Dietary Approach of Patients with Hormone-Related Cancer Based on the Glycemic Index and Glycemic Load Estimates
Abstract
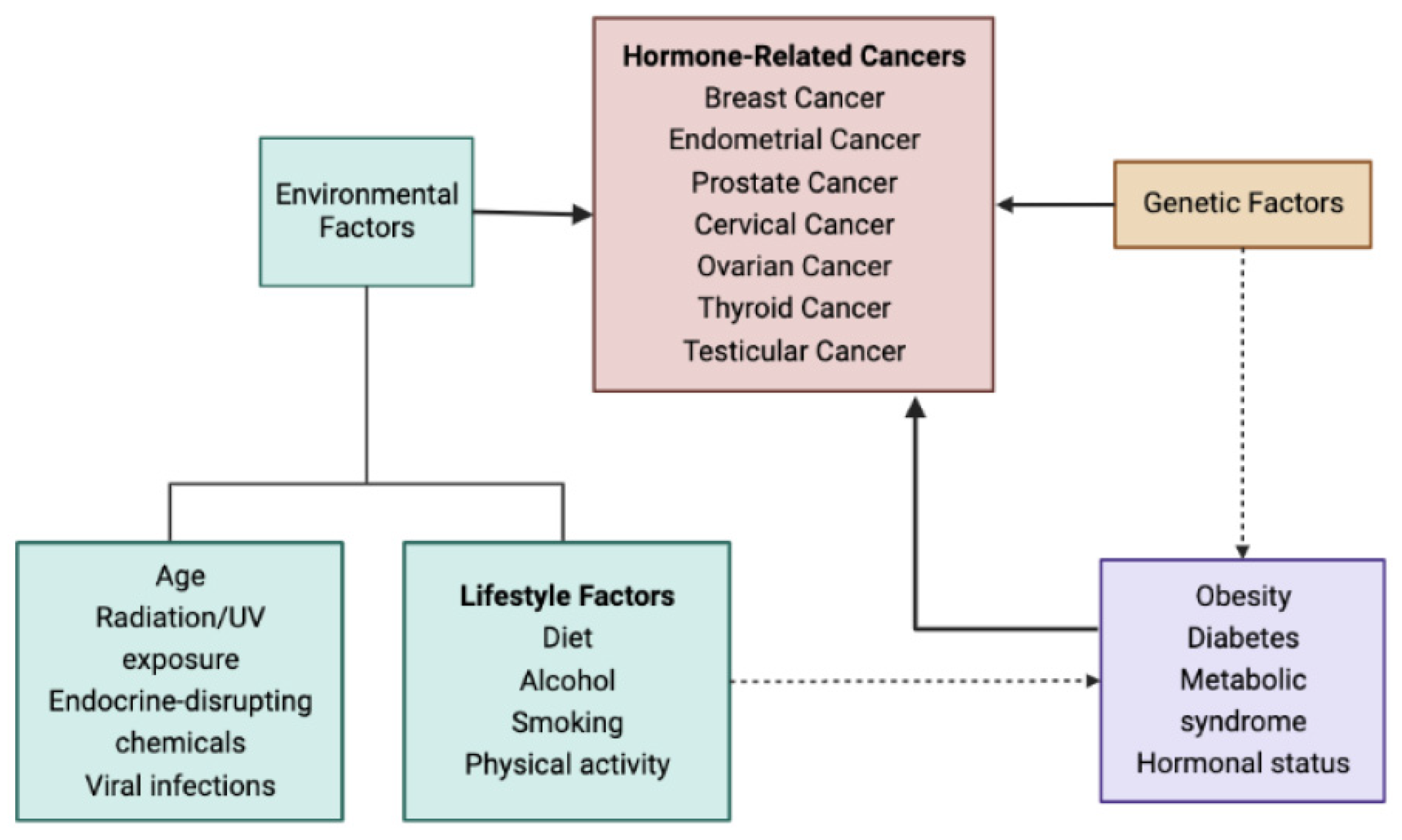
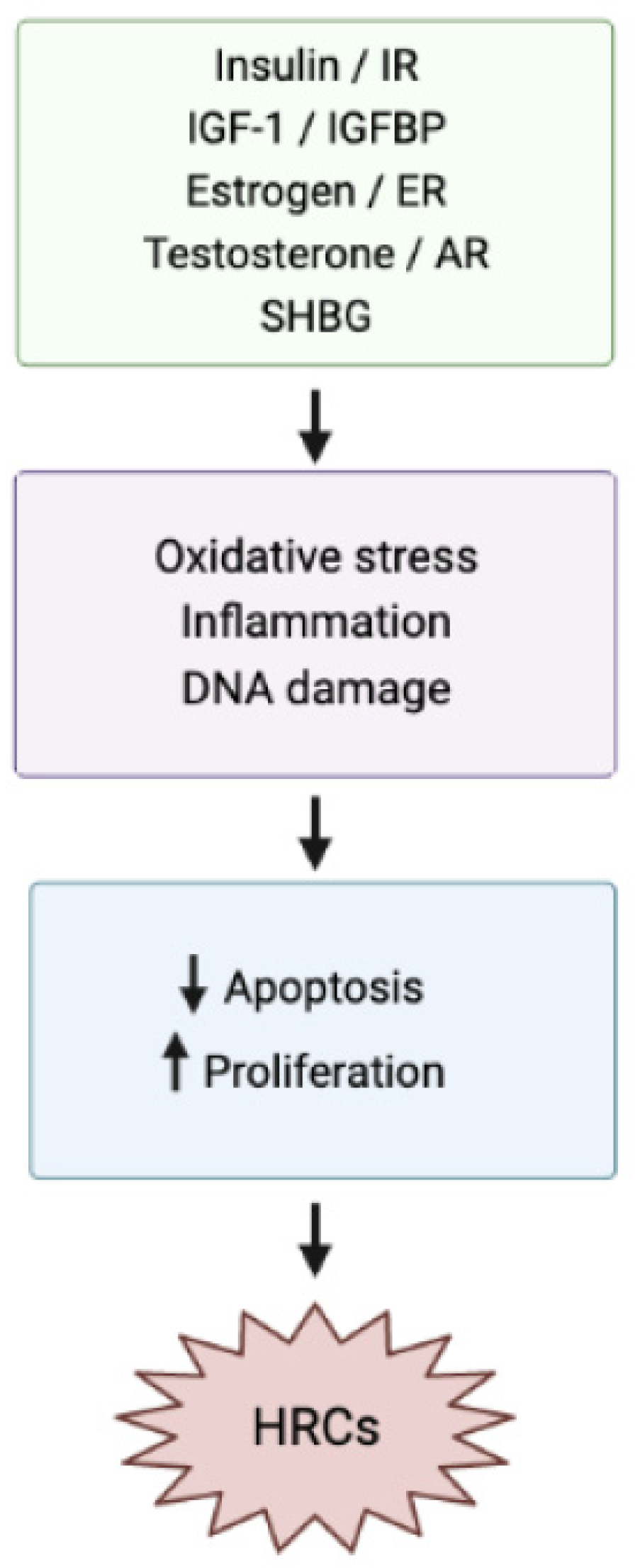
1. Introduction
2. Effects of Dietary GI and GL Indexes on HRCs
2.1. Effects of Dietary GI and GL Indexes on Breast Cancer
2.2. Effects of Dietary GI and GL Indexes on Endometrial Cancer
2.3. Effects of Dietary GI and GL Indexes on Prostate Cancer
2.4. Effects of Dietary GI and GL Indexes on Ovarian Cancer
2.5. Effects of Dietary GI and GL Indexes on Cervical Cancer
2.6. Effects of Dietary GI and GL Indexes on Thyroid Cancer
2.7. Effects of Dietary GI and GL Indexes on Testicular Cancer
3. Conclusions
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henderson, B.E.; Feigelson, H.S. Hormonal Carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef]
- Keyvani, V.; Kheradmand, N.; Navaei, Z.N.; Mollazadeh, S.; Esmaeili, S.-A. Epidemiological Trends and Risk Factors of Gynecological Cancers: An Update. Med. Oncol. 2023, 40, 93. [Google Scholar] [CrossRef]
- Catalona, W.J. Prostate Cancer Screening. Med. Clin. N. Am. 2018, 102, 199–214. [Google Scholar] [CrossRef]
- Roman, B.R.; Morris, L.G.; Davies, L. The thyroid cancer epidemic, 2017 perspective. Curr. Opin. Endocrinol. Diabetes 2017, 24, 332–336. [Google Scholar] [CrossRef]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. Semin. Cancer Biol. 2016, 40–41, 82–99. [Google Scholar] [CrossRef]
- Kamal, N.; Ilowefah, M.A.; Hilles, A.R.; Anua, N.A.; Awin, T.; Alshwyeh, H.A.; Aldosary, S.K.; Jambocus, N.G.S.; Alosaimi, A.A.; Rahman, A.; et al. Genesis and Mechanism of Some Cancer Types and an Overview on the Role of Diet and Nutrition in Cancer Prevention. Molecules 2022, 27, 1794. [Google Scholar] [CrossRef]
- Shapira, N. The Potential Contribution of Dietary Factors to Breast Cancer Prevention. Eur. J. Cancer Prev. 2017, 26, 385–395. [Google Scholar] [CrossRef]
- Rossi, R.E.; Pericleous, M.; Mandair, D.; Whyand, T.; Caplin, M.E. The Role of Dietary Factors in Prevention and Progression of Breast Cancer. Anticancer Res. 2014, 34, 6861–6875. [Google Scholar]
- Rocha, P.R.S.; Oliveira, V.D.; Vasques, C.I.; dos Reis, P.E.D.; Amato, A.A. Exposure to Endocrine Disruptors and Risk of Breast Cancer: A Systematic Review. Crit. Rev. Oncol. Hematol. 2021, 161, 103330. [Google Scholar] [CrossRef]
- Peppa, M.; Mavroeidi, I. Endocrine Disruptors and Environmental Pollutants Associated with Gonadal and Adrenal Cancer. In Endocrinology. Environmental Endocrinology and Endocrine Disruptors: Endocrine and Endocrine-Targeted Actions and Related Human Disease; Pivonello, R., Diamanti-Kandarakis, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-38366-4. [Google Scholar]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-Derived Advanced Glycation End Products Are Major Contributors to the Body’s AGE Pool and Induce Inflammation in Healthy Subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M. Dietary AGEs and Their Role in Health and Disease; Uribarri, J., Ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315120041. [Google Scholar]
- Peppa, M.; Mavroeidi, I. Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease. Nutrients 2021, 13, 3467. [Google Scholar] [CrossRef]
- Cai, W.; Gao, Q.-D.; Zhu, L.; Peppa, M.; He, C.; Vlassara, H. Oxidative Stress-Inducing Carbonyl Compounds from Common Foods: Novel Mediators of Cellular Dysfunction. Mol. Med. 2002, 8, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef]
- Aglago, E.K.; Mayén, A.-L.; Knaze, V.; Freisling, H.; Fedirko, V.; Hughes, D.J.; Jiao, L.; Eriksen, A.K.; Tjønneland, A.; Boutron-Ruault, M.-C.; et al. Dietary Advanced Glycation End-Products and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Nutrients 2021, 13, 3132. [Google Scholar] [CrossRef]
- Maino Vieytes, C.A.; Taha, H.M.; Burton-Obanla, A.A.; Douglas, K.G.; Arthur, A.E. Carbohydrate Nutrition and the Risk of Cancer. Curr. Nutr. Rep. 2019, 8, 230–239. [Google Scholar] [CrossRef]
- Sieri, S.; Krogh, V. Dietary Glycemic Index, Glycemic Load and Cancer: An Overview of the Literature. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 18–31. [Google Scholar] [CrossRef]
- Jankovic, N.; Geelen, A.; Winkels, R.M.; Mwungura, B.; Fedirko, V.; Jenab, M.; Illner, A.K.; Brenner, H.; Ordóñez-Mena, J.M.; Kiefte de Jong, J.C.; et al. Adherence to the WCRF/AICR Dietary Recommendations for Cancer Prevention and Risk of Cancer in Elderly from Europe and the United States: A Meta-Analysis within the CHANCES Project. Cancer Epidemiol. Biomark. Prev. 2017, 26, 136–144. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, F.; Yin, M.; Lei, Q. Cancer metabolism and dietary interventions. Cancer Biol. Med. 2021, 19, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Jenkins, D.; Sievenpiper, J.; Shab-Bidar, S. Dietary Glycemic Index, Glycemic Load, and Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Crit. Rev. Food. Sci. Nutr. 2022, 62, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Mayne, S.T.; Leitzmann, M.F.; Park, Y.; Schatzkin, A.; Flood, A.; Hollenbeck, A.; Subar, A.F. Dietary Glycemic Index, Glycemic Load, and Risk of Cancer: A Prospective Cohort Study. Am. J. Epidemiol. 2008, 169, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Sieri, S.; Agnoli, C.; Pala, V.; Grioni, S.; Brighenti, F.; Pellegrini, N.; Masala, G.; Palli, D.; Mattiello, A.; Panico, S.; et al. Dietary Glycemic Index, Glycemic Load, and Cancer Risk: Results from the EPIC-Italy Study. Sci. Rep. 2017, 7, 9757. [Google Scholar] [CrossRef]
- Corpet, D.E.; Peiffer, G.; Taché, S. Glycemic Index, Nutrient Density, and Promotion of Aberrant Crypt Foci in Rat Colon. Nutr. Cancer 1998, 32, 29–36. [Google Scholar] [CrossRef]
- Turati, F.; Galeone, C.; Augustin, L.S.A.; La Vecchia, C. Glycemic Index, Glycemic Load and Cancer Risk: An Updated Meta-Analysis. Nutrients 2019, 11, 2342. [Google Scholar] [CrossRef]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Alboghobeish, Z.; Hosseini Balam, F.; Askari, F.; Rashidkhani, B. Dietary Carbohydrate Intake Glycemic Index and Glycemic Load and the Risk of Prostate Cancer among Iranian Men: A Case-Control Study. Nutr. Cancer 2022, 74, 882–888. [Google Scholar] [CrossRef]
- Lasorsa, F.; Rutigliano, M.; Milella, M.; Ferro, M.; Pandolfo, S.D.; Crocetto, F.; Tataru, O.S.; Autorino, R.; Battaglia, M.; Ditonno, P.; et al. Cellular and Molecular Players in the Tumor Microenvironment of Renal Cell Carcinoma. J. Clin. Med. 2023, 12, 3888. [Google Scholar] [CrossRef]
- Carneiro, L.; Leloup, C. Mens Sana in Corpore Sano: Does the Glycemic Index Have a Role to Play? Nutrients 2020, 12, 2989. [Google Scholar] [CrossRef]
- Cava, E.; Marzullo, P.; Farinelli, D.; Gennari, A.; Saggia, C.; Riso, S.; Prodam, F. Breast Cancer Diet “BCD”: A Review of Healthy Dietary Patterns to Prevent Breast Cancer Recurrence and Reduce Mortality. Nutrients 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Terranova, C.O.; Protani, M.M.; Reeves, M.M. Overall Dietary Intake and Prognosis after Breast Cancer: A Systematic Review. Nutr. Cancer 2018, 70, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pishdad, S.; Joola, P.; Bourbour, F.; Rastgoo, S.; Majidi, N.; Gholamalizadeh, M.; Ebrahimi, K.; Abbas Torki, S.; Akbari, M.E.; Montazeri, F.; et al. Association between Different Types of Dietary Carbohydrate and Breast Cancer. Clin. Nutr. ESPEN 2021, 46, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Barnett, J.B.; Spence, N.D.; Rosner, B.A.; Holmes, M.D. Types of Carbohydrate Intake and Breast Cancer Survival. Eur. J. Nutr. 2021, 60, 4565–4577. [Google Scholar] [CrossRef]
- McCann, S.E.; McCann, W.E.; Hong, C.-C.; Marshall, J.R.; Edge, S.B.; Trevisan, M.; Muti, P.; Freudenheim, J.L. Dietary Patterns Related to Glycemic Index and Load and Risk of Premenopausal and Postmenopausal Breast Cancer in the Western New York Exposure and Breast Cancer Study. Am. J. Clin. Nutr. 2007, 86, 465–471. [Google Scholar] [CrossRef][Green Version]
- Cho, E.; Spiegelman, D.; Hunter, D.J.; Chen, W.Y.; Colditz, G.A.; Willett, W.C. Premenopausal Dietary Carbohydrate, Glycemic Index, Glycemic Load, and Fiber in Relation to Risk of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1153–1158. [Google Scholar]
- Sieri, S.; Pala, V.; Brighenti, F.; Pellegrini, N.; Muti, P.; Micheli, A.; Evangelista, A.; Grioni, S.; Contiero, P.; Berrino, F.; et al. Dietary Glycemic Index, Glycemic Load, and the Risk of Breast Cancer in an Italian Prospective Cohort Study. Am. J. Clin. Nutr. 2007, 86, 1160–1166. [Google Scholar] [CrossRef]
- Wen, W.; Shu, X.O.; Li, H.; Yang, G.; Ji, B.-T.; Cai, H.; Gao, Y.-T.; Zheng, W. Dietary Carbohydrates, Fiber, and Breast Cancer Risk in Chinese Women. Am. J. Clin. Nutr. 2009, 89, 283–289. [Google Scholar] [CrossRef]
- Higginbotham, S.; Zhang, Z.-F.; Lee, I.-M.; Cook, N.R.; Buring, J.E.; Liu, S. Dietary Glycemic Load and Breast Cancer Risk in the Women’s Health Study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 65–70. [Google Scholar] [CrossRef]
- Sasanfar, B.; Toorang, F.; Mohebbi, E.; Zendehdel, K.; Azadbakht, L. Dietary Carbohydrate Quality and Risk of Breast Cancer among Women. Nutr. J. 2021, 20, 93. [Google Scholar] [CrossRef]
- Woo, H.D.; Park, K.-S.; Shin, A.; Ro, J.; Kim, J. Glycemic Index and Glycemic Load Dietary Patterns and the Associated Risk of Breast Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. 2013, 14, 5193–5198. [Google Scholar] [CrossRef]
- Amadou, A.; Degoul, J.; Hainaut, P.; Chajes, V.; Biessy, C.; Torres Mejia, G.; Huybrechts, I.; Moreno Macia, H.; Ortega, C.; Angeles-Llerenas, A.; et al. Dietary Carbohydrate, Glycemic Index, Glycemic Load, and Breast Cancer Risk Among Mexican Women. Epidemiology 2015, 26, 917–924. [Google Scholar] [CrossRef]
- McCann, S.E.; Liu, S.; Wang, D.; Shen, J.; Hu, Q.; Hong, C.-C.; Newman, V.A.; Zhao, H. Reduction of Dietary Glycaemic Load Modifies the Expression of MicroRNA Potentially Associated with Energy Balance and Cancer Pathways in Pre-Menopausal Women. Br. J. Nutr. 2013, 109, 585–592. [Google Scholar] [CrossRef]
- Frazier, A.L.; Li, L.; Cho, E.; Willett, W.C.; Colditz, G.A. Adolescent Diet and Risk of Breast Cancer. Cancer Causes Control. 2004, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Romanos-Nanclares, A.; Gea, A.; Martínez-González, M.Á.; Zazpe, I.; Gardeazabal, I.; Fernandez-Lazaro, C.I.; Toledo, E. Carbohydrate Quality Index and Breast Cancer Risk in a Mediterranean Cohort: The SUN Project. Clin. Nutr. 2021, 40, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Lajous, M.; Boutron-Ruault, M.-C.; Fabre, A.; Clavel-Chapelon, F.; Romieu, I. Carbohydrate Intake, Glycemic Index, Glycemic Load, and Risk of Postmenopausal Breast Cancer in a Prospective Study of French Women. Am. J. Clin. Nutr. 2008, 87, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Glycemic Load, Glycemic Index and Breast Cancer Risk in a Prospective Cohort of Swedish Women. Int. J. Cancer 2009, 125, 153–157. [Google Scholar] [CrossRef]
- Alboghobeish, Z.; Hekmatdoost, A.; Jalali, S.; Ahmadi, M.; Rashidkhani, B. Carbohydrate Intake, Glycemic Index, and Glycemic Load and the Risk of Breast Cancer among Iranian Women. Nutr. Cancer 2021, 73, 785–793. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Julia, C.; Kesse-Guyot, E.; Zelek, L.; Agaësse, C.; Druesne-Pecollo, N.; Andreeva, V.A.; Galan, P.; et al. Glycaemic Index, Glycaemic Load and Cancer Risk: Results from the Prospective NutriNet-Santé Cohort. Int. J. Epidemiol. 2022, 51, 250–264. [Google Scholar] [CrossRef]
- Silvera, S.A.N.; Jain, M.; Howe, G.R.; Miller, A.B.; Rohan, T.E. Dietary Carbohydrates and Breast Cancer Risk: A Prospective Study of the Roles of Overall Glycemic Index and Glycemic Load. Int. J. Cancer 2005, 114, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.G.; Olsen, A.; Christensen, J.; Overvad, K.; Tjønneland, A. Dietary Carbohydrate Intake Is Not Associated with the Breast Cancer Incidence Rate Ratio in Postmenopausal Danish Women. J. Nutr. 2005, 135, 124–128. [Google Scholar] [CrossRef]
- Romieu, I.; Ferrari, P.; Rinaldi, S.; Slimani, N.; Jenab, M.; Olsen, A.; Tjonneland, A.; Overvad, K.; Boutron-Ruault, M.-C.; Lajous, M.; et al. Dietary Glycemic Index and Glycemic Load and Breast Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2012, 96, 345–355. [Google Scholar] [CrossRef]
- Shikany, J.M.; Redden, D.T.; Neuhouser, M.L.; Chlebowski, R.T.; Rohan, T.E.; Simon, M.S.; Liu, S.; Lane, D.S.; Tinker, L. Dietary Glycemic Load, Glycemic Index, and Carbohydrate and Risk of Breast Cancer in the Women’s Health Initiative. Nutr. Cancer 2011, 63, 899–907. [Google Scholar] [CrossRef]
- Masala, G.; Assedi, M.; Bendinelli, B.; Ermini, I.; Occhini, D.; Sieri, S.; Brighenti, F.; Turco, M.R.D.; Ambrogetti, D.; Palli, D. Glycemic Index, Glycemic Load and Mammographic Breast Density: The EPIC Florence Longitudinal Study. PLoS ONE 2013, 8, e70943. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic Density and Breast Cancer Risk: Current Understanding and Future Prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Masala, G.; Assedi, M.; Sera, F.; Ermini, I.; Occhini, D.; Castaldo, M.; Pierpaoli, E.; Caini, S.; Bendinelli, B.; Ambrogetti, D.; et al. Can Dietary and Physical Activity Modifications Reduce Breast Density in Postmenopausal Women? The DAMA Study, a Randomized Intervention Trial in Italy. Cancer Epidemiol. Biomark. Prev. 2019, 28, 41–50. [Google Scholar] [CrossRef]
- Sieri, S.; Pala, V.; Brighenti, F.; Agnoli, C.; Grioni, S.; Berrino, F.; Scazzina, F.; Palli, D.; Masala, G.; Vineis, P.; et al. High Glycemic Diet and Breast Cancer Occurrence in the Italian EPIC Cohort. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 628–634. [Google Scholar] [CrossRef]
- Augustin, L.S.; Dal Maso, L.; La Vecchia, C.; Parpinel, M.; Negri, E.; Vaccarella, S.; Kendall, C.W.; Jenkins, D.J.; Francesch, S. Dietary Glycemic Index and Glycemic Load, and Breast Cancer Risk: A Case-Control Study. Ann. Oncol. 2001, 12, 1533–1538. [Google Scholar] [CrossRef]
- Farvid, M.S.; Tamimi, R.M.; Poole, E.M.; Chen, W.Y.; Rosner, B.A.; Willett, W.C.; Holmes, M.D.; Eliassen, A.H. Postdiagnostic Dietary Glycemic Index, Glycemic Load, Dietary Insulin Index, and Insulin Load and Breast Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Lajous, M.; Willett, W.; Lazcano-Ponce, E.; Sanchez-Zamorano, L.M.; Hernandez-Avila, M.; Romieu, I. Glycemic Load, Glycemic Index, and the Risk of Breast Cancer Among Mexican Women. Cancer Causes Control 2005, 16, 1165–1169. [Google Scholar] [CrossRef]
- Rigi, S.; Salari-Moghaddam, A.; Benisi-Kohansal, S.; Azadbakht, L.; Esmaillzadeh, A. Dietary Glycaemic Index and Glycaemic Load in Relation to Risk of Breast Cancer. Public Health Nutr. 2022, 25, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Haluszka, E.; Niclis, C.; Diaz, M.d.P.; Osella, A.R.; Aballay, L.R. Higher Dietary Glycemic Index, Intake of High-Glycemic Index Foods, and Insulin Load Are Associated with the Risk of Breast Cancer, with Differences According to Body Mass Index in Women from Córdoba, Argentina. Nutr. Res. 2022, 104, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Bandera, E.V.; Lin, Y.; Jacques, P.F.; Hayes, R.B.; Parekh, N. Carbohydrate Nutrition and Risk of Adiposity-Related Cancers: Results from the Framingham Offspring Cohort (1991–2013). Br. J. Nutr. 2017, 117, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.H.; Gamboa-Loira, B.; Mérida-Ortega, Á.; López-Carrillo, L. Dietary Glycemic Index and Glycemic Load and Risk of Breast Cancer by Molecular Subtype in Mexican Women. Nutr. Cancer 2019, 71, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.J.; Mattei, J.; Hughes, M.D.; Hu, F.B.; Willett, W.C. Dietary Diabetes Risk Reduction Score, Race and Ethnicity, and Risk of Type 2 Diabetes in Women. Diabetes Care 2015, 38, 596–603. [Google Scholar] [CrossRef]
- Turati, F.; Bravi, F.; Rossi, M.; Serraino, D.; Mattioli, V.; Augustin, L.; Crispo, A.; Giacosa, A.; Negri, E.; La Vecchia, C. Diabetes Risk Reduction Diet and the Risk of Breast Cancer. Eur. J. Cancer Prev. 2022, 31, 339–345. [Google Scholar] [CrossRef]
- Kang, J.H.; Peng, C.; Rhee, J.J.; Farvid, M.S.; Willett, W.C.; Hu, F.B.; Rosner, B.A.; Tamimi, R.; Eliassen, A.H. Prospective Study of a Diabetes Risk Reduction Diet and the Risk of Breast Cancer. Am. J. Clin. Nutr. 2020, 112, 1492–1503. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Bagheri, A.; Benisi-Kohansal, S.; Azadbakht, L.; Esmaillzadeh, A. Consumption of “Diabetes Risk Reduction Diet” and Odds of Breast Cancer Among Women in a Middle Eastern Country. Front. Nutr. 2022, 9, 744500. [Google Scholar] [CrossRef]
- Wang, T.; Farvid, M.S.; Kang, J.H.; Holmes, M.D.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Diabetes Risk Reduction Diet and Survival after Breast Cancer Diagnosis. Cancer Res. 2021, 81, 4155–4162. [Google Scholar] [CrossRef]
- Farvid, M.S.; Eliassen, A.H.; Cho, E.; Chen, W.Y.; Willett, W.C. Adolescent and Early Adulthood Dietary Carbohydrate Quantity and Quality in Relation to Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1111–1120. [Google Scholar] [CrossRef]
- Jonas, C.R.; McCullough, M.L.; Teras, L.R.; Walker-Thurmond, K.A.; Thun, M.J.; Calle, E.E. Dietary Glycemic Index, Glycemic Load, and Risk of Incident Breast Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2003, 12, 573–577. [Google Scholar]
- Giles, G.G.; Simpson, J.A.; English, D.R.; Hodge, A.M.; Gertig, D.M.; MacInnis, R.J.; Hopper, J.L. Dietary Carbohydrate, Fibre, Glycaemic Index, Glycaemic Load and the Risk of Postmenopausal Breast Cancer. Int. J. Cancer 2006, 118, 1843–1847. [Google Scholar] [CrossRef]
- Castro-Quezada, I.; Sánchez-Villegas, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Estruch, R.; Schröder, H.; Álvarez-Pérez, J.; Ruiz-López, M.D.; Artacho, R.; et al. Glycemic Index, Glycemic Load and Invasive Breast Cancer Incidence in Postmenopausal Women: The PREDIMED Study. Eur. J. Cancer Prev. 2016, 25, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Imani, H.; Sheikhhossein, F.; Majdi, M.; Ghanbari, M.; Shab-Bidar, S. Dietary Carbohydrate Quality and Quantity and Risk of Breast Cancer among Iranian Women. Nutr. Cancer 2022, 74, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Kim, K.; Nam, S.J.; Kong, G.; Kim, M.K. The Association of Carbohydrate Intake, Glycemic Load, Glycemic Index, and Selected Rice Foods with Breast Cancer Risk: A Case-Control Study in South Korea. Asia Pac. J. Clin. Nutr. 2010, 19, 383–392. [Google Scholar]
- Watanabe, Y.; Katagiri, R.; Goto, A.; Shimazu, T.; Yamaji, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Tsugane, S. Dietary Glycemic Index, Glycemic Load, and Endometrial Cancer Risk: The Japan Public Health Center-based Prospective Study. Cancer Sci. 2021, 112, 3682–3690. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Biel, R.K.; Lau, D.C.W.; Csizmadi, I.; Courneya, K.S.; Magliocco, A.M.; Yasui, Y.; Cook, L.S. Case–Control Study of the Metabolic Syndrome and Metabolic Risk Factors for Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2384–2395. [Google Scholar] [CrossRef]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 13, 1178. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Nagle, C.M.; Olsen, C.M.; Ibiebele, T.I.; Spurdle, A.B.; Webb, P.M. Glycemic Index, Glycemic Load and Endometrial Cancer Risk: Results from the Australian National Endometrial Cancer Study and an Updated Systematic Review and Meta-Analysis. Eur. J. Nutr. 2013, 52, 705–715. [Google Scholar] [CrossRef]
- Friberg, E.; Wallin, A.; Wolk, A. Sucrose, High-Sugar Foods, and Risk of Endometrial Cancer—A Population-Based Cohort Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Gallus, S.; Bosetti, C.; Levi, F.; Negri, E.; Franceschi, S.; Dal Maso, L.; Jenkins, D.J.A.; Kendall, C.W.C.; La Vecchia, C. Glycemic Index and Glycemic Load in Endometrial Cancer. Int. J. Cancer 2003, 105, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Friberg, E.; Wolk, A. Carbohydrate Intake, Glycemic Index and Glycemic Load in Relation to Risk of Endometrial Cancer: A Prospective Study of Swedish Women. Int. J. Cancer 2006, 120, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Demissie, Z.; Harnack, L. Glycemic Index, Glycemic Load, and Incidence of Endometrial Cancer: The Iowa Women’s Health Study. Nutr. Cancer 2003, 46, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Xiang, Y.-B.; Zhang, X.; Ruan, Z.; Cai, H.; Zheng, W.; Shu, X.-O. Association of Dietary Glycemic Index and Glycemic Load with Endometrial Cancer Risk Among Chinese Women. Nutr. Cancer 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Haynes, R.B.; Wilczynski, N.; McKibbon, K.A.; Walker, C.J.; Sinclair, J.C. Developing Optimal Search Strategies for Detecting Clinically Sound Studies in MEDLINE. J. Am. Med. Inform. Assoc. 1994, 1, 447–458. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Gandini, S.; La Vecchia, C.; Maisonneuve, P. Glycemic Index, Glycemic Load, and Cancer Risk: A Meta-Analysis. Am. J. Clin. Nutr. 2008, 87, 1793–1801. [Google Scholar] [CrossRef]
- Hartman, T.J.; McCullough, M.L.; Hodge, J.M.; Gaudet, M.M.; Wang, Y.; Gapstur, S.M. Dietary Energy Density, Glycemic Load, Glycemic Index, and Risk for Endometrial Cancer in the CPS-II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2018, 27, 113–115. [Google Scholar] [CrossRef]
- Brenner, D.R.; Speidel, T.; Csizmadi, I.; Biel, R.K.; Cook, L.S.; Courneya, K.S.; Friedenreich, C.M. Glycemic Load and Endometrial Cancer Risk in a Case-Control Study of Canadian Women. Cancer Epidemiol. 2015, 39, 170–173. [Google Scholar] [CrossRef]
- Coleman, H.G.; Kitahara, C.M.; Murray, L.J.; Dodd, K.W.; Black, A.; Stolzenberg-Solomon, R.Z.; Cantwell, M.M. Dietary Carbohydrate Intake, Glycemic Index, and Glycemic Load and Endometrial Cancer Risk: A Prospective Cohort Study. Am. J. Epidemiol. 2014, 179, 75–84. [Google Scholar] [CrossRef]
- Silvera, S.A.; Rohan, T.E.; Jain, M.; Terry, P.D.; Howe, G.R.; Miller, A.B. Glycaemic Index, Glycaemic Load and Risk of Endometrial Cancer: A Prospective Cohort Study. Public Health Nutr. 2005, 8, 912–919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galeone, C.; Augustin, L.S.A.; Filomeno, M.; Malerba, S.; Zucchetto, A.; Pelucchi, C.; Montella, M.; Talamini, R.; Franceschi, S.; La Vecchia, C. Dietary Glycemic Index, Glycemic Load, and the Risk of Endometrial Cancer. Eur. J. Cancer Prev. 2013, 22, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Aronson, W.J. Dietary Intervention Strategies to Modulate Prostate Cancer Risk and Prognosis. Curr. Opin. Urol. 2009, 19, 263–267. [Google Scholar] [CrossRef]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and Prostate Cancer: Weighing the Evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef]
- Lin, P.-H.; Aronson, W.; Freedland, S.J. An Update of Research Evidence on Nutrition and Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 387–401. [Google Scholar] [CrossRef]
- Drake, I.; Sonestedt, E.; Gullberg, B.; Ahlgren, G.; Bjartell, A.; Wallström, P.; Wirfält, E. Dietary Intakes of Carbohydrates in Relation to Prostate Cancer Risk: A Prospective Study in the Malmö Diet and Cancer Cohort. Am. J. Clin. Nutr. 2012, 96, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Kenfield, S.; Jensen, M.K.; Stampfer, M.J.; Franz, M.; Sampson, L.; Brand-Miller, J.C.; Willett, W.C.; Giovannucci, E. Dietary Glycemic Index, Glycemic Load, Insulin Index, Fiber and Whole-Grain Intake in Relation to Risk of Prostate Cancer. Cancer Causes Control 2011, 22, 51–61. [Google Scholar] [CrossRef]
- Ferro, M.; Terracciano, D.; Buonerba, C.; Lucarelli, G.; Bottero, D.; Perdonà, S.; Autorino, R.; Serino, A.; Cantiello, F.; Damiano, R.; et al. The Emerging Role of Obesity, Diet and Lipid Metabolism in Prostate Cancer. Future Oncol. 2017, 13, 285–293. [Google Scholar] [CrossRef]
- Vidal, A.C.; Williams, C.D.; Allott, E.H.; Howard, L.E.; Grant, D.J.; McPhail, M.; Sourbeer, K.N.; Hwa, L.P.; Boffetta, P.; Hoyo, C.; et al. Carbohydrate Intake, Glycemic Index and Prostate Cancer Risk. Prostate 2015, 75, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.W.; Neuhouser, M.L.; Schenk, J.M.; Coleman, I.M.; Hawley, S.; Gifford, D.; Hung, H.; Knudsen, B.S.; Nelson, P.S.; Kristal, A.R. Low-Fat, Low-Glycemic Load Diet and Gene Expression in Human Prostate Epithelium: A Feasibility Study of Using CDNA Microarrays to Assess the Response to Dietary Intervention in Target Tissues. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2150–2154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, N.; Barnard, R.J.; Said, J.; Hong-Gonzalez, J.; Corman, D.M.; Ku, M.; Doan, N.B.; Gui, D.; Elashoff, D.; Cohen, P.; et al. Effect of Low-Fat Diet on Development of Prostate Cancer and Akt Phosphorylation in the Hi-Myc Transgenic Mouse Model. Cancer Res. 2008, 68, 3066–3073. [Google Scholar] [CrossRef]
- Hu, J.; La Vecchia, C.; Augustin, L.S.; Negri, E.; de Groh, M.; Morrison, H.; Mery, L. Glycemic Index, Glycemic Load and Cancer Risk. Ann. Oncol. 2013, 24, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Sadeghi, O.; Khodadost, M.; Pirouzi, A.; Hosseini, B.; Saedisomeolia, A. Dietary Glycemic Index and Glycemic Load and the Risk of Prostate Cancer: An Updated Systematic Review and Dose–Response Meta-Analysis. Nutr. Cancer 2020, 72, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.; Cheng, I.; Witte, J.S. Impact of Consumption of Vegetable, Fruit, Grain, and High Glycemic Index Foods on Aggressive Prostate Cancer Risk. Nutr. Cancer 2011, 63, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Shikany, J.M.; Flood, A.P.; Kitahara, C.M.; Hsing, A.W.; Meyer, T.E.; Willcox, B.J.; Redden, D.T.; Ziegler, R.G. Dietary Carbohydrate, Glycemic Index, Glycemic Load, and Risk of Prostate Cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) Cohort. Cancer Causes Control 2011, 22, 995–1002. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Kalli, K.R. The Insulin-like Growth Factor Insulin System in Epithelial Ovarian Cancer. Front. Biosci. 2003, 8, 1034. [Google Scholar] [CrossRef]
- Lukanova, A.; Kaaks, R. Endogenous Hormones and Ovarian Cancer: Epidemiology and Current Hypotheses. Cancer Epidemiol. Biomark. Prev. 2005, 14, 98–107. [Google Scholar] [CrossRef]
- Cheng, E.; Kirley, J.; Cespedes Feliciano, E.M.; Caan, B.J. Adiposity and Cancer Survival: A Systematic Review and Meta-Analysis. Cancer Causes Control 2022, 33, 1219–1246. [Google Scholar] [CrossRef]
- Gaitskell, K.; Hermon, C.; Barnes, I.; Pirie, K.; Floud, S.; Green, J.; Beral, V.; Reeves, G.K. Ovarian Cancer Survival by Stage, Histotype, and Pre-Diagnostic Lifestyle Factors, in the Prospective UK Million Women Study. Cancer Epidemiol. 2022, 76, 102074. [Google Scholar] [CrossRef]
- Cuello, M.A.; Gómez, F.; Wichmann, I.; Suárez, F.; Kato, S.; Orlandini, E.; Brañes, J.; Ibañez, C. Body Composition and Metabolic Dysfunction Really Matter for the Achievement of Better Outcomes in High-Grade Serous Ovarian Cancer. Cancers 2023, 15, 1156. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M. The Effects of the Dietary and Nutrient Intake on Gynecologic Cancers. Healthcare 2019, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Gong, T.-T.; Ma, Q.-P.; Wei, Y.-F.; Du, Z.-D.; Zhao, J.-Q.; Zou, B.-J.; Yan, S.; Liu, F.-H.; Sun, M.-L.; et al. The association of macronutrient quality and its interactions with energy intake with survival among patients with ovarian cancer: Results from a prospective cohort study. Am. J. Clin. Nutr. 2023, 117, 1362–1371. [Google Scholar] [CrossRef]
- Nagle, C.M.; Kolahdooz, F.; Ibiebele, T.I.; Olsen, C.M.; Lahmann, P.H.; Green, A.C.; Webb, P.M. Carbohydrate Intake, Glycemic Load, Glycemic Index, and Risk of Ovarian Cancer. Ann. Oncol. 2011, 22, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Moorman, P.G.; Alberg, A.J.; Barnholtz-Sloan, J.S.; Bondy, M.; Cote, M.L.; Funkhouser, E.; Peters, E.S.; Schwartz, A.G.; Terry, P.; et al. Dietary Carbohydrate Intake, Glycaemic Load, Glycaemic Index and Ovarian Cancer Risk in African-American Women. Br. J. Nutr. 2016, 115, 694–702. [Google Scholar] [CrossRef]
- Silvera, S.A.; Jain, M.; Howe, G.R.; Miller, A.B.; Rohan, T.E. Glycaemic Index, Glycaemic Load and Ovarian Cancer Risk: A Prospective Cohort Study. Public Health Nutr. 2007, 10, 1076–1081. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Polesel, J.; Bosetti, C.; Kendall, C.W.C.; La Vecchia, C.; Parpinel, M.; Conti, E.; Montella, M.; Franceschi, S.; Jenkins, D.J.A.; et al. Dietary Glycemic Index, Glycemic Load and Ovarian Cancer Risk:A Case–Control Study in Italy. Ann. Oncol. 2003, 14, 78–84. [Google Scholar] [CrossRef]
- Playdon, M.C.; Nagle, C.M.; Ibiebele, T.I.; Ferrucci, L.M.; Protani, M.M.; Carter, J.; Hyde, S.E.; Neesham, D.; Nicklin, J.L.; Mayne, S.T.; et al. Pre-Diagnosis Diet and Survival after a Diagnosis of Ovarian Cancer. Br. J. Cancer 2017, 116, 1627–1637. [Google Scholar] [CrossRef]
- Mongiovi, J.M.; Freudenheim, J.L.; Moysich, K.B.; McCann, S.E. Glycemic Index, Glycemic Load, and Risk of Ovarian Cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cohort. J. Nutr. 2021, 151, 1597–1608. [Google Scholar] [CrossRef]
- Johnson, C.A.; James, D.; Marzan, A.; Armaos, M. Cervical Cancer: An Overview of Pathophysiology and Management. Semin. Oncol. Nurs. 2019, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gnade, C.M.; Hill, E.K.; Botkin, H.E.; Hefel, A.R.; Hansen, H.E.; Sheets, K.A.; Mott, S.L.; Hardy-Fairbanks, A.J.; Stockdale, C.K. Effect of Obesity on Cervical Cancer Screening and Outcomes. J. Low Genit. Tract Dis. 2020, 24, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Scalisi, A.; Agodi, A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients 2018, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.; Karhu, E. The Role of Plant-Based Nutrition in Cancer Prevention. J. Unexplored Med. Data 2018, 3, 9. [Google Scholar] [CrossRef]
- Nomelini, R.S.; Neto, A.S.L.; Capuci, K.A.; Murta, B.M.T.; Murta, E.F.C. Relationship between Plasma Glucose Levels and Malignant Uterine Cervical Neoplasias. Clin. Med. Insights Oncol. 2011, 5, CMO.S6916. [Google Scholar] [CrossRef]
- Sreeja, S.R.; Seo, S.S.; Kim, M.K. Associations of Dietary Glycemic Index, Glycemic Load and Carbohydrate with the Risk of Cervical Intraepithelial Neoplasia and Cervical Cancer: A Case-Control Study. Nutrients 2020, 12, 3742. [Google Scholar] [CrossRef]
- Knudsen, N.; Brix, T.H. Genetic and Non-Iodine-Related Factors in the Aetiology of Nodular Goitre. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 495–506. [Google Scholar] [CrossRef]
- Franchini, F.; Palatucci, G.; Colao, A.; Ungaro, P.; Macchia, P.E.; Nettore, I.C. Obesity and Thyroid Cancer Risk: An Update. Int. J. Env. Res. Public Health 2022, 19, 1116. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, N.; Zhu, C.; Ni, X.; Ko, J.; Huang, H.; Ma, S.; Udelsman, R.; Zhang, Y. Dietary Patterns and Thyroid Cancer Risk: A Population-Based Case-Control Study. Am. J. Transl. Res. 2020, 12, 180–190. [Google Scholar]
- Markaki, I.; Linos, D.; Linos, A. The Influence of Dietary Patterns on the Development of Thyroid Cancer. Eur. J. Cancer 2003, 39, 1912–1919. [Google Scholar] [CrossRef]
- Przybylik-Mazurek, E.; Hubalewska-Dydejczyk, A.; Kuźniarz-Rymarz, S.; Kieć-Klimczak, M.; Skalniak, A.; Sowa-Staszczak, A.; Gołkowski, F.; Kostecka-Matyja, M.; Pach, D. Dietary Patterns as Risk Factors of Differentiated Thyroid Carcinoma. Postep. Hig. Med. Dosw. 2012, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, L.; Laouali, N.; Dossus, L.; Shivappa, N.; Hébert, J.R.; Agudo, A.; Tjonneland, A.; Halkjaer, J.; Overvad, K.; Katzke, V.A.; et al. Inflammatory Potential of the Diet and Association with Risk of Differentiated Thyroid Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Eur. J. Nutr. 2022, 61, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.A.; Sampaio, A.C.; Geloneze, B.; Vasques, A.C.J.; Assumpção, L.V.M.; Ward, L.S. Obesity and Excess Protein and Carbohydrate Consumption Are Risk Factors for Thyroid Cancer. Nutr. Cancer 2012, 64, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Rinaldi, S.; Tsilidis, K.K.; Weiderpass, E.; Boutron-Ruault, M.-C.; Rostgaard-Hansen, A.L.; Tjønneland, A.; Clavel-Chapelon, F.; Mesrine, S.; Katzke, V.A.; et al. Energy and Macronutrient Intake and Risk of Differentiated Thyroid Carcinoma in the European Prospective Investigation into Cancer and Nutrition Study. Int. J. Cancer 2016, 138, 65–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Randi, G.; Ferraroni, M.; Talamini, R.; Garavello, W.; Deandrea, S.; Decarli, A.; Franceschi, S.; La Vecchia, C. Glycemic Index, Glycemic Load and Thyroid Cancer Risk. Ann. Oncol. 2008, 19, 380–383. [Google Scholar] [CrossRef]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P. EAU Guidelines on Testicular Cancer: 2011 Update. Eur. Urol. 2011, 60, 304–319. [Google Scholar] [CrossRef]
- Papavasileiou, G.; Tsilingiris, D.; Spyrou, N.; Vallianou, N.G.; Karampela, I.; Magkos, F.; Dalamaga, M. Obesity and Main Urologic Cancers: Current Systematic Evidence, Novel Biological Mechanisms, Perspectives and Challenges. Semin. Cancer Biol. 2023, 91, 70–98. [Google Scholar] [CrossRef]
- Manecksha, R.P.; Fitzpatrick, J.M. Epidemiology of Testicular Cancer. BJU Int. 2009, 104, 1329–1333. [Google Scholar] [CrossRef]
- Signal, V.; Huang, S.; Sarfati, D.; Stanley, J.; McGlynn, K.A.; Gurney, J.K. Dairy Consumption and Risk of Testicular Cancer: A Systematic Review. Nutr. Cancer 2018, 70, 710–736. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Trabert, B. Adolescent and Adult Risk Factors for Testicular Cancer. Nat. Rev. Urol. 2012, 9, 339–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peppa, M.; Manta, A.; Mavroeidi, I.; Nastos, C.; Pikoulis, E.; Syrigos, K.; Bamias, A. Dietary Approach of Patients with Hormone-Related Cancer Based on the Glycemic Index and Glycemic Load Estimates. Nutrients 2023, 15, 3810. https://doi.org/10.3390/nu15173810
Peppa M, Manta A, Mavroeidi I, Nastos C, Pikoulis E, Syrigos K, Bamias A. Dietary Approach of Patients with Hormone-Related Cancer Based on the Glycemic Index and Glycemic Load Estimates. Nutrients. 2023; 15(17):3810. https://doi.org/10.3390/nu15173810
Chicago/Turabian StylePeppa, Melpomeni, Aspasia Manta, Ioanna Mavroeidi, Constantinos Nastos, Emmanouil Pikoulis, Konstantinos Syrigos, and Aristotelis Bamias. 2023. "Dietary Approach of Patients with Hormone-Related Cancer Based on the Glycemic Index and Glycemic Load Estimates" Nutrients 15, no. 17: 3810. https://doi.org/10.3390/nu15173810
APA StylePeppa, M., Manta, A., Mavroeidi, I., Nastos, C., Pikoulis, E., Syrigos, K., & Bamias, A. (2023). Dietary Approach of Patients with Hormone-Related Cancer Based on the Glycemic Index and Glycemic Load Estimates. Nutrients, 15(17), 3810. https://doi.org/10.3390/nu15173810







