Pilot Study on the Relationship between Malnutrition and Grip Strength with Prognosis in Diabetic Foot
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
- -
- Pain: assessed using a 0–10 visual analog scale (VAS), with 10 indicating the most severe pain.
- -
- Quality of life: at admission, quality of life was measured using the DFS-SF scale (Diabetic Foot Ulcer Scale Short Spanish Validated Form) [31]. The DFS-SF is a disease-specific questionnaire that assesses the impact of DF ulcers on quality of life. The scale consists of 29 items (scored on a scale of 1–5) based on the following 5 subscales: leisure, physical health, dependence/daily living, negative emotions, and bothered by ulcer care. The score is calculated based on a minimum value of 29 (better quality of life) and a maximum of 145 (worse quality of life). The version used is a shortened version of the original, which contains 58 items and 11 domains.
- -
- Mobility: measured by the ability to walk and transfer, this determines whether the patient was fully independent, required assistance to walk, was confined to bed and chair, or was completely bedridden.
- -
- Length of hospital stay was also recorded (measured in days from admission to discharge), as well as whether the patient died during the follow-up period and if there were any readmissions.
2.2. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. Geriatric Syndromes and Functional Assessment (Table 2)
| n | Mean ± SD | % | |
|---|---|---|---|
| Geriatric syndrome | |||
| Cognitive impairment (Pfeiffer) | 45 | 2.56 ± 2.33 | |
| Sarcopenia | 12 | 26.7 | |
| Mobility | |||
| Independent | 9 | 20 | |
| Needs assistance | 24 | 53.3 | |
| Confined to bed + chair | 12 | 26.7 | |
| Urinary incontinence | 20 | 44.4 | |
| Fecal incontinence | 5 | 11.1 | |
| Polypharmacy | 44 | 97.8 | |
| Altered albumin levels | 31 | 68.9 | |
| Altered total protein levels | 30 | 66.7 | |
| Positive CIPA | 13 | 28.9 | |
| BMI | 45 | 30.29 ± 5.88 | |
| Underweight | 3 | 6.7 | |
| Normal weigh | 8 | 17.8 | |
| Overweight | 13 | 28.9 | |
| Obesity I | 13 | 28.9 | |
| Obesity II | 4 | 8.9 | |
| Obesity III | 4 | 8.9 | |
| Functional assessment | |||
| Basic activities (Barthel) | 45 | 60.67 ± 27.99 | |
| Instrumental activities (Lawton–Brody) | 45 | 3.87 ± 2.34 |
3.3. Three-Month Follow-Up Results
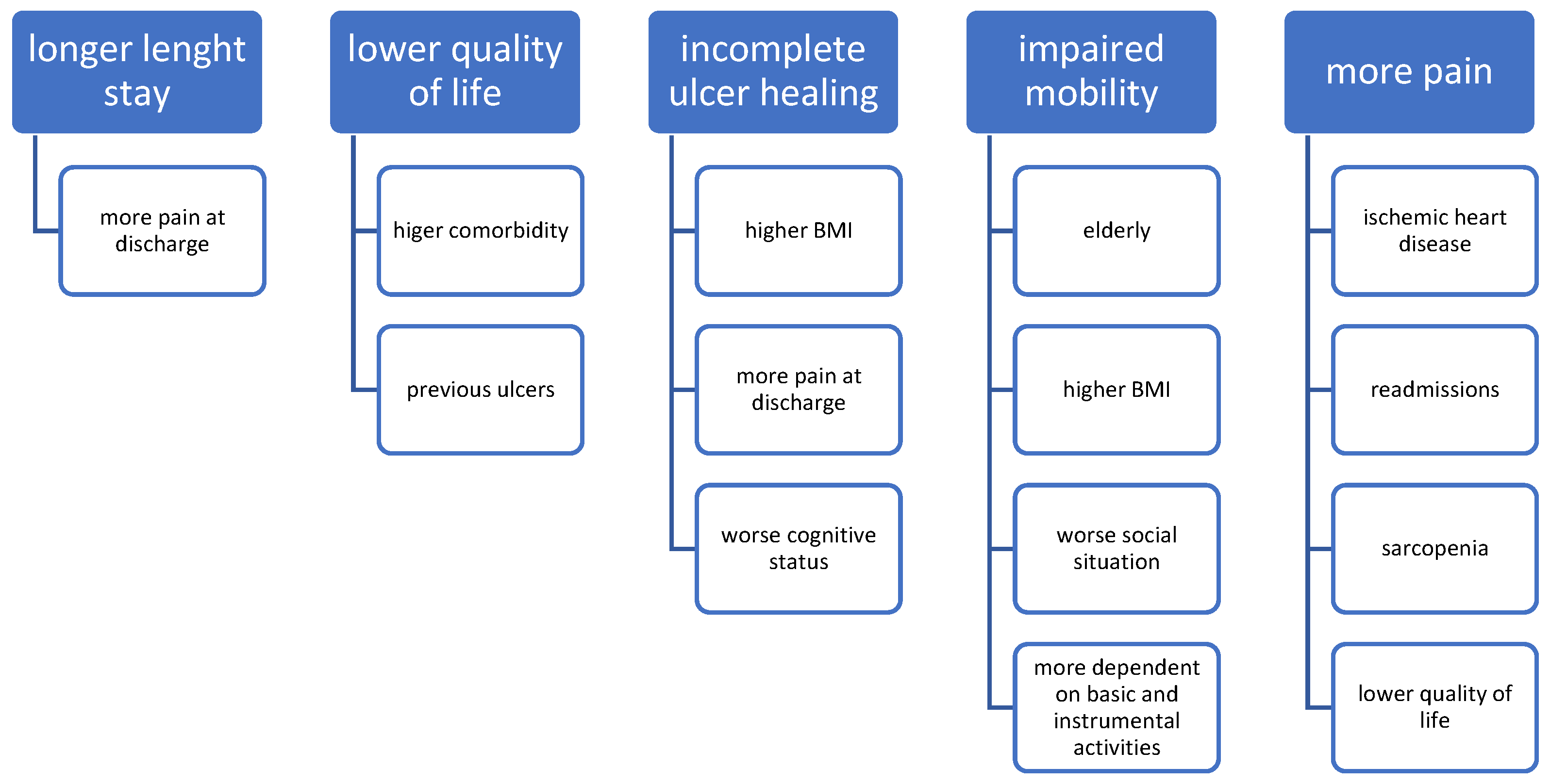
3.4. Association between Variables and Patient Outcomes at Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, T. Pie diabético. Fisiopatología y consecuencias. Rev. Colomb. Ortop. Traumatol. 2014, 18, 143–153. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lazzarini, P.A.; McPhail, S.M.; van Netten, J.J.; Armstrong, D.G.; Pacella, R.E. Global Disability Burdens of Diabetes-Related Lower-Extremity Complications in 1990 and 2016. Diabetes Care 2020, 43, 964–974. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Abordaje del Pie Diabético. Estrategia de Diabetes del Sistema Nacional de Salud; Ministerio de Sanidad: Madrid, Spain, 2022. [Google Scholar]
- BDCAP—Base de Datos Clínicos de Atención Primaria. Prevalencia de Diabetes Mellitus. 2020. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/estadisticas/estMinisterio/SIAP/home.htm (accessed on 1 June 2023).
- Instituto Nacional de Estadística. Available online: https://www.ine.es (accessed on 1 June 2023).
- Ahmed, M.U.; Tannous, W.K.; Agho, K.E.; Henshaw, F.; Turner, D.; Simmons, D. Social determinants of diabetes-related foot disease among older adults in New South Wales, Australia: Evidence from a population-based study. J. Foot Ankle Res. 2021, 14, 65. [Google Scholar] [CrossRef]
- Kim, T.G.; Moon, S.Y.; Park, M.S.; Kwon, S.S.; Jung, K.J.; Lee, T.; Kim, B.K.; Yoon, C.; Lee, K.M. Factors Affecting Length of Hospital Stay and Mortality in Infected Diabetic Foot Ulcers Undergoing Surgical Drainage without Major Amputation. J. Korean Med. Sci. 2016, 31, 120–124. [Google Scholar] [CrossRef]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef]
- Yesil, S.; Akinci, B.; Yener, S.; Bayraktar, F.; Karabay, O.; Havitcioglu, H.; Yapar, N.; Atabey, A.; Kucukyavas, Y.; Comlekci, A.; et al. Predictors of amputation in diabetics with foot ulcer: Single center experience in a large Turkish cohort. Hormones 2009, 8, 286–295. [Google Scholar] [CrossRef]
- Brennan, M.B.; Hess, T.M.; Bartle, B.; Cooper, J.M.; Kang, J.; Huang, E.S.; Smith, M.; Sohn, M.W.; Crnich, C. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 556–561. [Google Scholar] [CrossRef]
- Sabater, A.M.; Ruiz, M.F.P. Valoración del riesgo de pie diabético en el paciente anciano en una consulta de enfermería. Gerokomos 2009, 20, 73–77. [Google Scholar]
- González De La Torre, H.; Perdomo Pérez, E.; Lorenzo, Q.; Luana, M.; Mosquera Fernández, A. Estratificación de riesgo en pie diabético. Gerokomos 2010, 21, 172–182. [Google Scholar] [CrossRef][Green Version]
- Cifuentes Hoyos, V.; Giraldo Hoyos, A.P. Factores de Riesgo Para pie Diabético en Pacientes con Diabetes Mellitus Tipo 2; Grupo Observatorio de la Salud Pública: Medellín, Colombia, 2010. [Google Scholar]
- Mader, J.K.; Haas, W.; Aberer, F.; Boulgaropoulos, B.; Baumann, P.; Pandis, M.; Horvath, K.; Aziz, F.; Köhler, G.; Pieber, T.R.; et al. Patients with healed diabetic foot ulcer represent a cohort at highest risk for future fatal events. Sci. Rep. 2019, 9, 10325. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.L.; Shamliyan, T.; Talley, K.; Pacala, J. The association between geriatric syndromes and survival. J. Am. Geriatr. Soc. 2012, 60, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Becker, I.; Siri, G.; Brinkkötter, P.T.; Benzing, T.; Pilotto, A.; Polidori, M.C. The prognostic significance of geriatric syndromes and resources. Aging Clin. Exp. Res. 2020, 32, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Forman, D.E.; Van Diepen, S.; Alexander, K.P.; Page, R.L.; Hummel, S.L.; Menon, V.; Katz, J.N.; Albert, N.M.; Afilalo, J.; et al. American Heart Association Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement from the American Heart Association. Circulation 2020, 141, e6–e32. [Google Scholar]
- Alarcón, M.T.; González, J.I. La escala sociofamiliar de Gijón, instrumento útil en el hospital general. Rev. Esp. Geriatr. Gerontol. 1998, 33, 178–180. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–375. [Google Scholar] [CrossRef]
- Wagner, F.W. The Dysvascular Foot: A System for Diagnosis and Treatment. Foot Ankle 1981, 2, 64–122. [Google Scholar] [CrossRef]
- Van Acker, K.; De Block, C.; Abrams, P.; Bouten, A.; De Leeuw, I.; Droste, J.; Weyler, J.; Peter-Riesch, B. The choice of diabetic foot ulcer classification in relation to the final Outcome. Wounds 2002, 14, 16–25. [Google Scholar]
- de la Iglesiaa, J.M.; DueñasHerrerob, R.; Vilchesa, M.C.O.; Tabernéa, C.A.; Colomerc, C.A.; Luquec, R.L. Adaptación y validación al castellano del cuestionario de Pfeiffer (SPMSQ) para detectar la existencia de deterioro cognitivo en personas mayores de 65 años. Med. Clínica 2001, 117, 129–134. [Google Scholar] [CrossRef]
- Mateo Lázaro, M.L.; Berisa Losantos, F.; Plaza Bayo, A. Nuevas tablas de fuerza de la mano para población adulta de Teruel. Nutr. Hosp. 2008, 23, 35–40. [Google Scholar] [PubMed]
- Hospitalario. Gobierno de Canarias; Ministerio de Sanidad, Servicios Sociales e Lgualdad. Herramientas para la Detección Precoz de la Desnutrición Relacionada con la Enfermedad para Población Adulta en el Ámbito. 2017. Available online: https://www.researchgate.net/publication/343576504_Herramientas_para_la_deteccion_precoz_de_la_desnutricion_relacionada_con_la_enfermedad_para_poblacion_adulta_en_el_ambito_hospitalario (accessed on 1 June 2023).
- Castro-Vega, I.; Veses-Martín, S.; Cantero-Llorca, J.; Salom-Vendrell, C.; Bañuls, C.; Hernández-Mijares, A. Validación del cribado nutricional Malnutrition Screening Tool comparado con la valoración nutricional completa y otros cribados en distintos ámbitos sociosanitarios. Nutr. Hosp. 2018, 35, 351–358. [Google Scholar] [PubMed]
- Planas, M. Valoración nutricional en el anciano. In Recomendaciones Prácticas de los Expertos en Geriatría y Nutrición; Galénitas-Nigra Trea: Bilbao, Spain, 2009. [Google Scholar]
- Mahoney, F.J.; Barthel, D.W. Functional evaluation: The Barthel Index. A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Rubio, E.; Comín, M.; Montón, G.; Martínez, T.; Magallón, R.; García-Campayo, J. Capacidad funcional en las personas mayores según el género. Gerokomos 2013, 24, 69–73. [Google Scholar] [CrossRef][Green Version]
- Martinez-Gonzalez, D.; Dòria, M.; Martínez-Alonso, M.; Alcubierre, N.; Valls, J.; Verdú-Soriano, J.; Granado-Casas, M.; Mauricio, D. Adaptation and Validation of the Diabetic Foot Ulcer Scale-Short Form in Spanish Subjects. J. Clin. Med. 2020, 9, 2497. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Péres, K.; Féart, C.; Amieva, H.; Harmand, M.G.; Helmer, C.; Salles, N.; Rainfray, M.; Dartigues, J.F. Prevalence and Co-Occurrence of Geriatric Syndromes in People Aged 75 Years and Older in France: Results from the Bordeaux Three-city Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 109–116. [Google Scholar] [CrossRef]
- Clerencia-Sierra, M.; Calderón-Larrañaga, A.; Martínez-Velilla, N.; Vergara-Mitxeltorena, I.; Aldaz-Herce, P.; Poblador-Plou, B.; Machón-Sobrado, M.; Egüés-Olazabal, N.; Abellán-van Kan, G.; Prados-Torres, A. Multimorbidity Patterns in Hospitalized Older Patients: Associations among Chronic Diseases and Geriatric Syndromes. PLoS ONE 2015, 10, e0132909. [Google Scholar] [CrossRef]
- Lewandowicz, A.; Skowronek, P.; Maksymiuk-Kłos, A.; Piątkiewicz, P. The Giant Geriatric Syndromes Are Intensified by Diabetic Complications. Gerontol. Geriatr. Med. 2018, 4, 2333721418817396. [Google Scholar] [CrossRef]
- Miguel, L.; Julia, A.H.; Mercè, P.; Abelardo, G.L.; Krysmaru, A.; Sebastián, C. Prevalence of hospital malnutrition in patients with diabetes mellitus: A sub-analysis of the PREDyCES study. SM J. Public. Health Epidemiol. 2015, 1, 1018. [Google Scholar]
- Lauwers, P.; Dirinck, E.; Van Bouwel, S.; Verrijken, A.; Van Dessel, K.; Van Gils, C.; Sels, M.; Peiffer, F.; Van Schil, P.; De Block, C.; et al. Malnutrition and its relation with diabetic foot ulcer severity and outcome: A review. Acta Clin. Belg. 2022, 77, 79–85. [Google Scholar] [CrossRef]
- Demir, O.; Sen, V.; Irer, B.; Bozkurt, O.; Esen, A. Prevalence and Possible Risk Factors for Urinary Incontinence: A Cohort Study in the City of Izmir. Urol. Int. 2017, 99, 84–90. [Google Scholar] [CrossRef]
- Areosa Sastre, A.; Vernooij, R.W.; González-Colaço Harmand, M.; Martínez, G. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst. Rev. 2017, 6, CD003804. [Google Scholar] [CrossRef]
- Bellia, C.; Lombardo, M.; Meloni, M.; Della-Morte, D.; Bellia, A.; Lauro, D. Diabetes and cognitive decline. Adv. Clin. Chem. 2022, 108, 37–71. [Google Scholar] [CrossRef] [PubMed]
- Sela, Y.; Grinberg, K.; Cukierman-Yaffe, T.; Natovich, R. Relationship between cognitive function in individuals with diabetic foot ulcer and mortality. Diabetol. Metab. Syndr. 2022, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Joshi, A.; Kapoor, N. Osteoporosis and diabetes: The dual pandemics. J. Pak. Med. Assoc. 2022, 72, 1663–1664. [Google Scholar] [PubMed]
- Karakousis, N.D.; Pyrgioti, E.E.; Georgakopoulos, P.N.; Papanas, N. Sarcopenia, Frailty and Diabetic Foot: A Mini Narrative Review. Int. J. Low. Extrem. Wounds. 2022, 15347346221111420. [Google Scholar] [CrossRef]
- Jung, S.Y.; Lee, M.J.; Lee, S.Y. Analysis of the Relationship Between Lower leg Muscle Mass and Preservation of Lower Extremity in Patients with Diabetic Foot Ulcer. Int. J. Low. Extrem. Wounds 2023, 22, 503–508. [Google Scholar] [CrossRef]
- Yang, Q.; Ni, X.; Zhang, Y.; Zhu, B.; Zeng, Q.; Yang, C.; Shi, J.; Zhang, C.; Cai, J.; Hu, J.; et al. Sarcopenia is an independent risk factor for all-cause mortality rate in patients with diabetic foot ulcers. Front. Nutr. 2023, 10, 1097008. [Google Scholar] [CrossRef]
- Cha, Y.H.; Song, S.Y.; Park, K.S.; Yoo, J.I. Relationship between pressure ulcer risk and sarcopenia in patients with hip fractures. J. Wound Care 2022, 31, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Trombini, K.C.B.; Martins, M.V.S.; Martins, H.R.F. Screening for sarcopenia and frailty in patients with chronic ulcers: A cross-sectional study. J. Vasc. Bras. 2020, 19, e20190054. [Google Scholar] [CrossRef]
- Altinkaynak, M.; Ozturk, C.K.; Goksoy, Y.; Eryigit, O.Y.; Akpinar, T.S.; Erten, S.N.; Saka, B. The prevalence of sarcopenic obesity and its relationship with type 2 diabetes in a nursing home. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2858–2864. [Google Scholar] [PubMed]
- Chuan, F.; Chen, S.; Ye, X.; Kang, S.; Mei, M.; Tian, W.; Liao, K.; Li, Y.; Gong, L.; Li, R.; et al. Sarcopenic obesity predicts negative health outcomes among older patients with type 2 diabetes: The Ageing and Body Composition of Diabetes (ABCD) cohort study. Clin. Nutr. 2022, 41, 2740–2748. [Google Scholar] [CrossRef]
- Munoz, N.; Posthauer, M.E. Nutrition strategies for pressure injury management: Implementing the 2019 International Clinical Practice Guideline. Nutr. Clin. Pract. 2022, 37, 567–582. [Google Scholar] [CrossRef]
- Jaul, E. Assessment and management of pressure ulcers in the elderly: Current strategies. Drugs Aging 2010, 27, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-López, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef]
- Söderström, L.; Rosenblad, A.; Thors Adolfsson, E.; Bergkvist, L. Malnutrition is associated with increased mortality in older adults regardless of the cause of death. Br. J. Nutr. 2017, 117, 532–540. [Google Scholar] [CrossRef]
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef]
- Cheng, Q.; Hu, J.; Yang, P.; Cao, X.; Deng, X.; Yang, Q.; Liu, Z.; Yang, S.; Goswami, R.; Wang, Y.; et al. Sarcopenia is independently associated with diabetic foot disease. Sci. Rep. 2017, 7, 8372. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and Its Impact on Quality of Life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [PubMed]
- Rizzoli, R.; Reginster, J.-Y.; Arnal, J.-F.; Bautmans, I.; Beaudart, C.; Bischoff-Ferrari, H.; Biver, E.; Boonen, S.; Brandi, M.-L.; Chines, A.; et al. Quality of Life in Sarcopenia and Frailty. Calcif. Tissue Int. 2013, 93, 101–120. [Google Scholar] [CrossRef]
- Wang, C.; Mai, L.; Yang, C.; Liu, D.; Sun, K.; Song, W.; Luo, B.; Li, Y.; Xu, M.; Zhang, S.; et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team in patient with diabetes foot ulcer. BMC Endocr. Disord. 2016, 16, 38. [Google Scholar] [CrossRef]
- Moore, Z.E.; Corcoran, M.A.; Patton, D. Nutritional interventions for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2020, 7, CD011378. [Google Scholar] [CrossRef] [PubMed]
- Bechara, N.; Gunton, J.E.; Flood, V.; Hng, T.-M.; McGloin, C. Associations between Nutrients and Foot Ulceration in Diabetes: A Systematic Review. Nutrients 2021, 13, 2576. [Google Scholar] [CrossRef]
| n | Mean ± SD | % | |
|---|---|---|---|
| Age (years) | 45 | 76.82 ± 8.08 | |
| Female sex | 13 | 28.9 | |
| Living alone | 10 | 22.2 | |
| Time since diagnosis | 45 | ||
| <5 years | 10 | 22.2 | |
| 5–10 years | 11 | 24.4 | |
| >10 years | 24 | 53.3 | |
| Social assessment (Gijón) | 45 | 10.33 ± 2.95 | |
| Normal | 23 | 51.1 | |
| At social risk | 17 | 37.8 | |
| Social problems | 5 | 11.1 | |
| Clinical problems | |||
| Hypertension | 41 | 91.1 | |
| Anemia | 13 | 28.9 | |
| Active smoking | 5 | 11.1 | |
| Prior ulcers | 29 | 64.4 | |
| Ischemic heart disease | 31 | 68.9 | |
| Diabetic neuropathy | 44 | 97.8 | |
| Diabetic kidney disease | 19 | 42.2 | |
| Diabetic retinopathy | 14 | 31.1 | |
| Dialysis | 11 | 24.4 | |
| Charlson index | 45 | 8.96 ± 2.71 | |
| Wagner–Merrit | |||
| Superficial ulcers | 1 | 2.2 | |
| Limited gangrene | 44 | 97.8 | |
| Van Ackers/Peters system | |||
| 2A | 1 | 2.2 | |
| 4E | 44 | 97.8 | |
| Ulcer treatment | |||
| Betadine | 41 | 91.1 | |
| Betadine + Hydration | 1 | 2.2 | |
| Negative pressure wound dressing (PICO) | 1 | 2.2 | |
| Prontosan + Purilon | 2 | 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Colaço Harmand, M.; Tejera Concepción, A.; Farráis Expósito, F.J.; Domínguez González, J.; Ramallo-Fariña, Y. Pilot Study on the Relationship between Malnutrition and Grip Strength with Prognosis in Diabetic Foot. Nutrients 2023, 15, 3710. https://doi.org/10.3390/nu15173710
González-Colaço Harmand M, Tejera Concepción A, Farráis Expósito FJ, Domínguez González J, Ramallo-Fariña Y. Pilot Study on the Relationship between Malnutrition and Grip Strength with Prognosis in Diabetic Foot. Nutrients. 2023; 15(17):3710. https://doi.org/10.3390/nu15173710
Chicago/Turabian StyleGonzález-Colaço Harmand, Magali, Alicia Tejera Concepción, Francisco José Farráis Expósito, Jennifer Domínguez González, and Yolanda Ramallo-Fariña. 2023. "Pilot Study on the Relationship between Malnutrition and Grip Strength with Prognosis in Diabetic Foot" Nutrients 15, no. 17: 3710. https://doi.org/10.3390/nu15173710
APA StyleGonzález-Colaço Harmand, M., Tejera Concepción, A., Farráis Expósito, F. J., Domínguez González, J., & Ramallo-Fariña, Y. (2023). Pilot Study on the Relationship between Malnutrition and Grip Strength with Prognosis in Diabetic Foot. Nutrients, 15(17), 3710. https://doi.org/10.3390/nu15173710






