1.71 ± 0.75 | 1.83 ± 0.77 | 0.04 |
| LanosterolTC c | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.88 |
| Cholesterol absorption markers (µmol/mmol of TC b) | ||||
| CholestanolTC c | 1.13 ± 0.42 | 1.16 ± 0.46 | 1.06 ± 0.32 | 0.02 |
| CampesterolTC c | 1.13 ± 0.65 | 1.16 ± 0.68 | 1.07 ± 0.59 | 0.17 |
| β-SitosterolTC c | 1.22 ± 0.74 | 1.25 ± 0.77 | 1.15 ± 0.67 | 0.046 |
| StigmasterolTC c | 0.05 ± 0.04 | 0.05 ± 0.03 | 0.05 ± 0.05 | 0.72 |
| Cholesterol synthesis/absorption ratios (µmol/µmol) | ||||
| Lathosterol/Cholestanol | 1.72 ± 0.92 | 1.64 ± 0.89 | 1.87 ± 0.97 | <0.01 |
| Lathosterol/Campesterol | 1.88 ± 1.20 | 1.82 ± 1.22 | 2.00 ± 1.17 | 0.03 |
| Lathosterol/β-Sitosterol | 1.74 ± 1.07 | 1.65 ± 1.02 | 1.91 ± 1.14 | 0.01 |
| Lathosterol/Stigmasterol | 48.60 ± 49.35 | 48.57 ± 53.56 | 48.67 ± 39.81 | 0.48 |
| Per 1-SD of Log-Transformed Increase | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Δ8-CholestenolTC | 1.22 (0.98, 1.51) | 0.07 | 1.23 (0.99, 1.54) | 0.07 | 1.18 (0.95, 1.48) | 0.14 | 1.18 (0.94, 1.48) | 0.15 |
| SqualeneTC | 0.94 (0.73, 1.21) | 0.63 | 0.95 (0.74, 1.23) | 0.71 | 0.97 (0.75, 1.26) | 0.82 | 0.96 (0.74, 1.25) | 0.78 |
| DesmosterolTC | 1.24 (1.00, 1.54) | 0.047 | 1.25 (1.00, 1.56) | 0.047 | 1.22 (0.98, 1.53) | 0.08 | 1.23 (0.98, 1.54) | 0.07 |
| LathosterolTC | 1.26 (1.01, 1.59) | 0.04 | 1.27 (1.00, 1.60) | 0.047 | 1.22 (0.96, 1.54) | 0.10 | 1.21 (0.96, 1.53) | 0.11 |
| LanosterolTC | 0.98 (0.77, 1.24) | 0.88 | 1.01 (0.80, 1.29) | 0.91 | 1.00 (0.78, 1.27) | 0.97 | 1.01 (0.79, 1.29) | 0.96 |
| CholestanolTC | 0.76 (0.61, 0.96) | 0.02 | 0.77 (0.61, 0.96) | 0.02 | 0.79 (0.63, 1.00) | 0.045 | 0.79 (0.63, 1.00) | 0.047 |
| CampesterolTC | 0.86 (0.69, 1.07) | 0.17 | 0.84 (0.67, 1.04) | 0.12 | 0.85 (0.68, 1.06) | 0.16 | 0.85 (0.68, 1.06) | 0.16 |
| β-SitosterolTC | 0.80 (0.64, 1.00) | 0.046 | 0.80 (0.64, 1.00) | 0.048 | 0.81 (0.65, 1.01) | 0.07 | 0.81 (0.65, 1.01) | 0.07 |
| StigmasterolTC | 1.04 (0.84, 1.29) | 0.72 | 1.03 (0.82, 1.29) | 0.83 | 1.06 (0.84, 1.34) | 0.64 | 1.04 (0.83, 1.32) | 0.71 |
| Lathosterol/Cholestanol | 1.36 (1.09, 1.71) | <0.01 | 1.36 (1.08, 1.72) | <0.01 | 1.30 (1.03, 1.64) | 0.03 | 1.29 (1.02, 1.63) | 0.03 |
| Lathosterol/Campesterol | 1.29 (1.02, 1.62) | 0.03 | 1.32 (1.04, 1.68) | 0.02 | 1.27 (0.99, 1.62) | 0.06 | 1.26 (0.99, 1.61) | 0.06 |
| Lathosterol/β-Sitosterol | 1.35 (1.07, 1.71) | 0.01 | 1.36 (1.07, 1.73) | 0.01 | 1.31 (1.03, 1.66) | 0.03 | 1.30 (1.02, 1.66) | 0.03 |
| Lathosterol/Stigmasterol | 1.08 (0.87, 1.35) | 0.48 | 1.10 (0.88, 1.38) | 0.41 | 1.05 (0.83, 1.33) | 0.67 | 1.06 (0.84, 1.33) | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, Y.; Ge, Y.; Huang, S.; Yang, Y.; Zhang, Z.; Cui, N.; Yan, J.; Li, Y.; Luo, P.; et al. Pregnancy Cholesterol Metabolism Markers and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study. Nutrients 2023, 15, 3809. https://doi.org/10.3390/nu15173809
Li Y, Wu Y, Ge Y, Huang S, Yang Y, Zhang Z, Cui N, Yan J, Li Y, Luo P, et al. Pregnancy Cholesterol Metabolism Markers and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study. Nutrients. 2023; 15(17):3809. https://doi.org/10.3390/nu15173809
Chicago/Turabian StyleLi, Yan, Yuanjue Wu, Yanyan Ge, Shanshan Huang, Yang Yang, Zhen Zhang, Ningning Cui, Junan Yan, Yonggang Li, Ping Luo, and et al. 2023. "Pregnancy Cholesterol Metabolism Markers and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study" Nutrients 15, no. 17: 3809. https://doi.org/10.3390/nu15173809
APA StyleLi, Y., Wu, Y., Ge, Y., Huang, S., Yang, Y., Zhang, Z., Cui, N., Yan, J., Li, Y., Luo, P., Hao, L., Xiong, G., & Yang, X. (2023). Pregnancy Cholesterol Metabolism Markers and the Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study. Nutrients, 15(17), 3809. https://doi.org/10.3390/nu15173809







