Abstract
Mild traumatic brain injury (mTBI) represents a significant burden for individuals, economies, and healthcare systems worldwide. Recovery protocols focus on medication and physiotherapy-based interventions. Animal studies have shown that antioxidants, branched-chain amino acids and omega-3 fatty acids may improve neurophysiological outcomes after TBI. However, there appears to be a paucity of nutritional interventions in humans with chronic (≥1 month) symptomology post-mTBI. This systematic literature review aimed to consolidate evidence for nutrition and dietary-related interventions in humans with chronic mTBI. The review was registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021277780) and conducted following the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Three reviewers searched five databases (PubMed/MEDLINE, Web of Science, SPORTDiscus, CINAHL Complete and Cochrane), which yielded 6164 studies. Nine studies met the inclusion criteria. The main finding was the lack of interventions conducted to date, and a quality assessment of the included studies was found to be fair to good. Due to heterogeneity, a meta-analysis was not feasible. The six nutrition areas identified (omega-3 fatty acids, melatonin, Enzogenol®, MLC901, ketogenic diet and phytocannabinoids) were safe and well-tolerated. It was found that these nutritional interventions may improve cognitive failures, sleep disturbances, anxiety, physical disability, systolic blood pressure volume and sport concussion assessment tool scores following mTBI. Potential areas of improvement identified for future studies included blinding, reporting compliance, and controlling for confounders. In conclusion, further research of higher quality is needed to investigate the role of nutrition in recovery from mTBI to reduce the burden of chronic outcomes following mTBI.
1. Introduction
Traumatic brain injuries (TBIs) are often referred to as the ‘silent epidemic’. This is because many injuries go unrecognised and are excluded from epidemiological data [1]. These injuries represent a significant global health burden and substantial cost to economies and healthcare systems [2]. It has been estimated that the cost of new TBI incidence is USD 47.9 million in the first year. At the individual level, they can impact the long-term health of the person and cause disability. Epidemiological data estimate the incidence of TBI at 27.08 million cases annually, with age-standardised incidence rates at 369 per 100,000 population [3]. Other statistics suggest that incidence may be as high as 790 cases per 100,000 person-years [4]. However, it is suggested that the true incidence of TBIs may even be higher [5] as underreporting has been suggested at a factor of 6 to 10 times [6].
1.1. Traumatic Brain Injury—Definition and Diagnosing
Traumatic brain injuries can be categorised according to severity: mild, moderate, and severe injury, as defined by the Glasgow Coma Score (GCS) [7]. A mild traumatic brain injury (mTBI) is typically diagnosed with an initial Glasgow Coma Scale (GCS) of 13–15, loss of consciousness for up to 30 min, post-traumatic amnesia for less than 24 h and usually, the absence of positive neuroimaging or skull fractures [8]. The Centre for Disease Control and Prevention defines an mTBI as “a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces secondary to direct or indirect forces to the head” [9]. These physical forces can cause neurological dysfunction and neural cell death and may induce secondary long-lasting injuries [10]. In contrast, moderate and severe injuries are classified with a lower GCS and positive neuroimaging or skull fractures [4,11]. Although mTBIs account for 95% of TBIs and are 18 times more likely to occur, research focuses primarily on the severe category [4,11,12,13].
Often, the terms “mTBI” and “concussion” are used interchangeably, which is incorrect. Concussions are subsets of mTBI, and while all concussions are mTBIs, not all mTBIs are concussions [14]. Although the term concussion has been documented in sports for many years, it has become increasingly synonymous with sports recently. Noteworthy, only 15.5% are sports-related concussions (SRCs) [15]. Sport-related concussion refers to a TBI caused by biochemical forces to the head or surrounding areas experienced during a sporting activity [16]. A common feature of SRC is the rapid onset of neurological impairment, which tends to resolve spontaneously. Some of the acute symptoms are suggestive of a functional disturbance rather than a physical injury, which is reflected in negative neuroimaging [16,17]. The risk of SRC is highest in collision sports. Among the most common of these are American football, rugby, soccer and Australian Rules football [15]. Recent research found that the rate of concussion was 0.06 concussions per 1000 h. Additionally, players were 78 times more likely to sustain one during a match versus training [18].
1.2. Symptoms and Quality of Life Following mTBI
In those with mTBI, approximately three-quarters of people have symptom resolution within 4 weeks of injury. Almost all of these patients recover fully within 2 months [19,20]. However, recovery is not always straightforward. Some individuals can experience persistent symptoms or a collection of these symptoms, known as post-concussion syndrome (PCS) [21] or, more recently, as persistent post-concussion symptoms (PPCSs) [22]. Persistent symptoms can manifest as headaches, fatigue, dizziness, motion and light sensitivity, anxiety, depression, memory loss, personality changes and cognitive impairment [23]. Chronic symptoms are not the only aspect of individuals’ lives that may become affected following injury. Individuals’ quality of life (QoL) may be disrupted including relationship breakdown, unemployment, and disconnect from society [24]. Also, there is a growing concern that chronic and repeated mTBIs are associated with negative long-term outcomes [25,26].
1.3. Is There a Role for Nutrition in Recovery?
While it is well known that diet plays an essential role in maintaining neurological function, little research has focused on the role of nutrition in mTBI recovery and the alleviation of PCS symptoms [27,28]. Most research advises rest and medical and physiotherapy-led interventions in acute and chronic recovery [16,29]. Notably, nutrition was not included in the 2017 concussion consensus statement [16]. This systematic literature review (SLR) is based on the recent review by McGeown et al. [30], which detailed some promising results for antioxidants, creatine, omega-3 fatty acids (n-3FAs), multi-supplements, and the ketogenic diet in animal models following mTBI injury [31]. The aim of this review was to systematically consolidate evidence regarding the role of nutritional strategies in the chronic phase of mTBI in adult humans. Specifically, our question to be answered regarded adults with PCS/PPCS—do nutrition-related interventions, compared to placebo-control, relevant medication control or no comparator, improve recovery?
2. Materials and Methods
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) before commencing the search (registration number CRD42021277780). The review was conducted following the Preferred Reporting for Systematic Review and Meta-Analyses (PRISMA) guidelines (see Appendix A) [32]. As outlined in Table 1, the review methodology was developed as per the population, intervention, comparator and outcome (PICO) model.

Table 1.
PICO model and search strategy in accordance with PRISMA 2020 guidelines, where * indicates any potential ending to the phrase will also be searched [32].
2.1. Study Search and Selection Criteria
The five electronic databases that were chosen for this review were PubMed/MEDLINE, Web of Science, SPORTDiscus, CINAHL Complete and Cochrane Reviews. The review was limited to studies published from January 1998 to 2023 to emulate the work of McGeown et al. [30]. Also, it was reasoned that research on mTBI is constantly evolving, and most of the relevant research was published within the last 25 years. Both researchers (SN and LR) agreed on search terms for the review (Table 1). Group 1 and 2 terms were based on the terms used in McGeown et al. [30], with the addition of recovery terms (Group 3) selected from medical subject headings.
2.2. Study Selection
Eligible studies for this review were (1) peer-reviewed Randomised Controlled Trials (RCTs) and observational studies; (2) studies on adults (≥18 years), (3) studies in the chronic phase of mTBI, concussion or SRC, where chronic was defined as symptoms lasting at least 1 month, (4) studies that included a measure of recovery, (5) studies that included a nutrition-related or dietary-related intervention(s) administered in the chronic phase of injury and (6) studies in English. The exclusion criteria included (1) animal or cell studies, (2) studies exclusively in children and/or adolescents (aged ≤ 17 years), (3) studies exclusively on moderate and/or severe TBI, (4) studies on acute concussion (symptoms lasting less than 1 month), (5) non-nutrition or dietary-related intervention(s), (6) dietary-interventions with parenteral and/or enteral nutrition and (7) student theses, case reports, conference abstracts, limited review book chapters. The study designs that were eligible for inclusion were observational, descriptive and qualitative interventions, such as randomised trials, non-randomised trials, case-control studies, non-case-control studies, cohort studies and pilot studies. If applicable, SLRs were used to hand-search reference lists. Narrative reviews were excluded.
2.3. Data Extraction
The initial search and extraction were conducted in 2020. The search protocol was re-run and updated in 2023. EndNote V20 was used to collate the search results into a universal database. Two researchers (SN and LR) agreed on the exclusion criteria. Studies were excluded and coded accordingly if they were not a population, intervention, outcome, study or language of interest. Any queries or disagreements regarding the selected studies were discussed and, if required, a third reviewer contributed to the final decision. After screening based on titles and abstracts, full-text studies were then reviewed and assessed for inclusion. All studies that met the inclusion criteria were hand-searched to identify studies that may have been missed in the original search. The extracted data were then defined by the following headings: author, country of study, study design, participant characteristics, participant baseline and intervention data, intervention details, measures assessed and outcomes (see Table 2). The level of evidence was assessed based on the National Health and Medical Research Council guidelines [33].

Table 2.
Characteristics of the Included Studies.
2.4. Quality Assessment
The quality of the included studies was assessed using a modified Black and Downs quality rating tool [43]. This tool has a checklist of 27 questions with “yes”, “no”, “partially” and “unable to determine” answers. The questions are organised into 5 topics: study quality (questions 1–10), external validity (questions 11–13), internal validity and bias (questions 14–20), internal validity, confounding and selection bias (questions 21–26) and power of the study (question 27). In this modified version of the quality tool, question 27 was adapted so that the studies were rated on whether they had sufficient power to detect clinically important effects. If a study had sufficient power, it was awarded 1 point and if otherwise, no points were awarded. Accordingly, the maximum number of points to be awarded was 28 instead of 32. Table 3 details the classification list as seen in [44]. This modified version has been used in previous SLRs [44,45,46,47].

Table 3.
Modified Black and Downs Quality Classification [43].
3. Synthesis of Results
Due to the variety of nutritional therapies, participant characteristics and recovery outcomes across the studies, it was not possible to combine the quantitative data to conduct a meta-analysis. Thus, the findings are presented in narrative form. Table 2 details the study and participant characteristics. Table 4 outlines the main findings, and Table 5 summarises these findings for use by practitioners.

Table 4.
Summary of Outcomes Measured and Results.

Table 5.
Summary of main outcomes.
3.1. Article Selection and Quality Assessment
Figure 1 illustrates the PRISMA flow diagram. The initial search identified 6164 articles using database searches. Following the title and abstract screening, 91 entire papers were retrieved and assessed for inclusion, with 9 being eligible for inclusion [34,35,36,37,38,40,48]. Walton et al. [41] was excluded in the final review stage because it was unclear whether the participants (ex-National Football League players with a history of SRC) had chronic symptomology. Another paper [49] was excluded because it was unclear if participants had chronic symptoms. The participants had to make contact within 10 days of initial injury, and the intervention lasted 3 months. The majority of included studies were conducted after 2011 [34,35,37,38,40,41,48]. Of the nine studies, five were randomised-controlled trials (RCTs) [36,37,38,48]. Five papers had level II evidence [36,37,38,39,41,48,49] and three papers had level III evidence [34,35,40].
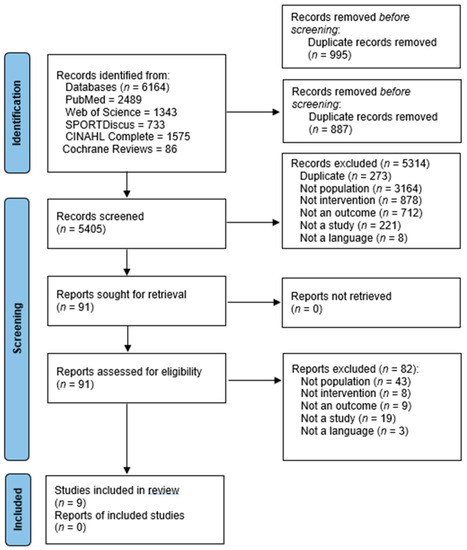
Figure 1.
PRISMA flow diagram showing the included studies [32].
3.2. Details of the Included Studies and Participants
The causes of mTBI in the studies analysed included road traffic accidents, SRCs, workplace accidents, falls and assaults. Two studies consisted entirely of a sporting population [34,39]. Theadom et al. [38] included six participants, from a total of 60, that had a sports-related injury. The study by Amen et al. [34] did not specify the severity of TBI. Both researchers from our group agreed to classify these as SRCs because the participants were retired NFL players who had experienced brain damage and/or cognitive impairment from numerous TBIs sustained while playing football. Moreover, most studies included participants with a history of mTBI or concussion exclusively. Some included moderate and severe TBIs in the overall cohort studied [36,37,48]. One study included post-menopausal women who were medically diagnosed by their physician as having PCS. Though the timeframe between experiencing a concussion and when the investigation took place was not directly stated in the paper, upon contacting the authors, it became clear that this study was still eligible for inclusion in this SLR [42]. Regarding the length of time since injury, the inclusion criteria ranged from at least one month to three years post-injury.
3.3. Participant Characteristics
Participants in RCTs ranged from 7 to 78, with an average of 47. In this review, commonly reported chronic symptoms were cognitive difficulties, functional disabilities, mood disorders and sleep disturbances. Two of the studies included male-only participants [34,36]. Grima et al. [37] included 67% male participants. Three studies included a relatively even gender split: Theadom et al. [38] had 43% male participants, Walter et al. [39] had 48%, and Theadom et al. [48] had 50%. The remaining studies by Fotuhi et al. [35] and Rippee et al. [40] had a majority of female subjects, with 35% male and 14% male participants, respectively. One study recruited all female subjects [42]. As the inclusion criteria were specific to adults only, all studies that included paediatric-only populations, classified as those less than 18 years old, were excluded. Two studies had a mixture of paediatric and adult populations [35,36].
3.4. Length of Follow-Up
The studies consisted of a variety of follow-up intervals. In two studies, the follow-up was 12 weeks [35,40]. Two studies, which investigated the role of melatonin, had a 10-week follow-up interval [36,37]. Another study had 4-month interval [38]. The remaining studies had 6-week [39], 6 month [48], 2–12 month [34] and 10–28 day [42] follow-up intervals.
As per Table 4, there were a variety of outcomes measured such as cognition, memory, attention, mood, motivation, sleep quality, QoL, balance and sport concussion assessment tool (SCAT) scores. Cognition and everyday memory were measured using the Microcog Assessment of Cognitive Functioning [34], Cognitive Failures Questionnaire [38,48], Central Nervous System Vital Signs [35,48] and Impact of Event Scale—Revised tool [40]. Other tests used to assess working memory were the Wechsler Adult Intelligence Scale and California Verbal Learning Test [38]. QoL was assessed using an adapted version of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) tool in Grima et al. [37]. In Theadom et al. [48], the “QoL after Brain Injury” tool was used to assess self-autonomy, cognition and physical domains of QoL. Mood disorders were measured using a range of questionnaires. The most frequently used tool to assess anxiety and depression was the Hospital Anxiety and Depression [36,37,38,48]. Other tools used were the General Anxiety Disorder and Patient Health Questionnaire and the SCAT tool [40,42].
Post-concussion symptoms were measured using the Rivermead Post-Concussion Symptoms Questionnaire [38,48] and the Post-Concussion Symptom Scale [40] Balance was assessed using a modified Balance Error Scoring System [40]. To assess sleep quality, the Epworth Sleepiness Scale was used in two studies to measure daytime sleepiness [35,37]. Sleep diaries that monitored sleep onset latency, duration, sleep quality and daytime alertness were kept in Kemp et al. [36]. The results were computed into averages for analysis. The Pittsburgh Sleep Quality Index was used to assess sleep onset latency and quality [37]. The Fatigue Severit Scale, and a modified version of this tool, was used by Grima et al. [37] and Theadom et al. [48], respectively, as an objective measure of fatigue during daily activities. Walter et al. [39] used electroencephalogram and virtual reality devices to assess brain activity during testing and spatial memory, reaction time and attention. One study included in-house assessments that were non-validated and may limit the generalisability of their findings [35]. Fotuhi et al. [35] used two in-house questionnaires that assessed lifestyle factors associated with brain health, neurocognitive and neurobehavioural symptoms associated with PCS/PPCS.
3.5. Details of Interventions and Findings
The most common intervention was dietary supplementation with a nutrient or commercial product. The included nutritional interventions can be divided into six areas: studies involving n-3FAs, melatonin, Enzogenol®, MLC901, dietary manipulation in terms of the ketogenic diet (KD) and CBD or a CBD:THC formula.
3.5.1. Studies Involving Omega-3 Fatty Acids
Two studies used n-3FA supplements in different dosages for chronic [34,35]. Those in Amen et al. [34] were given a fish oil supplement that contained 1720 mg eicosapentaenoic acid (EPA) and 1160 mg docosahexaenoic acid (DHA), a multiple vitamin and a “brain enhancement supplement” daily. The doses of the two latter products were not reported and compliance data were not available. Also, the participants received education regarding brain health advice such as smoking cessation, exercise and substance abuse. There was an improvement in cognition and brain perfusion, self-reported mood, motivation, sleep and memory after intervention. However, it was not possible to ascertain whether these findings were attributable to the n-3FAs, the other supplements or the synergistic relationship between all three products and the education element. As part of the multifaceted CRP analysed by Fotuhi et al. [35], the participants consumed n-3FA supplements containing 1000–1500 mg of EPA and DHA daily and followed a Mediterranean-style diet. Similarly, compliance data were not reported. Additionally, the participants had access to a “brain coach” for advice regarding exercise, stress relief and education. Notable results were improvements in cognition, attention, memory, executive functioning and reaction time.
3.5.2. Melatonin
Melatonin was included as a nutrition-related intervention because it is often referred to as a dietary supplement and is sold over the counter and in health food shops internationally. It was used in two studies to investigate its potential to improve disturbances post-TBI [36,37]. Notably, both studies included a spectrum of TBI severities and had two participants with mTBI. Each used a randomised, double-blind, controlled and cross-over design and chose the same follow-up period (10 weeks). Different doses of melatonin were used: 5 mg in Kemp et al. [36] versus 2 mg in Grima et al. [37]. The studies reported a reduction in anxiety; however, this was not significant in Kemp et al. [36]. Noteworthy, there were seven participants in total in this study, and no power calculation was provided. Also, melatonin was compared to amitriptyline, a known antidepressant, which may have reduced the study’s ability to produce significant findings. Grima et al. [37] found that melatonin was associated with a significant reduction in self-reported anxiety, p = 0.006. No significant difference was found in either study for depression. Grima et al. [37] reported a moderate and significant reduction in PSQI, which signified improved sleep quality. Interestingly, no significant association between TBI severity and treatment effect for sleep onset latency or PSQI were found. The use of actigraphy technology reported a significant increase in sleep efficiency. In contrast, no improvement in sleep quality, duration, latency or daytime alertness was reported after melatonin use [36].
3.5.3. Enzogenol®
Enzogenol® is a supplement associated with antioxidant and anti-inflammatory properties. Two RCTs used 1000 mg daily [38,39]. Theadom et al. [38] observed that self-reported cognitive shortcomings improved after supplementation, which was indicative of better daily cognition. However, it did not improve episodic memory. Its treatment effects stabilised after 11 weeks, and improvements in anxiety and depression levels were found after the intervention. This was the only study in this review to conduct a sensitivity analysis, and the results did not significantly change after a sensitivity analysis was conducted. Walter et al. [39] reported that Enzogenol® improved self-reported sleep disturbances and mental fatigue. The supplement was generally well tolerated, except for some reports of blurred vision, sleep disturbances and headaches.
3.5.4. The Ketogenic Diet (KD)
One study investigated the role of the KD in treating symptoms of chronic concussion [40]. The participants were instructed to consume a diet of 70–75% energy from fat, 20–25% energy from protein and 5–10% energy from carbohydrates. This study found that visual memory and symptoms of PCS/PPCS may benefit from 2 months of consuming this high-fat, low-carbohydrate diet. As this was a feasibility study, it was cautioned that these findings may have been coincidental and limited by the low number of participants (n = 11) who completed the trial. No significant changes were reported for depression, anxiety or postural stability. The KD required continuous monitoring and input from the researchers and a dietitian, which may limit feasibility and increase cost in a real-life setting.
3.5.5. MLC901 Capsules
MLC901 capsules, which contain herbal components, such as radix astragali, prunus persica and radix polygalae, were used in Theadom et al. [48]. These are commonly used in Chinese medicine and have antioxidant properties. Adherence to the supplement remained over 85% at 6 months. Those who were randomised to the MLC901 group (n = 36) reported significant improvements in executive functioning and complex attention. These improvements slowed after 6–9 months of ceasing the treatment, which supports an ongoing treatment effect. No other significant differences were found for cognitive functioning, neurobehavioural sequelae of fatigue, mood or QoL. It is unclear if earlier administration of this product would have caused greater treatment effects, as most participants were 5–6 months post-injury. Overall, MLC901 was well-tolerated and safe for those with mild and moderate TBI. Some side-effects reported were headache, sore tongue and itchiness.
3.5.6. Phytocannabinoids
Singh et al. [42] investigated the effects of phytocannabinoids on heart rate variability (HRV) and either low-frequency (LF) or high-frequency (HF) domain blood pressure variability (BPV) in post-menopausal females diagnosed with PCS by their physician. With participants self-administering either full spectrum CBD or a 20:1 CBD:THC formula under the guidance of their physician, the dosing varied widely. From the first to forth follow-up visits, participant 1 took 100 mg, 200 mg, 400 mg and 400 mg CBD, respectively; participant 2 took 100 mg CBD at visit 2 and 200 mg CBD at visits 3–5; participant 3 took 25 mg and 50 mg at visits 2 and 3–4, respectively; and finally, participant 4 took 40:2, 40:2, 20:1 and 24:1.2 mg CBD:THC at visits 2–5, respectively. For all participants, systolic HF BPV increased throughout the duration of the study. HRV did change, but not in the same pattern as seen for BPV. Though the LF/HF ratio decreased throughout each visit for participant 1, the opposite was observed for participants 2, 3 and 4. The authors suggested that this may be related to the increase in HF power for participant 1, while participants 2 and 4 experienced reduced HR power, and participant 3 experienced decreased LF power in comparison to their baseline measurements. Regarding SCAT scores, participants 1, 3 and 4 began with scores of 88, 47 and 8, respectively, while participant 2, who experienced a minor head injury 10 days prior to follow-up visit 3, was unable to quantify her symptoms using a Likert scale. Participant 1′s SCAT score dropped on visit 2 and began to increase from then onwards. Participant 3 and 4 both experienced a drop in SCAT scores from baseline to their final visit from 47 and 8 to 2 and 2, respectively. The authors consider their investigation on the effects of phytocannabinoids on HRV and BPV as the first of its kind to observe these beneficial effects. They concluded that administering phytocannabinoids had a beneficial effect on systolic BPV. However, with all participants administering various amounts, a personalised approach may be required for treatment rather than a blanket recommendation.
3.6. Quality and Risk of Bias Assessment
The results of the modified Black and Downs [43] quality tool are summarised in Table 6 and Figure 2. The average score of the studies was 19. Four of the included studies had above average scores [36,37,38,48]. One study was deemed as “excellent” [37]. Two of the studies that investigated n-3FAs scored ≤14 [34,35]. Most studies were classified as “good” with scores between 20 and 25 [36,38,48]. Notably, questions 11 and 12, which assessed external validity, found that most studies did “not meet” the criteria or were “unable to determine” if the criteria was met. Questions 14 to 26 assessed the risk of bias (RoB). Most studies suitably blinded the participants and researchers (questions 14 and 15). Notably, all studies met the criteria that examined whether the main outcomes measured were accurate and reliable (question 20). Question 25 assessed if an adequate adjustment was made for confounding. It was reported that four studies did not meet this criterion, and one study was unable to be determined. Only one study in this review reported conducting a sensitivity analysis [48].

Table 6.
Black and Downs [43] for the 10 included studies. Filled circles indicate that the criteria were met, empty circles indicate the criteria were not met, diamonds indicate that the criteria were partially met and question marks indicate that was unable to be determined.
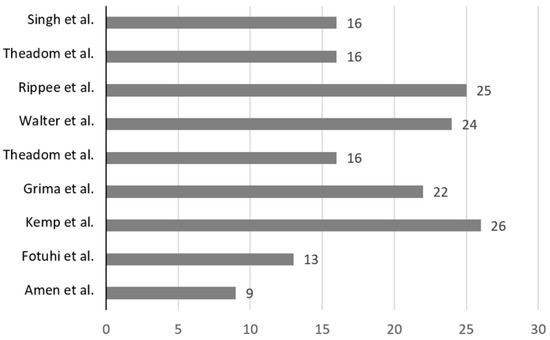
Figure 2.
Visual representation of relative study quality, with study first author and year of publication, with the average value for the 9 included studies (18.6) [34,35,36,37,38,39,40,42,48].
4. Discussion
This review highlighted the dearth of high-quality prospective interventions and clinical trials in the area of nutritional interventions for chronic mTBI in humans. The lack of evidence is surprising considering the promising results using animal models of mTBI [30,31]. In this review, a meta-analysis was not possible because of heterogeneity in treatments, which may have affected the strength of the generated findings.
4.1. Participant and Study Characteristics
It is generally accepted that a gender bias exists in the scientific literature, and this bias extends into the domains of sports science and neuroscience. Epidemiological data suggests that males account for most of the recorded TBI statistics [4,50]. In this review, both genders were represented, despite two studies with 100% male participants and one study with 100% female participants [34,36,42]. Emerging research suggests females tend to suffer worse symptoms and longer recovery time [51]. In this systematic review, studies did not examine if gender differences existed, with some suggesting that low participant numbers did not allow for this [39]. In terms of intervention administration, participants in most studies were fed ad libitum with the exception of Rippee et al. [40]. Although, this is representative of the habitual intake of the target audience of this review, it may have led to high variability in what was consumed outside of the interventions and possible nutrient interactions between other foods eaten. Notably, studies with artificial nutrition were excluded from the search, and although these would have allowed for less diversity in intake, the findings would have been more representative of those with moderate or severe injury.
4.2. The Possible Role of Nutrition in Recovery from mTBI
Despite limitations in the number of studies to date, the data provide novel findings regarding nutritional recovery for humans with chronic symptoms post-mTBI. To date, any knowledge relating to nutritional interventions has mainly been based on animal models. The nutritional areas examined in this review were associated with improved cognitive and memory function [34,35,38,40,48], better overall sleep quality [37,39], improved mood disorders [31,38] and a beneficial effect on systolic BPV and SCAT scores [42]. The common themes in these interventions were that they were feasible, well-tolerated and safe to use. Findings from preclinical trials have reported that n-3FAs in clinically high doses from 10 mg/kg/day to 370 mg/kg/day improved cognitive and neurological performance post-mTBI (pooled ES of 1.52–3.55) [31]. A case study in severe head trauma reported that 19,212 mg of n-3FAs daily with enteral feeding showed no adverse side effects [52]. Although these dosages are in excess of most national guidelines, they have been suggested in ‘real-life’ scenarios, such as in the National Collegiate Athletic Association, where 9000 mg/day of n-3FAs is recommended [31]. Due to differences in the studies investigating n-3FAs in this review, an optimal dosage in humans cannot yet be recommended.
Moreover, interest in antioxidant-based interventions has grown due to promising research suggesting that their administration following mTBI may ameliorate oxidative stress and inflammation and alter the clinical progression of these injuries [53]. This systematic review builds on the findings from a similar review in which antioxidants following TBI were associated with improved recovery and cognitive function, as well as reduced TBI sequelae and decreased mortality risk [54]. In addition, the finding that melatonin is associated with improved post-traumatic sleep quality is positive, as it is estimated that 50% of individuals following TBI experience sleep disturbance [55]. Its use may extend to the treatment of other PCS/PCSS symptoms, such as headaches [56]. In that cohort study, melatonin significantly improved post-concussion headaches in 75% of those taking it. The specific variables of interest included improved alertness, improved sleep duration, improved sleep quality and reduced latency. In addition, two studies [34,35] included in this review may provide evidence to suggest the benefits of combining nutritional interventions with either another nutrition-related supplement or a lifestyle modification. Previous studies have reported added benefits of multiple interventions in treating TBI [57,58]. The latter suggests a beneficial additive effect of combining supplements. In this case, the addition of curcumin to DHA significantly improved learning latency in rats following TBI [58]. The inclusion of concurrent dietary and exercise interventions was previously found to further compound the benefits of nutritional interventions [58,59].
In their 2023 review of nutrition for cognitive health, Puri et al. [60] highlight the evidence supporting various nutritional strategies that have the potential to protect against cognitive decline. Among these, micronutrients like iron and B groups, as well as high-protein and low-fat diets have shown a positive effect on maintaining cognitive health. Regarding the impact of dietary habits on sleep quality, many of the studies in this area are of poor-to-fair quality, making it a challenge to establish a definitive causal relationship. However, current research indicated that diets higher in processed and free-sugar rich foods are associated with a decrease in sleep quality [61]. Termed “nutritional psychiatry”, the concept of mood disorder treatment with nutritional practices is an emerging area of research. Though research is limited, Martins et al. [62], highlight that the findings of observational studies suggest that a high-quality diet, characterized as high in fruits, vegetables, legumes, nuts, whole grains and high-quality protein sources, have a counteractive effect on mood disorders, particularly major depressive disorder and bipolar disorder. A recent review reported more specific findings, noting that although more clinical trials are needed, probiotic supplementation seemed to be a positive add-on therapy for major depressive disorder treatment [63]. Research into specific nutrients and their mechanisms of action in this area is still a relatively new area with much work remaining until definitive recommendations can be established. Future research should focus on single nutrients, particularly protein, probiotics and those that are available in significantly higher levels in whole-food compared with highly processed diets.
The Black and Downs [43] quality tool is designed mainly for RCTs and non-RCTs, which may have led to these designs scoring higher. Most studies were considered fair- to good-quality, with these being primarily RCTs that detailed patient characteristics, study design and outcome measures, as well as reporting exact p-values. Some studies included a flow diagram to depict participant flow and attrition throughout the study, which enabled methodological transparency [37,38,48]. The RoB components in the quality tool identified areas that should be considered in future studies, such as blinding, selecting appropriate statistical tests, reporting compliance, adverse events, external validity and power. Notably, all studies reported a positive effect of the interventions on recovery outcomes, which may indicate possible publication bias.
4.3. Limitations
There were limitations in this review that are worth noting. All the research included in this review recruited adults, and thus, it should not be assumed that these findings may be generalized to children or adolescents. Similarly, the participants were in the chronic phase of mTBI, and additional research is required to investigate whether the same findings would apply during the acute phase of such injury. Heterogeneity may have impacted the strength of evidence. These differences included population variability, different dosage of nutrients, diversity of injury and outcome measurements. In addition to this, the variety of nutritional therapies in a small number of studies may have reduced the potential to find the most effective treatment. Thirdly, the search strategy had limitations because it excluded paediatric and adolescent populations, those with moderate and severe TBI, those in the acute phase of mTBI and those receiving enteral or parenteral nutrition. Thus, the results should not be generalisable to these populations. Moreover, limitations of the individual studies should be considered before interpreting the findings. Only a single study included in this review reported conducting a sensitivity analysis. The studies reported small sample size [35,36,39] and smaller than intended sample size [37], the absence of a control group [35,40] and the non-randomised, open-label design used by Amen et al. [34] as limitations to findings. Selection bias was included as a possible limitation in Theadom et al. [38].
4.4. Practical Applications and Future Directions
It is the role of a multidisciplinary team, in the clinical and high-performance setting, to ensure recovery is optimised for patients and athletes after mTBI or concussion. Table 5 may be useful for practitioners in this field. Due to inherent limitations within the evidence presented, it was not possible to suggest one nutritional intervention as the single most promising remedy for chronic mTBI. The evidence suggests the need for more research on humans to improve the biofidelity of these findings and to translate findings from animal models into real-life settings. These trials should ensure sufficient participant numbers and standardised protocols to provide clinically effective results. These trials should also consider determining the therapeutic dose of such interventions. Also, the possible role of nutrition as a prophylactic treatment for mTBI recovery is an interesting concept with some positive outcomes, such as improving learning disability as noted in animals [64]. Observational studies may provide interesting data in the context of pre-treating these injuries.
5. Conclusions
A main finding of this review is the lack of available evidence that examines the role of nutrition in chronic recovery from mTBI. This review contributes to previous animal research on the potential for nutritional interventions in treating chronic symptomology following brain injury. Dietary adaptations have the potential to play a role in improving cognitive failures, sleep disturbances and other mood disorders following injury as well as individual LF, BPV and SCAT scores. It is promising that these interventions are considered safe and tolerated in most humans. In conclusion, this review presents opportunities for future clinical trials to optimise recovery for those with PCS/PPCS, particularly in the provision of accessible “over-the-counter” solutions in an effort to reduce the global health burden of this condition.
Author Contributions
The key author of this research (S.N.) developed the search strategy and eligibility criteria, which was then discussed and finalised by the research team. The initial database search was conducted in by S.N., who also extracted the resulting relevant data and wrote an original research paper. This search was then re-run in 2023 by the second author, T.R., who updated the original review. Authors E.D., A.J.P. and L.R. reviewed the updated document and provided feedback and guidance for its development. Once the relevant changes were made, the final document was again reviewed by the research team before being deemed acceptable for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Cover page |
| ABSTRACT | |||
| Abstract | 2 | See PRISMA 2020 for Abstract checklist. | P1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | P2–4 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | P4 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for syntheses. | P4–6 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | P4 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | P4–6 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of this review, including how many reviewers screened each record and each report retrieved, whether they worked independently and, if applicable, details of automation tools used in the process. | P6 |
| Data collection process | 9 | Specify the methods used to collect data from the reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators and, if applicable, details of automation tools used in the process. | P6 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses) and, if not, the methods used to decide which results to collect. | P4–6 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | P4–6 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently and, if applicable, details of automation tools used in the process. | P7 |
| Effect measures | 12 | For each outcome, specify the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | P4–6 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics or data conversions. | ||
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | ||
| 13d | Describe any methods used to synthesize the results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity and software package(s) used. | ||
| 13e | Describe any methods used to explore possible causes of heterogeneity among the study results (e.g., subgroup analysis, meta-regression). | ||
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | ||
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | P7–9 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | P8 |
| 16b | Cite studies that might appear to meet the inclusion criteria but were excluded, and explain why they were excluded. | P8 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | P15–22 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | |
| Results of individual studies | 19 | For all outcomes, for each study, present: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was performed, present each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ||
| 20c | Present results for all investigations of possible causes of heterogeneity among study results. | ||
| 20d | Present results for all sensitivity analyses conducted to assess the robustness of the synthesized results. | ||
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | P32–34 |
| 23b | Discuss any limitations of the evidence included in this review. | P34 | |
| 23c | Discuss any limitations of the review processes used. | P34 | |
| 23d | Discuss implications of the results for practice, policy and future research. | P34 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for this review, including register name and registration number or state that this review was not registered. | P4 |
| 24b | Indicate where the review protocol can be accessed or state that a protocol was not prepared. | ||
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | MA | |
| Support | 25 | Describe sources of financial or non-financial support for this review, and the role of the funders or sponsors in this review. | P35 |
| Competing interests | 26 | Declare any competing interests of the review authors. | P35 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; and any other materials used in the review. | Available upon request |
References
- Rusnak, M. Traumatic brain injury: Giving voice to a silent epidemic. Nat. Rev. Neurol. 2013, 9, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Te Ao, B.; Brown, P.; Tobias, M.; Ameratunga, S.; Barker-Collo, S.; Theadom, A.; McPherson, K.; Starkey, N.; Dowell, A.; Jones, K.; et al. Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology 2014, 83, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Murray, C.J.L. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Theadom, A.; Barker-Collo, S.; Starkey, N.J.; McPherson, K.; Kahan, M.; Dowell, A.; Brown, P.; Parag, V.; Kydd, R.; et al. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013, 12, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.; Theadom, A.; Jones, K.; Feigin, V.L.; Kahan, M. Accuracy of an International Classification of Diseases Code Surveillance System in the Identification of Traumatic Brain Injury. Neuroepidemiology 2016, 47, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.F.; Clapperton, A.J.; McCrory, P. Increasing incidence of hospitalisation for sport-related concussion in Victoria, Australia. Med. J. Aust. 2013, 198, 427–430. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Kay, T.; Harrington, E.E.; Adams, R.; Anderson, T.; Berrol, S.; Cicerone, K.; Malec, J. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993, 8, 86–87. [Google Scholar] [CrossRef]
- Dadas, A.; Janigro, D. The role and diagnostic significance of cellular barriers after concussive head trauma. Concussion 2018, 3, CNC53. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Progressive damage after brain and spinal cord injury: Pathomechanisms and treatment strategies. Prog. Brain Res. 2007, 161, 125–141. [Google Scholar] [CrossRef]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.R.; Valadka, A.; Manley, G.T. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cui, D.; Gao, L. Traumatic brain injury: A review of characteristics, molecular basis and management. Front. Biosci. 2016, 21, 890–899. [Google Scholar] [CrossRef]
- Harmon, K.G.; Drezner, J.A.; Gammons, M.; Guskiewicz, K.M.; Halstead, M.; Herring, S.A.; Kutcher, J.S.; Pana, A.; Putukian, M.; Roberts, W.O. American Medical Society for Sports Medicine position statement: Concussion in sport. Br. J. Sports Med. 2013, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Sirois, E. The possible role of hydration in concussions and long-term symptoms of concussion for athletes. A review of the evidence. J. Concussion 2020, 4, 2059700220939404. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus statement on concussion in sport—The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Herring, S.; Kibler, W.B.; Putukian, M.; Solomon, G.S.; Boyajian-O’Neill, L.; Dec, K.L.; Franks, R.R.; Indelicato, P.A.; LaBella, C.R.; Leddy, J.J.; et al. Selected issues in sport-related concussion (SRC|mild traumatic brain injury) for the team physician: A consensus statement. Br. J. Sports Med. 2021, 55, 1251–1261. [Google Scholar] [CrossRef]
- McNeel, C.; Clark, G.M.; Davies, C.B.; Major, B.P.; Lum, J.A.G. Concussion incidence and time-loss in Australian football: A systematic review. J. Sci. Med. Sport 2020, 23, 125–133. [Google Scholar] [CrossRef]
- Eme, R. Neurobehavioral Outcomes of Mild Traumatic Brain Injury: A Mini Review. Brain Sci. 2017, 7, 46. [Google Scholar] [CrossRef]
- Kara, S.; Crosswell, H.; Forch, K.; Cavadino, A.; McGeown, J.; Fulcher, M. Less Than Half of Patients Recover Within 2 Weeks of Injury After a Sports-Related Mild Traumatic Brain Injury: A 2-Year Prospective Study. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2020, 30, 96–101. [Google Scholar] [CrossRef]
- Hiploylee, C.; Dufort, P.A.; Davis, H.S.; Wennberg, R.A.; Tartaglia, M.C.; Mikulis, D.; Hazrati, L.-N.; Tator, C.H. Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers. J. Neurotrauma 2017, 34, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.J.; Tommerdahl, M.; King, D.A. Neurophysiological abnormalities in individuals with persistent post-concussion symptoms. Neuroscience 2019, 408, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Snell, D.; Macleod, A.; Anderson, T. Post-Concussion Syndrome after a Mild Traumatic Brain Injury: A Minefield for Clinical Practice. J. Behav. Brain Sci. 2016, 6, 227–232. [Google Scholar] [CrossRef]
- Reddy, R.P.; Rajeswaran, J.; Devi, B.I.; Kandavel, T. Cascade of Traumatic Brain Injury: A Correlational Study of Cognition, Postconcussion Symptoms, and Quality of Life. Indian J. Psychol. Med. 2017, 39, 32–39. [Google Scholar] [CrossRef]
- Alosco, M.L.; Tripodis, Y.; Baucom, Z.H.; Mez, J.; Stein, T.D.; Martin, B.; Haller, O.; Conneely, S.; McClean, M.; Nosheny, R.; et al. Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 2020, 95, e793–e804. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. The combined effects of exercise and foods in preventing neurological and cognitive disorders. Prev. Med. 2011, 52, S75–S80. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. Treatment of persistent post-concussion syndrome due to mild traumatic brain injury: Current status and future directions. Expert Rev. Neurother. 2016, 16, 875–887. [Google Scholar] [CrossRef]
- Mohamadpour, M.; Whitney, K.; Bergold, P.J. The Importance of Therapeutic Time Window in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2019, 13, 7. [Google Scholar] [CrossRef]
- McGeown, J.P.; Hume, P.A.; Theadom, A.; Quarrie, K.L.; Borotkanics, R. Nutritional interventions to improve neurophysiological impairments following traumatic brain injury: A systematic review. J. Neurosci. Res. 2021, 99, 573–603. [Google Scholar] [CrossRef]
- Patch, C.S.; Hill-Yardin, E.L.; Lewis, M.; Ryan, L.; Daly, E.; Pearce, A.J. The More, the Better: High-Dose Omega-3 Fatty Acids Improve Behavioural and Molecular Outcomes in Preclinical Models in Mild Brain Injury. Curr. Neurol. Neurosci. Rep. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- NHMRC. A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines; National Health and Medical Research Council: Canberra, Australia, 1990.
- Amen, D.G.; Wu, J.C.; Taylor, D.; Willeumier, K. Reversing brain damage in former NFL players: Implications for traumatic brain injury and substance abuse rehabilitation. J. Psychoact. Drugs 2011, 43, 1–5. [Google Scholar] [CrossRef]
- Fotuhi, M.; Dwivedy, P.; Yeom, L.H.; Nadeem, I.; Ebadi, A.Y.; Miles, M.; Tittle, R.K. Retrospective Analysis of a Comprehensive Concussion Recovery Program. J. Rehabil. 2020, 86, 20–31. [Google Scholar]
- Kemp, S.; Biswas, R.; Neumann, V.; Coughlan, A. The value of melatonin for sleep disorders occurring post-head injury: A pilot RCT. Brain Inj. 2004, 18, 911–919. [Google Scholar] [CrossRef]
- Grima, N.A.; Rajaratnam, S.M.W.; Mansfield, D.; Sletten, T.L.; Spitz, G.; Ponsford, J.L. Efficacy of melatonin for sleep disturbance following traumatic brain injury: A randomised controlled trial. BMC Med. 2018, 16, 8. [Google Scholar] [CrossRef]
- Theadom, A.; Mahon, S.; Barker-Collo, S.; McPherson, K.; Rush, E.; Vandal, A.C.; Feigin, V.L. Enzogenol for cognitive functioning in traumatic brain injury: A pilot placebo-controlled RCT. Eur. J. Neurol. 2013, 20, 1135–1144. [Google Scholar] [CrossRef]
- Walter, A.; Finelli, K.; Bai, X.; Arnett, P.; Bream, T.; Seidenberg, P.; Lynch, S.; Johnson, B.; Slobounov, S. Effect of Enzogenol® Supplementation on Cognitive, Executive, and Vestibular/Balance Functioning in Chronic Phase of Concussion. Dev. Neuropsychol. 2017, 42, 93–103. [Google Scholar] [CrossRef]
- Rippee, M.A.; Chen, J.; Taylor, M.K. The Ketogenic Diet in the Treatment of Post-concussion Syndrome-A Feasibility Study. Front. Nutr. 2020, 7, 160. [Google Scholar] [CrossRef]
- Walton, S.R.; Kerr, Z.Y.; Brett, B.L.; Chandran, A.; DeFreese, J.D.; Smith-Ryan, A.E.; Stoner, L.; Echemendia, R.J.; McCrea, M.; Meehan Iii, W.P.; et al. Health-promoting behaviours and concussion history are associated with cognitive function, mood-related symptoms and emotional-behavioural dyscontrol in former NFL players: An NFL-LONG Study. Br. J. Sports Med. 2021, 55, 683–690. [Google Scholar] [CrossRef]
- Singh, J.; Bhagaloo, L.; Pisorski, J.; Neary, P. Effects of phytoccannabinoids on heart rate variability and blood pressure in female post-concussion syndrome patients: Case series. Can. J. Physiol. Pharmacol. 2021, 100, 192–196. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Hooper, P.; Jutai, J.W.; Strong, G.; Russell-Minda, E. Age-related macular degeneration and low-vision rehabilitation: A systematic review. Can. J. Ophthalmol. 2008, 43, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.; Barton, C.J.; Rice, S.; Morrissey, D. Risk factors and successful interventions for cricket-related low back pain: A systematic review. Br. J. Sports Med. 2014, 48, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.A.; Fukuchi, R.K.; Ezzat, A.; Schneider, K.; Schneider, G.; Emery, C.A. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J. Orthop. Sports Phys. Ther. 2013, 43, 515-B19. [Google Scholar] [CrossRef] [PubMed]
- Simic, M.; Hinman, R.S.; Wrigley, T.V.; Bennell, K.L.; Hunt, M.A. Gait modification strategies for altering medial knee joint load: A systematic review. Arthr. Care Res. 2011, 63, 405–426. [Google Scholar] [CrossRef]
- Theadom, A.; Barker-Collo, S.; Jones, K.M.; Parmar, P.; Bhattacharjee, R.; Feigin, V.L. MLC 901 (NeuroAiD II™) for cognition after traumatic brain injury: A pilot randomized clinical trial. Eur. J. Neurol. 2018, 25, 1055-e82. [Google Scholar] [CrossRef]
- Noguchi, H.; Nishi, D.; Matsumura, K.; Hamazaki, K.; Hamazaki, T.; Matsuoka, Y.J. Limited effect of omega-3 fatty acids on the quality of life in survivors of traumatic injury: A randomized, placebo-controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2017, 127, 1–5. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef]
- Merritt, V.C.; Padgett, C.R.; Jak, A.J. A systematic review of sex differences in concussion outcome: What do we know? Clin. Neuropsychol. 2019, 33, 1016–1043. [Google Scholar] [CrossRef]
- Lewis, M.; Ghassemi, P.; Hibbeln, J. Therapeutic use of omega-3 fatty acids in severe head trauma. Am. J. Emerg. Med. 2013, 31, 273.e5–273.e8. [Google Scholar] [CrossRef] [PubMed]
- Bains, M.; Hall, E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 2012, 1822, 675–684. [Google Scholar] [CrossRef]
- Shen, Q.; Hiebert, J.B.; Hartwell, J.; Thimmesch, A.R.; Pierce, J.D. Systematic Review of Traumatic Brain Injury and the Impact of Antioxidant Therapy on Clinical Outcomes. Worldviews Evid.-Based Nurs. 2016, 13, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.L.; Alvaro, P.K. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: A meta-analysis. Sleep Med. 2012, 13, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, A.; Crawford, S.; Bodell, L.; Dewey, D.; Barlow, K.M. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: A prospective cohort. Dev. Med. Child Neurol. 2013, 55, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Thau-Zuchman, O.; Gomes, R.N.; Dyall, S.C.; Davies, M.; Priestley, J.V.; Groenendijk, M.; De Wilde, M.C.; Tremoleda, J.L.; Michael-Titus, A.T. Brain Phospholipid Precursors Administered Post-Injury Reduce Tissue Damage and Improve Neurological Outcome in Experimental Traumatic Brain Injury. J. Neurotrauma 2019, 36, 25–42. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary strategy to repair plasma membrane after brain trauma: Implications for plasticity and cognition. Neurorehabil. Neural Repair 2014, 28, 75–84. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Exercise facilitates the action of dietary DHA on functional recovery after brain trauma. Neuroscience 2013, 248, 655–663. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Griver, B. Nutrition and cognitive health: A life course approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef]
- Martens, L.B.; Tibeas, J.R.B.; Sanches, M.; Jakca, F.; Berk, M.; Teixeira, A.L. Nutrition-based interventions for mood disorders. Expert Rev. Neurother. 2021, 21, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Bulter, M.I.; Lackner, S. Advances in the gut microbiome and mood disorders. Curr. Opin. Psychiatry 2023, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).