The Effect of Including eHealth in Dietary Interventions for Patients with Type 2 Diabetes with Overweight or Obesity: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Randomized controlled trial;
- ≥6 months in duration;
- Involving a dietary intervention (defined as an intervention on diet more than just diabetes guidelines);
- Performed in overweight or obese adults (defined as a body mass index (BMI) ≥ 25 kg/m2 in a Caucasian population or a BMI ≥ 23 kg/m2 in an Asian population) with type 2 diabetes or prediabetes;
- Using face-to-face combined with eHealth (blended) or eHealth only, compared to a control intervention;
- Report an outcome on weight loss, glycemic regulation and/or cost effectiveness.
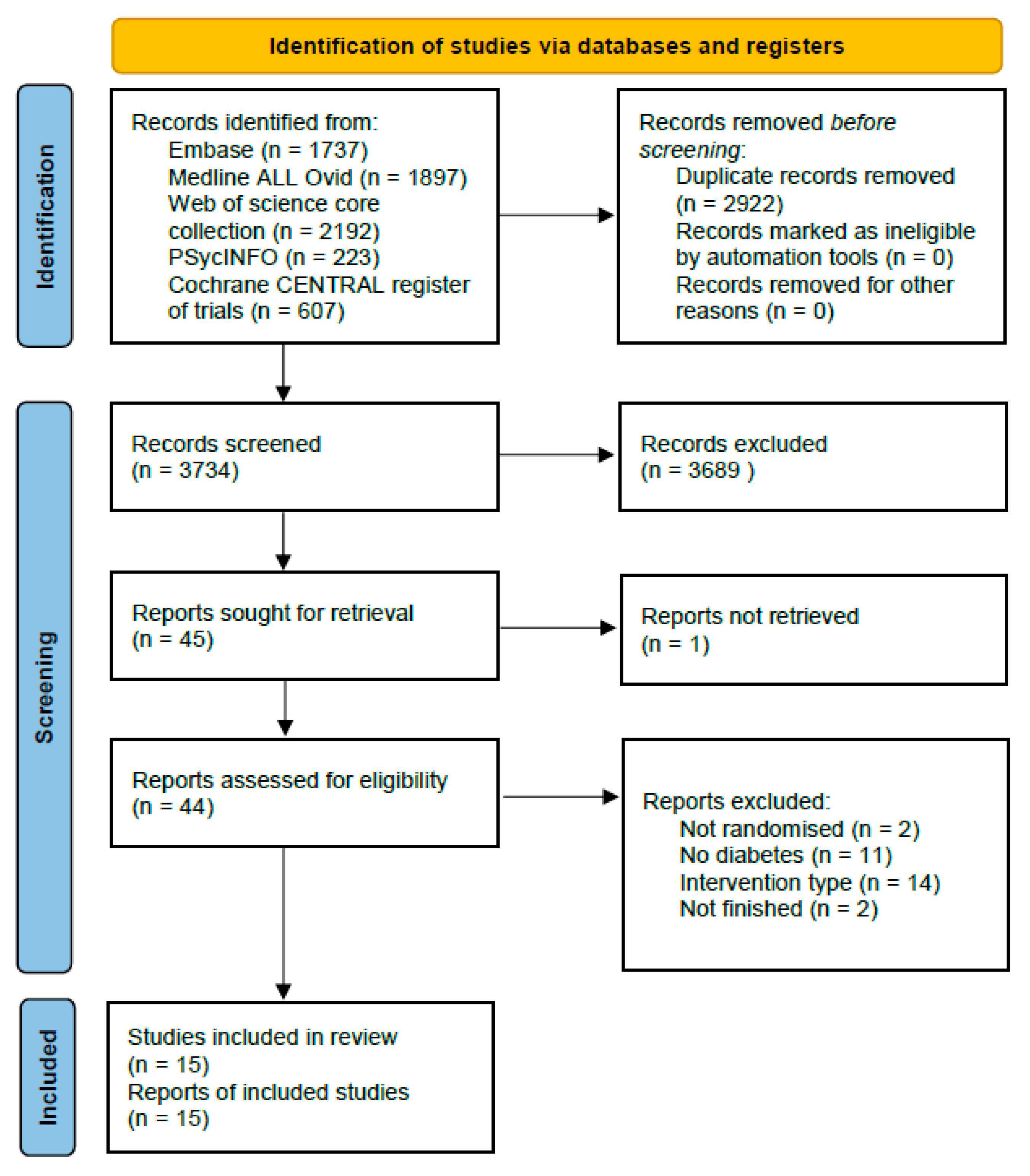
2.3. Study Selection
2.4. Data Collection Process
- Type of dietary intervention that was used;
- Type of eHealth that was used;
- Demographic variables of the study population;
- Duration of the study;
- The outcome variables weight loss and/or costs of the treatment;
- Other variables, when available (HbA1c and body composition measurements).
2.5. Risk of Bias in Individual Studies
2.6. Data Synthesis and Analysis
3. Results
3.1. Description of Studies
3.2. Quality Assessment of Included Studies
3.3. Study Population
3.4. Intervention Characteristics
3.5. Weight Loss
3.6. Costs
3.7. HbA1c
3.8. Fasting Blood Glucose
3.9. BMI
3.10. Waist Circumference
3.11. Fat Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Digital Health: World Health Organisation. 2023. Available online: https://www.who.int/health-topics/digital-health#tab=tab_1 (accessed on 15 March 2023).
- World Health Organisation. Global Strategy on Digital Health 2020–2025; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Eysenbach, G. What is e-health? J. Med. Internet Res. 2001, 3, E20. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.; Huygens, M.; Voogdt, H.; Meurs, M.; de Groot, J.; de Bruin, K.; Brabers, A.; Hofstede, C.; Friele, R.; van Gennip, L. Samen Aan Zet, Ehealth Monitor 2019; Nictiz: The Hague, The Netherlands; Nivel: Utrecht, The Netherlands, 2019. [Google Scholar]
- Wouters, M.; Huygens, M.; Voogdt, H.; Meurs, M.; de Groot, J.; de Bruin, K.; Brabers, A.; Hofstede, C.; Friele, R.; van Gennip, L. eHealth Monitor: Zelfmanagement en Telemonitoring; Thema Verdieping 3; Nictiz: The Hague, The Netherlands; Nivel: Utrecht, The Netherlands, 2019. [Google Scholar]
- Center for Disease Control and Prevention. World Diabetes Day: Center for Disease Control and Prevention. 2020, Updated 2 January 2020. Available online: https://www.cdc.gov/globalhealth/infographics/diabetes/world-diabetes-day.html (accessed on 15 March 2023).
- Wing, R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010, 170, 1566–1575. [Google Scholar] [PubMed]
- Tchero, H.; Kangambega, P.; Briatte, C.; Brunet-Houdard, S.; Retali, G.R.; Rusch, E. Clinical Effectiveness of Telemedicine in Diabetes Mellitus: A Meta-Analysis of 42 Randomized Controlled Trials. Telemed. J. E-Health 2019, 25, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- American Diabetes Association. Understanding A1C; Diagnosis American Diabetes Association. Available online: https://www.diabetes.org/a1c/diagnosis (accessed on 1 December 2021).
- Van De Schoot, R.; De Bruin, J.; Schram, R.; Zahedi, P.; De Boer, J.; Weijdema, F.; Kramer, B.; Huijts, M.; Hoogerwerf, M.; Ferdinands, G.; et al. An open source machine learning framework for efficient and transparent systematic reviews. Nat. Mach. Intell. 2021, 3, 125–133. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 15 February 2022).
- Castelnuovo, G.; Manzoni, G.M.; Cuzziol, P.; Cesa, G.L.; Corti, S.; Tuzzi, C.; Villa, V.; Liuzzi, A.; Petroni, M.L.; Molinari, E. TECNOB Study: Ad Interim Results of a Randomized Controlled Trial of a Multidisciplinary Telecare Intervention for Obese Patients with Type-2 Diabetes. Clin. Pract. Epidemilogy Ment. Health 2011, 7, 44–50. [Google Scholar] [CrossRef][Green Version]
- Dawes, D.; Ashe, M.; Campbell, K.; Cave, D.; Elley, C.R.; Kaczorowski, J.; Sohal, P.; Ur, E.; Dawes, M. Preventing diabetes in primary care: A feasibility cluster randomized trial. Can. J. Diabetes 2015, 39, 111–116. [Google Scholar] [CrossRef]
- Fischer, H.H.; Fischer, I.P.; Pereira, R.I.; Furniss, A.L.; Rozwadowski, J.M.; Moore, S.L.; Durfee, M.J.; Raghunath, S.G.; Tsai, A.G.; Havranek, E.P. Text Message Support for Weight Loss in Patients with Prediabetes: A Randomized Clinical Trial. Diabetes Care 2016, 39, 1364–1370. [Google Scholar] [CrossRef]
- Haste, A.; Adamson, A.J.; McColl, E.; Araujo-Soares, V.; Bell, R. Web-Based Weight Loss Intervention for Men with Type 2 Diabetes: Pilot Randomized Controlled Trial. JMIR Diabetes 2017, 2, e14. [Google Scholar] [CrossRef]
- Lutes, L.D.; Cummings, D.M.; Littlewood, K.; Dinatale, E.; Hambidge, B. A Community Health Worker-Delivered Intervention in African American Women with Type 2 Diabetes: A 12-Month Randomized Trial. Obesity 2017, 25, 1329–1335. [Google Scholar] [CrossRef]
- Ma, J.; Yank, V.; Xiao, L.; Lavori, P.W.; Wilson, S.R.; Rosas, L.G.; Stafford, R.S. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: A randomized trial. JAMA Intern. Med. 2013, 173, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Lopez, L.; Munoz-Torres, A.V.; Medina-Bravo, P.; Vilchis-Gil, J.; Klupsilonnder-Klupsilonnder, M.; Escobedo-de la Pena, J. Multimedia education program and nutrition therapy improves HbA1c, weight, and lipid profile of patients with type 2 diabetes: A randomized clinical trial. Endocrine 2017, 58, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, C.; Padhye, N.; Orlander, P.; Zare, M. A Behavioral Lifestyle Intervention Enhanced with Multiple-Behavior Self-Monitoring Using Mobile and Connected Tools for Underserved Individuals with Type 2 Diabetes and Comorbid Overweight or Obesity: Pilot Comparative Effectiveness Trial. JMIR Mhealth Uhealth 2018, 6, e92. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Azar, K.M.; Romanelli, R.J.; Block, T.J.; Hopkins, D.; Carpenter, H.A.; Dolginsky, M.S.; Hudes, M.L.; Palaniappan, L.P.; Block, C.H. Diabetes Prevention and Weight Loss with a Fully Automated Behavioral Intervention by Email, Web, and Mobile Phone: A Randomized Controlled Trial among Persons with Prediabetes. J. Med. Internet Res. 2015, 17, e240. [Google Scholar] [CrossRef]
- Katula, J.A.; Dressler, E.V.; Kittel, C.A.; Harvin, L.N.; Almeida, F.A.; Wilson, K.E.; Michaud, T.L.; Porter, G.C.; Brito, F.A.; Goessl, C.L.; et al. Effects of a Digital Diabetes Prevention Program: An RCT. Am. J. Prev. Med. 2022, 62, 567–577. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Hu, L.; Woolf, K.; Wang, C.; Goldfarb, D.S.; Katz, S.D.; Popp, C.; Williams, S.K.; Li, H.; Jagannathan, R.; et al. An Evaluation of Alternative Technology-Supported Counseling Approaches to Promote Multiple Lifestyle Behavior Changes in Patients with Type 2 Diabetes and Chronic Kidney Disease. J. Ren. Nutr. 2023, 33, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Toro-Ramos, T.; Michaelides, A.; Anton, M.; Karim, Z.; Kang-Oh, L.; Argyrou, C.; Loukaidou, E.; Charitou, M.M.; Sze, W.; Miller, J.D. Mobile Delivery of the Diabetes Prevention Program in People with Prediabetes: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e17842. [Google Scholar] [CrossRef]
- Al-Hamdan, R.; Avery, A.; Al-Disi, D.; Sabico, S.; Al-Daghri, N.M.; McCullough, F. Efficacy of lifestyle intervention program for Arab women with prediabetes using social media as an alternative platform of delivery. J. Diabetes Investig. 2021, 12, 1872–1880. [Google Scholar] [CrossRef]
- Lim, S.L.; Ong, K.W.; Johal, J.; Han, C.Y.; Yap, Q.V.; Chan, Y.H.; Chooi, Y.C.; Zhang, Z.P.; Chandra, C.C.; Thiagarajah, A.G.; et al. Effect of a Smartphone App on Weight Change and Metabolic Outcomes in Asian Adults with Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2112417. [Google Scholar] [CrossRef]
- Yin, W.; Liu, Y.; Hu, H.; Sun, J.; Liu, Y.; Wang, Z. Telemedicine management of type 2 diabetes mellitus in obese and overweight young and middle-aged patients during COVID-19 outbreak: A single-center, prospective, randomized control study. PLoS ONE 2022, 17, e0275251. [Google Scholar] [CrossRef]
- The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP) Description of lifestyle intervention. Diabetes Care 2002, 25, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Chrvala, C.A.; Sherr, D.; Lipman, R.D. Diabetes self-management education for adults with type 2 diabetes mellitus: A systematic review of the effect on glycemic control. Patient Educ. Couns. 2016, 99, 926–943. [Google Scholar] [CrossRef]
- Hanlon, P.; Daines, L.; Campbell, C.; McKinstry, B.; Weller, D.; Pinnock, H. Telehealth Interventions to Support Self-Management of Long-Term Conditions: A Systematic Metareview of Diabetes, Heart Failure, Asthma, Chronic Obstructive Pulmonary Disease, and Cancer. J. Med. Internet Res. 2017, 19, e172. [Google Scholar] [CrossRef] [PubMed]
- Wayne, N.; Perez, D.F.; Kaplan, D.M.; Ritvo, P. Health Coaching Reduces HbA1c in Type 2 Diabetic Patients from a Lower-Socioeconomic Status Community: A Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e224. [Google Scholar] [CrossRef] [PubMed]
- Young, H.M.; Miyamoto, S.; Dharmar, M.; Tang-Feldman, Y. Nurse Coaching and Mobile Health Compared with Usual Care to Improve Diabetes Self-Efficacy for Persons with Type 2 Diabetes: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e16665. [Google Scholar] [CrossRef]
- Hutchesson, M.J.; Gough, C.; Muller, A.M.; Short, C.E.; Whatnall, M.C.; Ahmed, M.; Pearson, N.; Yin, Z.; Ashton, L.M.; Maher, C.; et al. eHealth interventions targeting nutrition, physical activity, sedentary behavior, or obesity in adults: A scoping review of systematic reviews. Obes. Rev. 2021, 22, e13295. [Google Scholar] [CrossRef]
- Puigdomenech Puig, E.; Robles, N.; Saigi-Rubio, F.; Zamora, A.; Moharra, M.; Paluzie, G.; Balfego, M.; Cambra, G.C.; Garcia-Lorda, P.; Carrion, C. Assessment of the Efficacy, Safety, and Effectiveness of Weight Control and Obesity Management Mobile Health Interventions: Systematic Review. JMIR Mhealth Uhealth 2019, 7, e12612. [Google Scholar] [CrossRef]
- Sherrington, A.; Newham, J.J.; Bell, R.; Adamson, A.; McColl, E.; Araujo-Soares, V. Systematic review and meta-analysis of internet-delivered interventions providing personalized feedback for weight loss in overweight and obese adults. Obes. Rev. 2016, 17, 541–551. [Google Scholar] [CrossRef]
- World Health Organization. Mhealth: New Horizons for Health through Mobile Technologies: Second Global Survey on Ehealth; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Eng, T.R. The eHealth Landscape: A Terrain Map of Emerging Information and Communication Technologies in Health and Heath Care; Robert Wood Johnson Foundation: Princeton, NJ, USA, 2001. [Google Scholar]
- Hahn, S. Understanding noninferiority trials. Korean J. Pediatr. 2012, 55, 403–407. [Google Scholar] [CrossRef]
- Rompelberg, C.; Suijkerbuijk, A.; Wouters, M. Stand van Zaken E-Health in 2020; Rijks Instituut voor Volksgezondheid en Milieu: Utrecht, The Netherland, 2020. [Google Scholar]
- Nielen, M.; Poos, R.; Korevaar, J. Diabetes Mellitus in Nederland: Prevalentie en Incidentie: Heden, Verleden en Toekomst; Nivel: Utrecht, The Netherlands, 2020. [Google Scholar]
| Overall Risk-of-Bias Judgement | Criteria |
|---|---|
| Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Some concerns | The study is judged to raise some concerns in at least one domain for this result but not to be at high risk of bias for any domain. |
| High risk of bias | The study is judged to be at high risk of bias in at least one domain for this result. |
| Or | |
| The study is judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result. |
| Study | Participant Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| First Author; Year; Duration; Design | Intervention Groups * | Sample | Age (y) | Male (%) | BMI (kg/m2) | Anthropometric Measurements | Other Measurements | |
| Al Hamdan et al., 2021 [25] 6 months RCT | [1] Control group | 253 overweight or obese female with prediabetes | [1] 51 ± 7.1 | [A] 0 | [1] 31.6 ± 5.8 | Weight change (kg) | WC (cm) | HbA1c (%) |
| [1] −0.2 | [1] +0.2 | [1] −0.3 ** | ||||||
| [2] −5.8 ** | [2] −1.1 ** | [2] −0.7 ** | ||||||
| [3] −1.3 ** | [3] −3.5 ** | [3] −0.5 ** | ||||||
| [D] ** | [D] ** | [D] p = 0.33 | ||||||
| [2] eHealth education via app | [2] 44 ± 8.1 | [2] 30.0 ± 5.1 | BMI (kg/m2) | |||||
| [3] Face-to-face—group education | [3] 43 ± 12.2 | [3] 34.8 ± 9.0 | [1] −0.1 | |||||
| [2] −0.6 ** | ||||||||
| [3] −2.1 ** | ||||||||
| [D] ** | ||||||||
| Block et al., 2015 [21] 6 months RCT | [1] Control group | 340 overweight or obese male and female with prediabetes | [1] 55 (9.1) | [1] 69 | [1] 31.2 (4.3) | Weight change (kg) | WC (cm) | HbA1c (%) |
| [1] −1.26 (−1.27, −1.26) | [1] −2.22 (−2.36, −2.09) | [1] −0.18 (−0.19, −0.16) | ||||||
| [2] −3.26 (−3.26, −3.25) ** | [2] −4.56 (−4.69, −4.43) ** | [2] −0.26 (−0.27, −0.24) ** | ||||||
| [2] eHealth via web based program | [2] 55 (8.8) | [2] 68 | [2] 31.1 (4.5) | Weight change (%) | Fasting glucose (mmol/L) | |||
| [1] −1.32 (–1.36, −1.28) | [1] −0.12 (−0.15, −0.10) | |||||||
| [2] −3.60 (−3.63, −3.57) ** | [2] −0.41 (−0.44, −0.38) ** | |||||||
| BMI (kg/m2) | ||||||||
| [1] −0.39 (−0.39, −0.38) | ||||||||
| [2] −1.05 (−1.06, −1.05) ** | ||||||||
| Castelnuovo et al., 2011 [13] 12 months; RCT | [1] Control group | 34 obese male and female with type 2 diabetes | [1] 49 (46–57.5) | [1] 69 [2] 35 | - | Weight change (%) | ||
| [1] −4.1 (−15.3 to 3) | ||||||||
| [2] eHealth via web based program | [2] 54 (49–60) | [2] −6.2 (−10.6 to 16.7) | ||||||
| Dawes et al., 2015, [14] 6 months; RCT | [1] Control group | 59 overweight or obese male and female with prediabetes | 47% aged 35–64, | [A] 51 | [A] 29.1 ± 5.9 | Weight change (kg) | WC (cm) | Fasting glucose (mg/dL) |
| [1] −0.3 ± 1.8 | [1] −1 ± 5 | [1] −0.54 ± 8.47 | ||||||
| [2] −3.4 ± 3.1 | [2] −4 ± 5 | [2] −3.96 ± 7.39 | ||||||
| [D] −3.2 (−4.6 to −1.7) # | [D] −3 (−5.7 to −0.3) # | [D] −3.24 (−7.57 to 1.08) # | ||||||
| [2] eHealth with pedometer + telephone calls | 53% aged ≥ 65 | BMI (kg/m2) | HbA1c (%) | |||||
| [1] 0.0 ± 0.7 | [1] 0.03 ± 0.24 [2] −0.07 ± 0.21 | |||||||
| [2] −1.2 ± 1.1 | [D] −0.10 (−0.23 to 0.03) # | |||||||
| [D] −1.2 (−1.7 to −0.7) # | Mean cost per participant (C$) [2] 144. | |||||||
| Fischer et al., 2016 [15] 12 months; RCT | [1] Control group | 157 overweight or obese male and female with prediabetes | [1] 45 ± 10.6 | [1] 19 | - | Weight change (kg) | HbA1c (%) | |
| [1] −0.25 (−1.23 to 0.73) | [1] 0.19 (−0.1 to 0.5) | |||||||
| [2] −1.18 (−2.50 to 0.09) | [2] −0.09 (−0.2 to 0.0) | |||||||
| [2] eHealth by text messages | [2] 48 ± 12.4 | [2] 30 | [D] −0.95 (−2.54 to 0.64) | [D] −0.29 (−0.58 to 0.01) | ||||
| Total intervention program cost ($) | ||||||||
| [2] 22,113.61 | ||||||||
| Haste et al., 2017 [16] 12 months; pilot RCT | [1] Control group | 61 obese male with type 2 diabetes | [1] 61 {54.5, 66.8} | [A] 100 | [1] 34.6 ± 3.0 | Weight change (kg) | WC (cm) | |
| [1] −2.8 ± 4.4 | [1] –2.0 (–3.8 to –1) | |||||||
| [2] −5.4 ± 5.9 # | [2] –3.5 (–7 to –1.3) # | |||||||
| [2] eHealth by web based program | [2] 58 {50, 67.5} | [2] 33.9 ± 2.6 | BMI (kg/m2) | |||||
| [1] −0.9 ± 1.4 | ||||||||
| [2] −1.3 ± 2.0 # | ||||||||
| Katula et al., 2022 [22] 12 months single blind RCT | [1] Enhanced control group | 599 overweight or obese male and female with prediabetes | [1] 56 (12.6) | [1] 39 | [1] 36.1 (6.6) | Weight change (kg) | HbA1c (%) | |
| [1] −2.18 (2.97, 1.39) | [1] −0.16 (−0.19, −0.12) | |||||||
| [2] −5.52 (6.30, 4.75) | [2] −0.23 (−0.26, −0.20) | |||||||
| [D] 3.34 (4.39, 2.29) ** | [D] −0.08 (−0.12, −0.03) ** | |||||||
| [2] eHealth by web based program and pedometers | [2] 55 (12.9) | [2] 39 | [2] 35.8 (6.1) | Weight change (%) | ||||
| [1] 2.09 (2.82, 1.37) | [1] 1.70 (2.07, 1.33), | |||||||
| [2] 5.49 (6.20, 4.78) | [2] 2.52 (2.89, 2.16), | |||||||
| [D] 3.40 (4.36, 2.43) ** | [D] 0.82 (1.32, 0.32) ** | |||||||
| Lim et al., 2021 [26] 6 months Multi center RCT | [1] Control group | 204 overweight or obese male and female with type 2 diabetes | [1] 51 (10.0) | [1] 63 | [1] 30.9 (4.5) | Weight change (kg) | HbA1c (%): | |
| [1] −1.2 (3.6) | [1] −0.3 (1.0) | |||||||
| [2] −3.6 (4.7) | [2] −0.7 (1.2) | |||||||
| [D] −2.4 (−3.5 to −1.3) ** | [D] −0.4 (−0.7 to −0.1)** | |||||||
| [2] Control group + app | [2] 52 (9.4) | [2] 67 | [2] 30.3 (4.0) | Weight change (%) [1] −1.4 (4.2), [2] −4.3 (5.4), [D] −2.9 (−4.2 to −1.6) ** | Fasting glucose (mg/dL) [1] −1.8 (25.2) | |||
| BMI (kg/m2) | ||||||||
| [1] −0.4 (1.3) | ||||||||
| [2] −1.3 (1.7) | [2] −14.4 (37.8) | |||||||
| [D] −0.9 (−1.3 to −0.5) ** | [D] −12.6 (−23.4 to −3.6) ** | |||||||
| Lutes et al., 2017 [17] 12 months; RCT | [1] Face-to-face group education [2] eHealth by email | 200 overweight or obese female with type 2 diabetes | [1] 53 ± 10.62 | [A] 0 | [1] 36.59 ± 7.48 | Weight change (kg) | HbA1c (%) | |
| [2] 54 ± 9.84 | [2] 38.80 ± 8.43 | [1] −1.35 ± 6.22 | [1] −0.29 ± 1.84 | |||||
| [A] 53 ± 10.24 | [A] 37.67 ± 8.02 | [2] −0.39 ± 4.57 ** | [2] 0.05 ± 1.61 | |||||
| Ma et al., 2013 [18] 15 months; RCT | [1] Control group | 241 overweight or obese male and female with prediabetes or metabolic syndrome | [1] 53 ± 10.9 | [A] 54 | [1] 32.4 ± 6.3 | Weight change (kg) | WC (cm) | Fasting glucose (mg/dL) |
| [1] −2.4 (0.9) | [1] −2.2 (1.1) | [1] 0.2 (1.7) | ||||||
| [2] −4.5 (0.9) | [2] −4.9 (1.0) | [2] −2.7 (1.6) | ||||||
| [3] −6.3 (0.9) | [3] −5.8 (1.0) | [3] −4.2 (1.6) | ||||||
| [D] ** | ||||||||
| [2] eHealth by Digital Versatile Disk (DVD) and pedometer | [2] 52 ± 9.9 | [2] 31.7 ± 4.7 | Weight change (%) | |||||
| [1] −2.6 (0.9) | ||||||||
| [2]−5.0 (0.9) | ||||||||
| [3] −6.6 (0.9) | ||||||||
| [3] Face-to-face group education | [3] 55 ± 11.0 [A] 53 ± 10.6 | [3] 31.8 ± 5.1 [A] 32.0 ± 5.4 | BMI (kg/m2) | |||||
| [1] −0.9 (0.3) | ||||||||
| [2]−1.6 (0.3) | ||||||||
| [3] −2.2 (0.3) | ||||||||
| [D] ** | ||||||||
| St-Jules et al., 2022 [23] 6 months 2 × 2 factorial RCT | [1] Written information and telephone calls on education | 256 overweight or obese male and female with type 2 diabetes | [1] 67 (9.0) | [1] 48 | [1] 33.3 (4.5) | Weight change (kg) [1] −1.2 (4.3) | HbA1c (%) [1] −0.3 (1.1) | |
| [2] eHealth by video conferencing focused on education and a web based program | [2] 65 (10.0) | [2] 56 | [2] 34.4 (5.5) | [2] −2.3 (3.4) | [2] −0.3 (0.9) | |||
| [3] eHealth by video conferencing focused on education and behaviour | [3] 64 (9.0) | [3] 42 | [3] 34.2 (5.7) | [3] −1.4 (3.1) | [3] −0.1 (1.0) | |||
| [4] eHealth by video conferencing focused on education and behaviour and a web based program | [4] 64.0 (8.0) | [4] 53 | [4] 33.2 (4.4) | [4] −2.7 (4.4) | [4] −0.3 (0.9) | |||
| Toro-Ramos et al., 2020 [24] 12 months; RCT | [1] enhanced control group | 202 overweight or obese male and female with prediabetes | [1] 58 (12.5) | [1] 31 | [1] 30.9 (7.2) | Weight change (kg) | HbA1c (%) | |
| [1] −0.09 (−1.30 to 1.11) | [1] −0.16 (−0.27 to −0.05) ** | |||||||
| [2] −2.22 (−3.31 to −1.13) ** | [2] −0.23 (−0.32 to −0.14) ** | |||||||
| [D] −1.8 ** | ||||||||
| [2] eHealth by Noom web-based program and app | [2] 56 (13.6) | [2] 26 | [2] 31.3 (6.4) | Weight change (%) [1] 0.33 (−1.06 to 1.72) | ||||
| [2]−2.54 (−3.74 to −1.33) ** | ||||||||
| BMI (kg/m2) [1]−0.04 (−0.47 to 0.39) | ||||||||
| [2]−0.88 (−1.31 to 0.44) ** | ||||||||
| [D] −0.58 ** | ||||||||
| Velázquez-López et al., 2017 [19] 21 months; RCT | [1] Control group | 351 overweight or obese male and female with type 2 diabetes | [1] 54 ± 8.8 | [1] 34 | [1] 30.4 ± 5.0 | Weight change (kg) | WC (cm) | Fasting glucose (mg/dL) |
| [1] −0.61 (−1.47 to 0.25) | [1] −4.32 (−5.59 to −3.04) | [1] −6.50 (−17.8 to −4.70) | ||||||
| [2] −1.23 (−2.29 to −0.16) | [2] −5.50 (−6.89 to −4.12) | [2] −36.6 (−46.6 to −26.60) | ||||||
| [D] −0.62 (−1.97 to 0.74) | [D] −1.19 (−3.06 to 0.68) | [D] −18.1 (−29.7 to −6.4) ** | ||||||
| [2] Usual care and eHealth by web-based program | [2] 55 ± 8.8 | [2] 30 | [2] 30.8 ± 5.9 | BMI (kg/m2) | Fat mass (%) | HbA1c (%) | ||
| [1] −0.07 (−0.39 to 0.25) | [1] −0.27 (−1.57 to 1.03) | [1] −1.33 (−1.65 to −1.01) | ||||||
| [2] −0.42 (−0.86 to 0.01) | [2] −0.96 (−2.17 to 0.26) | [2] −1.48 (−1.91 to −1.04) | ||||||
| [D] −0.36 (−0.89 to 0.18) | [D] −0.69 (−2.46 to 1.08) | [D] −0.11 (−0.70 to 0.48) | ||||||
| Wang et al., 2018 [20] 6 months; pilot RCT | [1] Control group | 26 overweight or obese male and female with type 2 diabetes | [1] 49 ± 10.2 | [1] 80 | [1] 33.7 ± 2.7 | Weight change (%) [1] 1.6 {−4.1, 3.8} | HbA1c (%) [1] 8.9 ± 1.6 | |
| [2] Usual care and face-to-face group education and eHealth by app | [2] 59 ± 5.9 | [2] 18 | [2] 38.9 ± 9.0 | [2] −1.8 {−4.2, −0.3} | [2] 6.9 ± 1.0 | |||
| [3] Usual care and face-to-face group education | [3] 56 ± 5.4 | [3] 44 | [3] 40.1 ± 7.0 | [3] 0.4 {−2.3, 1.5} | [3] 9.1 ± 1.8 [D] ** | |||
| Yin et al., 2022 [27] 6 months RCT | [1] Control group | 120 overweight or obese male and female with type 2 diabetes | [1] 47 (42, 51) | [1] 38 | [1] 29.05 (3.31) | BMI (kg/m2) [1] −1.68 (3.95) | HbA1c (%) [1] −1.84 (1.55) [2] −2.41 (1.38) | |
| [2] eHealth by app | [2] 48 (43, 51) | [2] 43 | [2] 29.25 (2.93) | [2] −3.77 (3.38) ** | Fasting glucose (mmol/L) [1] −2.83 (2.03) [2] −2.74 (1.96) | |||
| Study ID | Randomissation Process | Deviations from Intended Intervention | Missing Outcome Data | Measurement of the Outcome | Selection of Reported Result | Overall |
|---|---|---|---|---|---|---|
| Castelnuovo | + | + | + | + | ! | ! |
| Dawes | ! | + | + | + | + | + |
| Fischer | + | + | + | + | + | + |
| Haste | + | ! | - | + | + | - |
| Lutes | + | + | + | + | ! | + |
| Ma | + | + | + | + | + | + |
| Velasquez | ! | + | + | + | ! | ! |
| Wang | + | + | + | + | + | + |
| Al Hamdan | - | - | + | + | + | - |
| Block | + | + | + | + | + | + |
| Katula | + | + | + | + | + | + |
| Lim | ! | + | + | + | + | ! |
| St Jules | + | + | + | + | + | + |
| Toro Ramos | + | + | + | + | + | + |
| Yin | + | - | + | + | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geurts, K.A.M.; Woodcock-Nekeman, S.; Hummel, M.; Dietvorst, C.A.W.; van Rossum, E.F.C.; Berk, K.A. The Effect of Including eHealth in Dietary Interventions for Patients with Type 2 Diabetes with Overweight or Obesity: A Systematic Review. Nutrients 2023, 15, 3776. https://doi.org/10.3390/nu15173776
Geurts KAM, Woodcock-Nekeman S, Hummel M, Dietvorst CAW, van Rossum EFC, Berk KA. The Effect of Including eHealth in Dietary Interventions for Patients with Type 2 Diabetes with Overweight or Obesity: A Systematic Review. Nutrients. 2023; 15(17):3776. https://doi.org/10.3390/nu15173776
Chicago/Turabian StyleGeurts, Karlijn A. M., Sandra Woodcock-Nekeman, Mitchell Hummel, Carmen A. W. Dietvorst, Elisabeth F. C. van Rossum, and Kirsten A. Berk. 2023. "The Effect of Including eHealth in Dietary Interventions for Patients with Type 2 Diabetes with Overweight or Obesity: A Systematic Review" Nutrients 15, no. 17: 3776. https://doi.org/10.3390/nu15173776
APA StyleGeurts, K. A. M., Woodcock-Nekeman, S., Hummel, M., Dietvorst, C. A. W., van Rossum, E. F. C., & Berk, K. A. (2023). The Effect of Including eHealth in Dietary Interventions for Patients with Type 2 Diabetes with Overweight or Obesity: A Systematic Review. Nutrients, 15(17), 3776. https://doi.org/10.3390/nu15173776






