Abstract
Acute leukemia commonly occurs in young children with peak incidence at the age of 2–5 years. However, the etiology is still unclear and many preventable risk factors still deserve to be reviewed. The focus of this systematic review and meta-analysis is to summarize the evidence concerning early life nourishment (breastfeeding, early life diet), neonatal vitamin K administration and the risk of acute leukemia. All epidemiological studies published up to June 2023 and assessing diet-related risk factors for childhood acute leukemia were identified in two electronic databases (PubMed and Web of Science), with no limits on publication year or language. A total of 38 studies (37 case–control studies and 1 study with pooled analysis) were included. The published risk estimates were combined into a meta-analysis using the Generic Inverse Variance method. The current evidence shows that breastfeeding (yes vs. no) has a protective effect against acute lymphoblastic leukemia (odds ratio = 0.85; 95% CI, 0.76–0.94). Evidence related to the role of other studied factors (foods and supplements) is inconclusive. Further research into the potential role of diet in early life and the risk of acute leukemia is needed to develop prevention strategies at population level. Review Registration: PROSPERO registration no. CRD42019128937.
1. Introduction
Childhood cancer is a notable cause of morbidity and mortality, with incidence rising from 124 to 140 per million person years between the eighties and 2000s in populations covered by cancer registration [1]. Acute leukemia (AL) is the most common type of cancer in children under 15 years old in most populations. The reported world age-standardized incidence rate of leukemia is 46.4 per million per year in children aged 0–14 years, and 28.5 per million in adolescents aged 15–19 years [1]. As AL is a rare disease, retrospective case–control design was used to study potential risk factors. Case control studies are known to be subjected to bias in exposure assessment [2,3]. The methodological limitations of retrospective case–control studies can partially explain the inconsistent results of the association between diverse exposures and leukemia etiology. Thus, apart from exposure to ionizing radiation, specific types of chemicals (e.g., benzene, chemotherapy), and certain genetic variations and syndromes, our understanding of the causes of AL in children is limited [2,4,5].
Almost 80% of AL cases are lymphoblastic lineage (ALL) and 15% are myeloid lineage (AML) in children from 0 to 14 years old [6], although incidences may vary by diverse factors (e.g., region, ethnicity) [7]. Age standardized rates of leukemia varies from 12.5 (Sub-Saharan Africa) to 65.4 (white Hispanics in USA) per million person years [1]. It is noteworthy that the differential incidence across geographic regions may be due to underdiagnosis in low-income settings [8]. In addition, considering a higher incidence around two to five years of age, it is reasonable to hypothesize that exposures (lifestyle and environmental exposures) during the perinatal and early infancy period could contribute in the pathogenesis of childhood leukemia [1,2,9]. Diet and supplementation provide nutrients for our daily life, but they are also implied in complex pathways (e.g., cellular replication, hormone regulation, immune response) that may modulate the hazard of developing any cancer, including leukemia [2,9].
Therefore, in this systematic review, we focused on the likely role of breastfeeding, early child’s diet, and neonatal administration of vitamin K on the risk of AL in children. The combined evidence could be used to assess the development of preventive strategies on a population level to reduce the incidence and mortality of leukemia.
2. Materials and Methods
2.1. Definition of the Outcome
For the systematic review, the incidence of AL in children aged 0–14 years and adolescents aged 15–19 years was the main outcome. Acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) were included as subtypes of leukemia [6,10,11].
2.2. Definition of the Risk Factors
The considered risk factors were breastfeeding (breastfed, lactation, infant feeding), early child’s diet circa first 3 years of life (diet, food intake, nutrition, supplement), and the neonatal administration of vitamin K.
2.3. Systematic Review Registration
PROSPERO was used to record the protocol details of this systematic review on registration number CRD42019128937.
2.4. Search Strategy
To identify early life nutritional factors that have been studied in relation to leukemia risk, we conducted a comprehensive multi-level literature search using Medline (via PubMed) and Web of Science. The first level of analysis for this systematic review consisted of an exploratory search for studies on leukemia in infants, children and adolescents and early life exposures (pregnancy to early life—first three years). This first search was conducted in December 2017, without being limited by language or publication date. A total of 66 studies were identified in this search; at this stage, exposures during pregnancy and early life were included. Based on these results, and after sub-grouping the exposures, we were able to further restrict the keywords and carry out a more targeted search, updated in June 2023, which focused on the nutritional exposures identified in early life, described in Supplementary Table S1. For all concepts, database-specific thesauri were used to identify relevant synonyms. The final search strategy is illustrated in Figure 1.
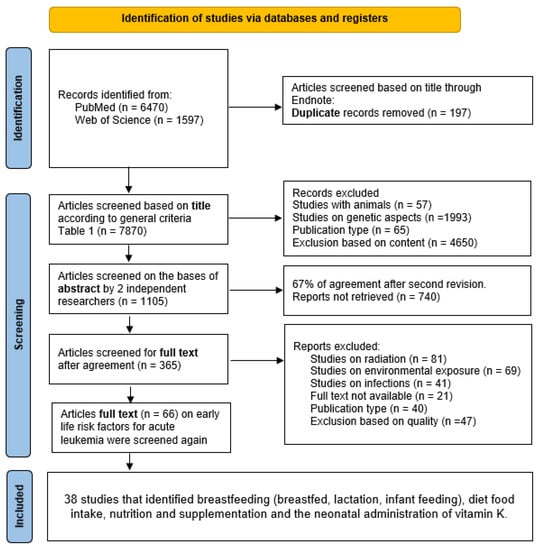
Figure 1.
Final search strategy.
2.5. Selection of the Studies and Quality Assessment
Studies were included if they provided estimates of effect (relative risk (RR), odds ratio (OR) or hazard ratio (HR) and 95% confidence intervals (CI)), or if they provided sufficient data to calculate estimates for different exposure levels.
Table 1 lists the inclusion and exclusion criteria used in the review. PRISMA guidelines were followed [12] (Supplementary Table S2). All references found through the search questions (Figure 1) were imported into Endnote software (Version x8, Clarivate Analytics, Philadelphia, PA, USA). Two independent authors (AK-K and JB-L) searched for publications in each database (PubMed and Web of Science). Any uncertainty regarding the selection of a given article was resolved by a third reviewer (IH) and discussed within the working group, if necessary. The PICOS criteria for included studies are listed in Table 2, and the selected studies included for each extracted factor are summarized in Table 3. Twenty-one articles were excluded because the full text was not available; it was not possible to obtain a copy, even when requesting the authors.
Quality control of the selected articles was then carried out using the checklist established by Fowkes et al. to ensure the inclusion of high-quality studies [13]. This checklist includes questions on study design and sample, characteristics of the control group, quality of measurements and results, completeness, and influence of bias. A major flaw identified for a given criterion is indicated by a ++ sign. The + sign has been applied to a criterion with a minor defect. In the absence of a defect, the sign 0 (nil) was applied. If a study obtained a score of ++ for more than one criterion in this checklist, it was excluded. The results of the quality control are shown in Supplementary Table S3, and details of the excluded studies are shown in Supplementary Table S4.




Table 1.
Inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria.
| Inclusion and Exclusion Criteria |
|---|
| General inclusion criteria |
|
| General exclusion criteria |
|
| Exclusion criteria based on content |
|
| Exclusion criteria based on study scope and data quality |
|

Table 2.
PICOS criteria for inclusion and exclusion of studies.
Table 2.
PICOS criteria for inclusion and exclusion of studies.
| Parameter | Criterion |
|---|---|
| Participants | Children and adolescents diagnosed with acute leukemia |
| Interventions | Early life nourishment (including breastfeeding and early child’s diet) and vitamin K administration |
| Control/comparator group | Healthy children and adolescents |
| Outcomes | Childhood acute leukemia (AL, ALL, AML) |
| Study design | Observational studies with a comparison group (cohort studies, case–control studies) |

Table 3.
Overview of the included studies.
Table 3.
Overview of the included studies.
| Extracted Factors (Early Life Nutrition Factors) | Number of Included Articles 1 | References |
|---|---|---|
| Breastfeeding | 25 | [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] |
| Early child’s diet | 6 | [16,23,39,40,41,42] |
| Vitamin K administration | 9 | [43,44,45,46,47,48,49,50,51] |
1 A total of 37 case–control studies and one study with pooled analysis were included.
2.6. Data Extraction
Table 4 summarizes the included studies according to name, design, sample size, age at diagnosis and country of study population. Key data extracted from all included studies, effect estimates (RR, OR or HR, 95% CI) and confounding variables tested are presented in Supplementary Tables S5–S7.

Table 4.
Characteristics of the included studies that included early life nutrition factors and risk of acute leukemia in children.
2.7. Quantitative Meta-Analysis
A meta-analysis was performed to estimate which factors had an impact on AL, ALL or AML. For each factor included, the choice to perform an additional quantitative analysis was based on the number (at least two studies) and homogeneity of the included studies (e.g., studies limited to infants or univariate analyses without adjustments were not pooled), and data from overlapping studies were also excluded. We used the reported adjusted odds ratio (OR) in case–control studies and the relative risk (RR) in cohort studies to calculate the overall effect. Analyses were performed using the generic inverse variance method, in which the weight of each study is equivalent to the inverse of the variance of the effect estimate [52]. A random-effects model was used where there was evidence of heterogeneity. For exposures reported at different levels, we pooled an estimate for a binary exposure status (exposed vs. unexposed). Heterogeneity was assessed using the I2 statistic, where 0% indicates perfect homogeneity and 100% complete heterogeneity [53]. Data were analyzed using Review Manager (RevMan) V.5.4.1, Cochrane, London, UK [54]. The results are summarized in Table 5.

Table 5.
Summary Odds Ratios (ORs) and 95% Confidence Intervals (Cis) obtained in the meta-analysis of data from referenced published studies of association of childhood leukemia with early life nutrition factors.
3. Results
3.1. Selected Studies
The results of the search strategy and the process of selecting studies for review are illustrated in Figure 1. The preliminary search yielded 7870 studies, which were selected based on their titles. After evaluation of titles and abstracts, 365 articles met the criteria for full-text review. Consequent review of the full-text reports of the 66 selected publications identified 38 eligible studies (37 case–control studies and one study with pooled analysis).
3.2. Characteristics of the Included Studies
Table 3 shows the number of studies included in each extracted exposure. The detailed characteristics (author, study period and location, year of publication, number and age of participants, study design, control source and extracted exposure variables) of each included study are presented in Table 4.
3.3. Quality Assessment
The quality assessment [13] of the included studies is presented in Supplementary Table S3. A common weakness detected in some studies was the lack of adjustment for possible confounding factors such as parental smoking status, maternal alcohol consumption and perinatal factors (e.g., birth weight, type of delivery). Supplementary Table S4 contains a list of the excluded studies. The confounding factors used in the analysis for each included study are presented in Supplementary Tables S5–S7.
3.4. Expousures and Detected Association with the Outcomes
3.4.1. Breastfeeding
Twenty-five case–control studies studied the link between breastfeeding and risk of AL in children [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Results are reported in Supplementary Table S5.
From eleven case–control studies [14,16,19,22,24,28,30,31,33,35,37] that studied breastfeeding and AL, only five [16,19,22,31,37] reported a statistically significant protective effect of breastfeeding (most of them with never breastfed as reference). An increased risk for AL among not breastfed children (compared to children breastfed for >13 months) was obtained in another study [22].
Eighteen case control studies evaluated the association of breastfeeding with ALL [15,17,18,20,21,23,24,25,26,27,29,31,32,34,35,36,37,38]; of them, only nine [15,17,18,23,25,31,34,36,37] reported a statistically significative protective effect (most of them when compared with never breastfed as reference). The strongest association was found for 7–12 months of breastfeeding duration [36].
Nine case control studies [15,21,24,27,31,32,35,36,37] appraised the association of breastfeeding with AML. In one of them, a breastfeeding period of 7–9 months found to reduce the risk of AML [36], and other study described an increased risk of AML in children breastfed less than 6 months [15].
A meta-analysis of nine case–control studies comparing children breastfed (any duration) versus children not breastfed was performed (6400 children with ALL, 17,642 children without ALL). The age of participants ranged between less than 14 years old and less than 16 years old [21,23,26,27,31,32,34,36,37]. A decreased risk of ALL was found for breastfed children (OR = 0.85, 95% CI: 0.76–0.94, I2 = 47%) (Figure 2), and for AML (OR = 0.83, 95% CI: 0.69–0.99, I2 = 0%) (Figure 3. All the results are included in Table 5 and Supplementary Figures S1–S6.
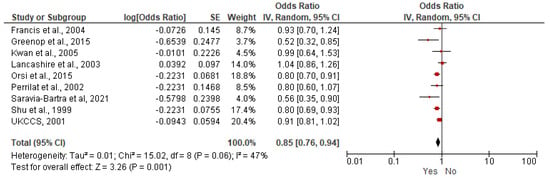
Figure 2.
Random-effects model examining the association between breastfeeding (Yes versus No) and risk of childhood ALL [21,23,26,27,31,32,34,36,37]. Note: the individual estimate (OR) from the studies is represented by the red box, and the black diamond represent the estimate of the meta-analysis.
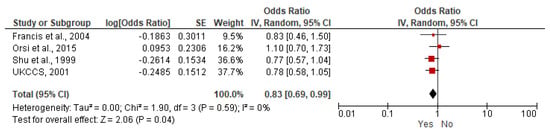
Figure 3.
Random-effects model examining the association between breastfeeding (Yes versus No) and risk of childhood AML [21,31,36,37]. Note: the individual estimate (OR) from the studies is represented by the red box, and the black diamond represent the estimate of the meta-analysis. For each factor included, quantitative analysis was performed with at least two studies, results from studies limited to infants, univariate analysis without adjustment and overlapping data were excluded.
3.4.2. Early Life Diet
Six case control studies explored the association between children’s early diet and the risk of AL [16,23,39,40,41,42]. Results are reported in Supplementary Table S6.
Three case control studies [16,40,41] investigated the association between early diet (during the first 2 years of life) and the risk of childhood AL. One of them stated a lowered risk of AL with regular consumption of oranges/bananas and orange juice [40]. Another study reported a lower risk of AL with the frequent (weekly) consumption of bean-curd and vegetables, and the risk increased with frequent consumption of cured meat/fish [41]. Iron supplementation was shown beneficial in one [16]. This study also assessed the impact of early diet on a combined risk of developing AL and lymphoma, and found an increased risk when processed food was consumed (processed meats, salty snacks, and candy ≥ 3 times/week), and a reduced risk when diet included vegetables at least three times per week.
Among the three case control studies that investigated the association between food consumption and ALL [23,39,42], one reported an increased risk for added lipids (butter, margarine, seed and olive oils, and olives), and no significant association with consumption of fruits [39]. Another study found no association with food or supplements with the risk of ALL [23].
Our meta-analysis, performed on two studies [23,39], evaluated the association between the consumption of fruits and vegetables and the risk of ALL: none was found (Table 5 and Supplementary Figures S7 and S8).
3.4.3. Neonatal Vitamin K Administration
Eight case–control studies [43,44,45,46,47,48,49,51] and one study with pooled analysis [50] investigated the association between neonatal intramuscular application of vitamin K and risk of AL in children (Supplementary Table S7).
The study with the pooled analysis [50] included some of the revised case control studies [43,46,47,48,49,51]. In two of these revised studies, vitamin K administration was found to increase the risk of AL [46] and ALL [48]. However, the pooled study did not find any statistically significative associations between the intramuscular administration of vitamin K and leukemia.
In a case–control study assessing intramuscular and oral administration of vitamin K, no association with the risk of ALL or AL was found in children aged one year or older [45]. Non-significant results were reported in another case–control study conducted in Scotland [44]. In contrast with these findings, intramuscular vitamin K in children younger than eight years of age was found to lower the risk of ALL, although this effect was no longer significant after adjustments [55].
Our meta-analysis, performed on two studies [45,50], evaluated the association between the neonatal administration of vitamin K and the risk of ALL: none was found (Table 5 and Supplementary Figure S9).
4. Discussion
With this systematic review and meta-analysis, we aimed to integrate the available scientific research on nutritional factors in early life (birth to age 3) and the impact of vitamin K administration that could be linked to the development of childhood acute leukemia.
Our systematic review and meta-analyses indicate a protective effect of breastfeeding against the risk of ALL (the minimum duration of breastfeeding in the included studies was less than 1 month or less than 3 months). All case–control studies that have investigated the impact of breastfeeding have found a decreased risk for breastfed children. In line with our findings, a meta-analysis of 33 studies published in 2021 compared systematic breastfeeding with ever-breastfed versus non- or occasional breastfeeding (OR = 0.77, 95% CI: 0.65–0.91) and longer breastfeeding with shorter breastfeeding versus shortest and longest duration (OR = 0.77, 95% CI: 0.63–0.94), showing a protective effect [56]. Similar results were obtained in a previous meta-analysis of 17 studies published in 2015, which showed that, compared with no breastfeeding or shorter breastfeeding, any breastfeeding for 6 months or more was associated with a 20% lower risk of childhood leukemia (OR = 0.80, 95% CI: 0.72–0.90). In the same study, the meta-analysis of 15 studies indicated that always having been breastfed versus never having been breastfed was associated with an 119% lower risk of childhood leukemia (OR = 0.91, 95% CI: 0.80–1.04) [57]. Another meta-analysis showed that breastfeeding for at least one month could protect against childhood cancer (OR = 0.75, 95% CI: 0.63–0.89) [58].
In our meta-analyses, breastfeeding of any duration had a protective effect against ALL (OR = 0.85; 95% CI: 0.76–0.94) and for AML (OR = 0.83; 95% CI: 0.69–0.99). This protective effect of breastfeeding on both ALL and AML risk can be explained by the complex composition of the human milk. Breastfeeding provides nutrients (including antioxidants, fatty acids), maternal antibodies and immunological components (cytokines, immunoglobulins, etc.) and many other components that contribute to the advancement of the immune system and microbiota of the infant [9,59]. Breastfeeding not only contributes to the well-being of the infant but also has benefits for the mother with a protective effect against breast cancer (OR = 0.71, 95% CI: 0.59–0.84) [60]. In a larger context, breastfeeding has many other benefits for child health [61]. Breastfeeding was evaluated in multiple settings (low and high-income countries), some of the studies are recent, and findings are consistent with the protective effect.
Regarding children’s early diet, only a few studies evaluated the potential role of the food and supplements in leukemogenesis. The results from our meta-analysis, and those from a previous one, were not conclusive [62]. As mentioned in a previous publication, evaluating dietary intake is challenging, and some of the studies reported their findings by specific items instead of food groups which difficult comparisons. Most of the publications are case–control studies, and with this, design risk factors are evaluated retrospectively, which might be affected by bias recall. Although, the potential link of the maternal diet during gravidity on the risk of ALL among their offspring was demonstrated [11].
Supplementing neonates with vitamin K is a common practice that was introduced to prevent the hemorrhagic disease of the newborn (HDN). This supplementation may be administrated as oral or intramuscular to all the newborns, or only to those at higher risk of HDN. During the nineties, many studies consider the potential association between the intramuscular administration of vitamin K and the risk of AL [43,44,46,47,48,49,51]. The evaluation of the effect of vitamin K in childhood AL is difficult since almost all newborns in western countries over the past 10 years received this prophylaxis at birth. Our review does not show convincing evidence on the link between neonatal intramuscular application of vitamin K and leukemia risk in children.
While studies of breastfeeding could partly rely on registered data (more reliable), all dietary information were collected through interviews and their integrity could not be evaluated. Therefore, the protective effect of breastfeeding is more convincing than the identified effects of diet.
We would like to highlight the need for studies that explore early life dietary factors and the risk of developing childhood AL in a comprehensive way (standardized questionnaires), including general information on the main food groups and supplements, but also other approaches that might be considered (e.g., degree of food processing). Micronutrient supplementation, including neonatal administration of vitamin K, needs to be evaluated in a more comprehensive way, including dose, dilutants, etc., to provide the best evidence on their potential role in developing leukemia. It should be noted that AL is a rare disease, therefore most of the results come from retrospective case–control studies often with a restricted number of cases. This is an important consideration when interpreting results. It is also important to mention the proposed mechanism for leukemogenesis, which involves early pre-leukemic genetic changes in utero, followed by triggers (environmental exposures, infections, etc.) in early life that induce secondary genetic changes and an immune response [9].
5. Conclusions
After reviewing the available evidence that evaluates early life nutritional factors and the risk of childhood acute leukemia, the most abundant and conclusive evidence points to the protective role of breastfeeding. However, the reviewed studies did not provide sufficient evidence on the role of early diet in the development of childhood acute leukemia. Although further research is needed to develop and test cost-effective, evidence-based prevention strategies to combat acute leukemia in children, breastfeeding can be recommended as a factor contributing to prevention of childhood leukemia.
Supplementary Materials
The following supporting information can be downloaded at:https://www.mdpi.com/article/10.3390/nu15173775/s1, Supplementary Table S1: Search strings used in literature searches; Supplementary Table S2: PRISMA 2020 checklist Early life nutrition factors and risk of Acute Leukemia in children; Supplementary Table S3: Quality assessment of the studies included in the systematic review evaluated by the checklist proposed by Fowkes et al. [10]. Supplementary Table S4: List of excluded articles, specifying the reason for exclusion. Supplementary Table S5: Association between breastfeeding and the risk of acute leukemia in children. Supplementary Table S6: Association between early life diet and the risk of acute leukemia in children. Supplementary Table S7: Association between neonatal vitamin K administration and the risk of acute leukemia in children. Supplementary Figure S1: Random-effects model examining the association between breastfeeding (≤6 months) and risk of childhood ALL. Supplementary Figure S2: Random-effects model examining the association between breastfeeding (≤6 months) and risk of childhood AML. Supplementary Figure S3: Random-effects model examining the association between breastfeeding with no vs. yes response and risk of childhood ALL. Supplementary Figure S4: Random-effects model examining the association between breastfeeding with no vs. yes response and risk of childhood AML. Supplementary Figure S5: Random-effects model examining the association between breastfeeding (<3 months) and risk of childhood ALL. Supplementary Figure S6: Random-effects model examining the association between breastfeeding (<1 month) and risk of childhood ALL. Supplementary Figure S7. Meta-analysis of the association between the foods (fruits) and acute lymphoblastic leukemia. Supplementary Figure S8. Meta-analysis of the association between the foods (vegetables) and acute lymphoblastic leukemia. Supplementary Figure S9. Meta-analysis between intramuscular vitamin K given in neonatal period and ALL.
Author Contributions
Conceptualization, I.H. and K.V.H.; search, data collection and quality assessment, S.P., C.C.B.A., I.I., A.K.K. and J.B.-L.; writing—original draft preparation, S.P. and C.C.B.A.; writing—review and editing, E.S.-F., C.S., M.J.G., E.J.L., R.D.B., I.I., Z.K., A.K.K. and J.B.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Hayley Wilson for the contribution in the elaboration of summary tables that were used for performing the meta-analyses and Anastasia Dolya for help with the management of references.
Conflicts of Interest
The authors declare they have no financial conflict of interest to disclose.
IARC Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Belson, M.; Kingsley, B.; Holmes, A. Risk factors for acute leukemia in children: A review. Environ. Health Perspect. 2007, 115, 138–145. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, I. Cancer Epidemiology: Principles and Methods; IARC Press: Lyon, France, 1999; pp. 1–34. [Google Scholar]
- Wiemels, J. Perspectives on the causes of childhood leukemia. Chem. Biol. Interact. 2012, 196, 59–67. [Google Scholar] [CrossRef]
- Tebbi, C.K. Etiology of Acute Leukemia. A review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Coebergh, J.W.; Reedijk, A.M.; de Vries, E.; Martos, C.; Jakab, Z.; Steliarova-Foucher, E.; Kamps, W.A. Leukaemia incidence and survival in children and adolescents in Europe during 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 2006, 42, 2019–2036. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.T.; Erdmann, F.; Newton, R.; Steliarova-Foucher, E.; Schuz, J.; Roman, E. Childhood cancer: Estimating regional and global incidence. Cancer Epidemiol. 2021, 71, 101662. [Google Scholar] [CrossRef]
- Atun, R.; Bhakta, N.; Denburg, A.; Frazier, A.L.; Friedrich, P.; Gupta, S.; Lam, C.G.; Ward, Z.J.; Yeh, J.M.; Allemani, C.; et al. Sustainable care for children with cancer: A Lancet Oncology Commission. Lancet Oncol. 2020, 21, e185–e224. [Google Scholar] [CrossRef]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef]
- Kramárová, E.; Stiller, C.A. The international classification of childhood cancer. Int. J. Cancer 1996, 68, 759–765. [Google Scholar] [CrossRef]
- Blanco-Lopez, J.; Iguacel, I.; Pisanu, S.; Almeida, C.C.B.; Steliarova-Foucher, E.; Sierens, C.; Gunter, M.J.; Ladas, E.J.; Barr, R.D.; Van Herck, K.; et al. Role of Maternal Diet in the Risk of Childhood Acute Leukemia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5428. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.; Fulton, P. Critical appraisal of published research: Introductory guidelines. Br. Med. J. 1991, 302, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Abudaowd, O.A.G.; Subki, S.; Alsaaedi, R.; Alsiyoufi, A.; Al-Khotany, B.; Altaifi, R.; Al-Kadi, H. Breast feeding and its association with childhood leukemia: A retrospective casecontrol study among children attending King Abdulaziz University Hospital in Jeddah, Saudi Arabia. Med. Sci. 2021, 25, 2624–2634. [Google Scholar]
- Altinkaynak, S.; Selimoglu, M.A.; Turgut, A.; Kilicaslan, B.; Ertekin, V. Breast-feeding Duration and Childhood Acute Leukemia and Lymphomas in a Sample of Turkish Children. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 568–572. [Google Scholar] [CrossRef]
- Amitay, E.L.; Dubnov Raz, G.; Keinan-Boker, L. Breastfeeding, Other Early Life Exposures and Childhood Leukemia and Lymphoma. Nutr. Cancer 2016, 68, 968–977. [Google Scholar] [CrossRef]
- Bener, A.; Denic, S.; Galadari, S. Longer breast-feeding and protection against childhood leukaemia and lymphomas. Eur. J. Cancer 2001, 37, 234–238. [Google Scholar] [CrossRef]
- Bener, A.; Hoffman, G.; Afifi, Z.; Rasul, K.; Tewfik, I. Does prolongued breastfeeding reduces the risk for childhood leukemia and lymphoma? Minerva Pediatr. 2008, 60, 155–161. [Google Scholar]
- Bonaventure, A.; Goujon-Bellec, S.; Rudant, J.; Orsi, L.; Leverger, G.; Baruchel, A.; Bertrand, Y.; Nelken, B.; Pasquet, M.; Michel, G.; et al. Maternal smoking during pregnancy, genetic polymorphisms of metabolic enzymes, and childhood acute leukemia: The ESCALE study (SFCE). Cancer Causes Control 2012, 23, 329–345. [Google Scholar] [CrossRef]
- Davis, M.; Savitz, D.; Graubard, B. Infant Feeding and Childhood Cancer. Lancet 1988, 2, 365–368. [Google Scholar] [CrossRef]
- Francis, S.S.; Selvin, S.; Metayer, C.; Wallace, A.D.; Crouse, V.; Moore, T.B.; Wiemels, J.L.; Buffler, P.A. Mode of delivery and risk of childhood leukemia. Cancer Epidemiol. Biomark. Prev. 2014, 23, 876–881. [Google Scholar] [CrossRef][Green Version]
- Gao, Z.; Wang, R.; Qin, Z.X.; Dong, A.; Liu, C.B. Protective effect of breastfeeding against childhood leukemia in Zhejiang Province, P.R. China: A retrospective case-control study. Libyan J. Med. 2018, 13, 1508273. [Google Scholar] [CrossRef]
- Greenop, K.R.; Bailey, H.D.; Miller, M.; Scott, R.J.; Attia, J.; Ashton, L.J.; Downie, P.; Armstrong, B.K.; Milne, E. Breastfeeding and nutrition to 2 years of age and risk of childhood acute lymphoblastic leukemia and brain tumors. Nutr. Cancer 2015, 67, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.D.A. Breast-feeding duration and the risk of malignant diseases in childhood in Sweden. Eur. J. Clin. Nutr. 2001, 55, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Infante-Rivard, C.; Fortier, I.; Olson, E. Markers of infection, breast-feeding and childhood acute lymphoblastic leukaemia. Br. J. Cancer 2000, 83, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Buffler, P.A.; Wiemels, J.L.; Metayer, C.; Selvin, S.; Ducore, J.M.; Block, G. Breastfeeding patterns and risk of childhood acute lymphoblastic leukaemia. Br. J. Cancer 2005, 93, 379–384. [Google Scholar] [CrossRef][Green Version]
- Lancashire, R.J.; Sorahan, T. Breastfeeding and childhood cancer risks: OSCC data. Br. J. Cancer 2003, 88, 1035–1037. [Google Scholar] [CrossRef]
- Lingappa, A.L.; Kalapalar, S.R.; Rudrappa, S.R.; Manjunatha, S.N. Breastfeeding and Its Associated Risk in Children with Acute Leukemia: A Retrospective Study. Indian J. Med. Paediatr. Oncol. 2018, 39, 312–315. [Google Scholar] [CrossRef]
- MacArthur, A.C.; McBride, M.L.; Spinelli, J.J.; Tamaro, S.; Gallagher, R.P.; Theriault, G.P. Risk of childhood leukemia associated with vaccination, infection, and medication use in childhood: The Cross-Canada Childhood Leukemia Study. Am. J. Epidemiol. 2008, 167, 598–606. [Google Scholar] [CrossRef]
- Mohammadi, M.; Naderi, M.; Ansari Moghaddam, A.; Mahdavifar, N.; Mohammadian, M. Investigation of the Relationship between Breastfeeding and Leukemia in Children. Iran. J. Pediatr. Hematol. Oncol. 2018, 8, 97–104. [Google Scholar]
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control 2015, 26, 1003–1017. [Google Scholar] [CrossRef]
- Perrillat, F.; Clavel, J.; Jaussent, I.; Baruchel, A.; Leverger, G.; Nelken, B.; Philippe, N.; Schaison, G.; Sommelet, D.; Vilmer, E.; et al. Breast-feeding, fetal loss and childhood acute leukaemia. Eur. J. Pediatr. 2002, 161, 235–237. [Google Scholar] [CrossRef]
- Petridou, E.; Trichopoulos, D.; Kalapothaki, V.; Pourtsidis, A.; Kogevinas, M.; Kalmanti, M.; Koliouskas, D.; Kosmidis, H.; Panagiotou, J.P.; Piperopoulou, F.; et al. The risk profile of childhood leukaemia in Greece: A nationwide case-control study. Br. J. Cancer 1997, 76, 1241–1247. [Google Scholar] [CrossRef]
- Saravia-Bartra, M.M.; Cazorla, P.; Ignacio-Cconchoy, F.L.; Cazorla-Saravia, P. Exclusive breastfeeding as a protective factor of acute lymphoblastic leukemia. Andes Pedriatica 2021, 92, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Clemens, J.; Zheng, W.; Ying, D.; Ji, B.; Jin, F. Infant breastfeeding and the risk of childhood lymphoma and leukaemia. Int. J. Epidemiol. 1995, 24, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Linet, M.S.; Steinbuch, M.; Wen, W.Q.; Buckley, J.D.; Neglia, J.P.; Potter, J.D.; Reaman, G.H.; Robison, L.L. Breast-feeding and risk of childhood acute leukemia. J. Natl. Cancer Inst. 1999, 91, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Investigators, U.C.C.S. Breastfeeding and childhood cancer. Br. J. Cancer 2001, 85, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, C.M.; van Steensel-Moll, H.A.; van der Does-vd Berg, A.; van Wering, E.R.; van Zanen, G.E.; Valkenburg, H.A.; Rammeloo, J.A. Infant feeding and childhood cancer. Lancet 1988, 2, 796–797. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diamantaras, A.A.; Dessypris, N.; Sergentanis, T.N.; Ntouvelis, E.; Athanasiadou-Piperopoulou, F.; Baka, M.; Fragandrea, I.; Moschovi, M.; Polychronopoulou, S.; Stiakaki, E.; et al. Nutrition in early life and risk of childhood leukemia: A case-control study in Greece. Cancer Causes Control 2013, 24, 117–124. [Google Scholar] [CrossRef]
- Kwan, M.L.; Block, G.; Selvin, S.; Month, S.; Buffler, P.A. Food consumption by children and the risk of childhood acute leukemia. Am. J. Epidemiol. 2004, 160, 1098–1107. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hsu, Y.H.; Wu, M.T.; Pan, P.C.; Ho, C.K.; Su, L.; Xu, X.; Li, Y.; Christiani, D.C. Cured meat, vegetables, and bean-curd foods in relation to childhood acute leukemia risk: A population based case-control study. BMC Cancer 2009, 9, 15. [Google Scholar] [CrossRef]
- Sarasua, S.; Savitz, D.A. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States). Cancer Causes Control 1994, 5, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ansell, P.; Bull, D.; Roman, E. Childhood leukaemia and intramuscular vitamin K: Findings from a case-control study. BMJ 1996, 313, 204–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekelund, H.; Finnstrom, O.; Gunnrskog, J.; Kallen, B.; Larsson, Y. Administration of vitamin K to newborn infants and childhood cancer. BMJ 1993, 307, 89–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fear, N.T.; Roman, E.; Ansell, P.; Simpson, J.; Day, N.; Eden, O.B. Vitamin K and childhood cancer: A report from the United Kingdom Childhood Cancer Study. Br. J. Cancer 2003, 89, 1228–1231. [Google Scholar] [CrossRef]
- Golding, J.; Greenwood, R.; Birmingham, K.; Mott, M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ 1992, 305, 341–346. [Google Scholar] [CrossRef] [PubMed]
- McKinney, P.A.; Juszczak, E.; Findlay, E.; Smith, K. Case-control study of childhood leukaemia and cancer in Scotland: Findings for neonatal intramuscular vitamin K. BMJ 1998, 316, 173–177. [Google Scholar] [CrossRef][Green Version]
- Parker, L.; Cole, M.; Craft, A.W.; Hey, E.N. Neonatal vitamin K administration and childhood cancer in the north of England: Retrospective case-control study. Br. Med. J. 1998, 316, 189–193. [Google Scholar] [CrossRef][Green Version]
- Passmore, S.J.; Daper, G.; Brownbill, P.; Kroll, M. Case-control studies of relation between childhood cancer and neonatal vitamin K administration. BMJ 1998, 316, 178–184. [Google Scholar] [CrossRef][Green Version]
- Roman, E.; Fear, N.T.; Ansell, P.; Bull, D.; Draper, G.; McKinney, P.; Michaelis, J.; Passmore, S.J.; von Kries, R. Vitamin K and childhood cancer: Analysis of individual patient data from six case-control studies. Br. J. Cancer 2002, 86, 63–69. [Google Scholar] [CrossRef]
- von Kries, R.; Gobel, U.; Hachmeister, A.; Kaletsch, U.; Michaelis, J. Vitamin K and childhood cancer: A population based case-control study in Lower Saxony, Germany. BMJ 1996, 313, 199–203. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S.; Cochrane, C. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Chichester, UK, 2008; pp. 245–246. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Review Manager Web (RevMan Web), Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Klebanoff, M.; Read, J.; Mills, J.; Shiono, P. The risk of childhood cancer after neonatal exposure to vitamin K. N. Engl. J. Med. 1993, 329, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Sun, X.; Zhu, L.; Yan, Q.; Zheng, P.; Mao, Y.; Ye, D. Breastfeeding and the risk of childhood cancer: A systematic review and dose-response meta-analysis. BMC Med. 2021, 19, 90. [Google Scholar] [CrossRef]
- Amitay, E.L.; Keinan-Boker, L. Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review. JAMA Pediatr. 2015, 169, e151025. [Google Scholar] [CrossRef]
- Gong, Q.Q.; Quan, D.D.; Guo, C.; Zhang, C.; Zhang, Z.J. Association between maternal breastfeeding and risk of systemic neoplasms of offspring. Ital. J. Pediatr. 2022, 48, 98. [Google Scholar] [CrossRef]
- M’Rabet, L.V.A.; Boehm, G.; Garssen, J. Breast-feeding and its role in early development of the immune system in infants: Consequences for health later in life. J. Nutr. 2008, 138, 1782S–1790S. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Zhong, Y.; Hu, M.; Wu, B. Breastfeeding and Reduced Risk of Breast Cancer: A Systematic Review and Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 8500910. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; United Nations Children’s Fund. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Dessypris, N.; Karalexi, M.A.; Ntouvelis, E.; Diamantaras, A.A.; Papadakis, V.; Baka, M.; Hatzipantelis, E.; Kourti, M.; Moschovi, M.; Polychronopoulou, S.; et al. Association of maternal and index child’s diet with subsequent leukemia risk: A systematic review and meta analysis. Cancer Epidemiol. 2017, 47, 64–75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).