Six Weeks of Supplementation with Bovine Colostrum Effectively Reduces URTIs Symptoms Frequency and Gravity for Up to 20 Weeks in Pre-School Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment, Blinding and Randomization
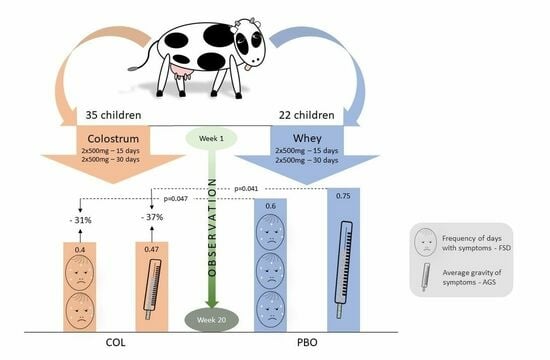
2.2. Outline of the Trial and Participation Issues
2.3. Supplementation Material
2.4. Surveys
2.5. Outcomes
2.6. Statistical Analysis
2.7. Bioethical Approval
3. Results
3.1. Study Group Characteristics
3.2. Colostrum Decreases Frequency of Days with URTI Symptoms
3.3. Colostrum Reduces Average Gravity Scores of URTI Symptoms
3.4. Colostrum Reduces Number of URTI Episodes
3.5. Colostrum Produced No Gastrointestinal Side Effects
4. Discussion
4.1. Colostrum Reduces URTI Frequency and Severity
4.2. URTI Episodes
4.3. How Colostrum Use May Prevent the URTIs
4.4. There Were No Side Effects from Colostrum Use
4.5. Strengths and Limitations
5. Conclusions
- Kindergarten children 4–7 years of age receiving the pre-season colostrum supplementation may have up to 31% less days with URTI self-reported symptoms over 20 weeks from beginning of supplementation than children receiving a placebo.
- These children tested within the same period (20 weeks) presented an even bigger median reduction in their URTI gravity score (−37%) in the COL vs. the PBO groups, which supposedly results not exclusively from sick days’ reduction but also from milder course of the URTIs.
- The number of episodes, defined as 3 consecutive days of second degree of URTI gravity separated from other episodes by at least 3 days, was reduced in the COL group by 50% as compared to the PBO group over the entire period of the trial (21 weeks).
- The observed effects of our study were obtained with main supplementation lasting for less than just 1/3 of the trial period.
- There were no significant side effects observed during the trial, neither in the COL nor in the PBO groups. Moreover, there was no statistically significant difference between these groups in regard to gastrointestinal symptoms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heikkinen, T.; Järvinen, A. The Common Cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hak, E.; Rovers, M.; Kuyvenhoven, M.; Schellevis, F.; Verheij, T. Incidence of GP-Diagnosed Respiratory Tract Infections According to Age, Gender and High-Risk Co-Morbidity: The Second Dutch National Survey of General Practice. Fam. Pract. 2006, 23, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Grief, S.N. Upper Respiratory Infections. Prim. Care Clin. Off. Pract. 2013, 40, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B. Epidemiology, Pathogenesis, and Treatment of the Common Cold. Ann. Allergy Asthma Immunol. 1997, 78, 531–540. [Google Scholar] [CrossRef]
- Kardos, P.; Malek, F.A. Common Cold—An Umbrella Term for Acute Infections of Nose, Throat, Larynx and Bronchi. Pneumologie 2017, 71, 221–226. [Google Scholar] [CrossRef]
- Thomas, A.O.; Lemanske, R.F., Jr.; Jackson, D.J. Infections and Their Role in Childhood Asthma Inception. Pediatr. Allergy Immunol. 2014, 25, 122–128. [Google Scholar] [CrossRef]
- Queen, J.; Zhang, J.; Sears, C.L. Oral Antibiotic Use and Chronic Disease: Long-Term Health Impact beyond Antimicrobial Resistance and Clostridioides Difficile. Gut Microb. 2020, 11, 1092–1103. [Google Scholar] [CrossRef]
- Ballengee, C.R.; Turner, R.B. Supportive Treatment for Children with the Common Cold. Curr. Opin. Pediatr. 2014, 26, 114. [Google Scholar] [CrossRef]
- Caruso, T.J.; Gwaltney, J.M., Jr. Treatment of the Common Cold with Echinacea: A Structured Review. Clin. Infect. Dis. 2005, 40, 807–810. [Google Scholar] [CrossRef]
- Allan, G.M.; Arroll, B. Prevention and Treatment of the Common Cold: Making Sense of the Evidence. CMAJ 2014, 186, 190–199. [Google Scholar] [CrossRef]
- Science, M.; Johnstone, J.; Roth, D.E.; Guyatt, G.; Loeb, M. Zinc for the Treatment of the Common Cold: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. CMAJ 2012, 184, E551–E561. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Oude Elberink, H.; Pali-Schöll, I.; et al. Nutrient Supplementation for Prevention of Viral Respiratory Tract Infections in Healthy Subjects: A Systematic Review and Meta-Analysis. Allergy 2022, 77, 1373–1388. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2013, 1, CD000980. [Google Scholar] [CrossRef]
- Morris, P.S. Upper Respiratory Tract Infections (Including Otitis Media). Pediatr. Clin. N. Am. 2009, 56, 101–117. [Google Scholar] [CrossRef]
- Zicari, A.M.; Castro, G.D.; Brindisi, G.; Papale, M.; Marinelli, G.; Licari, A.; Ciprandi, G. Respiratory Infections in Allergic Children: The Preventive Role of a Multicomponent Nutraceutical. Acta Biomed. 2020, 91, e2020072. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, A.; Poggi, E.; Trapani, G.; Muia, C.; Zanino, L.; Landi, M.; Ciprandi, G. Primary Care Experience on Stimunex® Gocce in Children with Recurrent Respiratory Infections: A Real-World Study during the COVID-19 Pandemic Era. Allergol. Immunopathol. 2022, 50, 8–14. [Google Scholar] [CrossRef]
- Kenealy, T.; Arroll, B. Antibiotics for the Common Cold and Acute Purulent Rhinitis. Cochrane Database Syst. Rev. 2013, 6, CD000247. [Google Scholar] [CrossRef] [PubMed]
- De Sutter, A.I.; De Meyere, M.J.; Christiaens, T.C.; Van Driel, M.L.; Peersman, W.; De Maeseneer, J.M. Does Amoxicillin Improve Outcomes in Patients with Purulent Rhinorrhea? A Pragmatic Randomized Double-Blind Controlled Trial in Family Practice. J. Fam. Pract. 2002, 51, 317–323. [Google Scholar] [PubMed]
- Spinks, A.; Glasziou, P.P.; Mar, C.B.D. Antibiotics for Sore Throat. Cochrane Database Syst. Rev. 2013, 11, CD000023. [Google Scholar] [CrossRef]
- Nokso-Koivisto, J.; Hovi, T.; Pitkäranta, A. Viral Upper Respiratory Tract Infections in Young Children with Emphasis on Acute Otitis Media. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Fashner, J.; Ericson, K.; Werner, S. Treatment of the Common Cold in Children and Adults. Am. Fam. Physician 2012, 86, 153–159. [Google Scholar] [PubMed]
- Jones, A.W.; Cameron, S.J.S.; Thatcher, R.; Beecroft, M.S.; Mur, L.A.J.; Davison, G. Effects of Bovine Colostrum Supplementation on Upper Respiratory Illness in Active Males. Brain Behav. Immun. 2014, 39, 194–203. [Google Scholar] [CrossRef]
- Jones, A.W.; March, D.S.; Curtis, F.; Bridle, C. Bovine Colostrum Supplementation and Upper Respiratory Symptoms during Exercise Training: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMC Sport. Sci. Med. Rehabil. 2016, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, M.; Baśkiewicz-Hałasa, M.; Jamioł-Milc, D.; Maciejewska-Markiewicz, D.; Skonieczna-Żydecka, K. Bovine Colostrum Supplementation in Prevention of Upper Respiratory Tract Infections—Systematic Review, Meta-Analysis and Meta-Regression of Randomized Controlled Trials. J. Funct. Foods 2022, 99, 105316. [Google Scholar] [CrossRef]
- Ameli, F.; Ciprandi, G. Sinerga May Prevent Recurrent Respiratory Infections in Allergic Children. J. Biol. Regul. Homeost. Agents 2019, 33, 601–607. [Google Scholar] [PubMed]
- Patıroğlu, T.; Kondolot, M. The Effect of Bovine Colostrum on Viral Upper Respiratory Tract Infections in Children with Immunoglobulin A Deficiency: Bovine Colostrum in Children with IgA Deficiency. Clin. Respir. J. 2013, 7, 21–26. [Google Scholar] [CrossRef]
- Baśkiewicz-Hałasa, M.; Stachowska, E.; Grochans, E.; Maciejewska-Markiewicz, D.; Bühner, L.; Skonieczna-Żydecka, K.; Hałasa, M. Moderate Dose Bovine Colostrum Supplementation in Prevention of Upper Respiratory Tract Infections in Medical University Students: A Randomized, Triple Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 1925. [Google Scholar] [CrossRef]
- EUSurvey—Welcome. Available online: https://ec.europa.eu/eusurvey/home/welcome (accessed on 13 April 2023).
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef]
- Hałasa, M.; Maciejewska-Markiewicz, D.; Baśkiewicz-Hałasa, M.; Safranow, K.; Stachowska, E. Post-Delivery Milking Delay Influence on the Effect of Oral Supplementation with Bovine Colostrum as Measured with Intestinal Permeability Test. Medicina 2020, 56, 495. [Google Scholar] [CrossRef]
- Saad, K.; Abo-Elela, M.G.M.; El-Baseer, K.A.A.; Ahmed, A.E.; Ahmad, F.-A.; Tawfeek, M.S.K.; El-Houfey, A.A.; Aboul_Khair, M.D.; Abdel-Salam, A.M.; Abo-elgheit, A.; et al. Effects of Bovine Colostrum on Recurrent Respiratory Tract Infections and Diarrhea in Children. Medicine 2016, 95, e4560. [Google Scholar] [CrossRef]
- Patel, K.; Rana, R. Pedimune in Recurrent Respiratory Infection and Diarrhoea—The Indian Experience—The PRIDE Study. Indian J. Pediatr. 2006, 73, 585–591. [Google Scholar] [CrossRef]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper Respiratory Tract Infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Struff, W.G.; Sprotte, G. Bovine Colostrum as a Biologic in Clinical Medicine: A Review—Part II: Clinical Studies. Int. J. Clin. Pharm. 2008, 46, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Davison, G. Bovine Colostrum and Immune Function after Exercise; Karger Publishers: Basel, Switzerland, 2012. [Google Scholar] [CrossRef]
- Rathe, M.; Müller, K.; Sangild, P.T.; Husby, S. Clinical Applications of Bovine Colostrum Therapy: A Systematic Review. Nutr. Rev. 2014, 72, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Rouse, B.T. 27—Immune Responses to Viruses. In Clinical ImmunologyI, 3rd ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Mosby: Edinburgh, UK, 2008; pp. 421–431. ISBN 978-0-323-04404-2. [Google Scholar]
- Rouse, B.T.; Sehrawat, S. Immunity and Immunopathology to Viruses: What Decides the Outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef]
- Immune Responses to Viruses|British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/pathogens-disease/immune-responses-viruses (accessed on 16 July 2023).
- Jones, A.W.; March, D.S.; Thatcher, R.; Diment, B.; Walsh, N.P.; Davison, G. The Effects of Bovine Colostrum Supplementation on in Vivo Immunity Following Prolonged Exercise: A Randomised Controlled Trial. Eur. J. Nutr. 2019, 58, 335–344. [Google Scholar] [CrossRef]
- Sangild, P.T.; Vonderohe, C.; Melendez Hebib, V.; Burrin, D.G. Potential Benefits of Bovine Colostrum in Pediatric Nutrition and Health. Nutrients 2021, 13, 2551. [Google Scholar] [CrossRef]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral Nanomedicine for Modulating Immunity, Intestinal Barrier Functions, and Gut Microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and Milk-Derived Peptide Growth Factors for the Treatment of Gastrointestinal Disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential Benefits of Colostrum in Gastrointestinal Diseases. FBS 2016, 8, 331–351. [Google Scholar] [CrossRef]





| The Outcome | Acronym | Method of Calculation | Assessed at Weeks |
|---|---|---|---|
| Frequency of days with URTI symptoms | FSD | Number of days with any URTI symptoms/days of analyzed period | 4, 8, 12, 14, 16, 18, 20, 21 |
| Average gravity score of URTI symptoms | AGS | Total URTI score/days of analyzed period | 4, 8, 12, 14, 16, 18, 20, 21 |
| Number of URTI episodes | NUE | Sequence of days with URTI score 2 lasting for at least 3 days with at least 3 days separation from other episodes | 21 |
| Variable | Colostrum | Placebo | p | ||||
|---|---|---|---|---|---|---|---|
| N | Median | IQR | n | Median | IQR | ||
| BMI (kg/m2) | 35 | 14.88 | 14.01–15.69 | 22 | 15.32 | 14.18–16.02 | 0.25 |
| Age (years) | 35 | 4 | 4.00–5.00 | 22 | 4 | 3.00–5.00 | 0.92 |
| Mass (kg) | 35 | 17 | 16.00–19.00 | 22 | 17 | 15.00–20.00 | 0.91 |
| Variable | COL | PBO | p | Median FSD Change COL vs. PBO | ||
|---|---|---|---|---|---|---|
| Median | 25–75 P | Median | 25–75 P | |||
| FSD 4 weeks | 0.50 | 0.232–0.812 | 0.75 | 0.536–0.893 | 0.092 | −33% |
| FSD 8 weeks | 0.45 | 0.286–0.714 | 0.63 | 0.464–0.839 | 0.036 | −30% |
| FSD 12 weeks | 0.44 | 0.318–0.658 | 0.66 | 0.429–0.821 | 0.047 | −33% |
| FSD 14 weeks | 0.45 | 0.304–0.663 | 0.68 | 0.439–0.827 | 0.050 | −34% |
| FSD 16 weeks | 0.42 | 0.288–0.643 | 0.68 | 0.411–0.759 | 0.025 | −37% |
| FSD 18 weeks | 0.41 | 0.258–0.603 | 0.65 | 0.413–0.722 | 0.027 | −37% |
| FSD 20 weeks | 0.41 | 0.232–0.598 | 0.59 | 0.386–0.693 | 0.047 | −31% |
| FSD 21 weeks | 0.43 | 0.221–0.588 | 0.58 | 0.367–0.673 | 0.052 | −26% |
| Variable | COL | PBO | p | Median AGS Change COL vs. PBO | ||
|---|---|---|---|---|---|---|
| Median | 25–75 P | Median | 25–75 P | |||
| AGS 4 weeks | 0.50 | 0.259–0.884 | 0.91 | 0.643–1.071 | 0.016 | −45% |
| AGS 8 weeks | 0.50 | 0.304–0.777 | 0.79 | 0.500–1.018 | 0.024 | −36% |
| AGS 12 weeks | 0.51 | 0.396–0.801 | 0.77 | 0.488–1.000 | 0.036 | −33% |
| AGS 14 weeks | 0.49 | 0.362–0.786 | 0.82 | 0.561–1.020 | 0.027 | −40% |
| AGS 16 weeks | 0.48 | 0.335–0.732 | 0.78 | 0.500–0.982 | 0.019 | −38% |
| AGS 18 weeks | 0.48 | 0.310–0.720 | 0.80 | 0.492–0.960 | 0.026 | −41% |
| AGS 20 weeks | 0.47 | 0.279–0.716 | 0.75 | 0.493–0.893 | 0.041 | −37% |
| AGS 21 weeks | 0.46 | 0.265–0.709 | 0.73 | 0.483–0.864 | 0.052 | −36% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hałasa, M.; Skonieczna-Żydecka, K.; Machaliński, B.; Bühner, L.; Baśkiewicz-Hałasa, M. Six Weeks of Supplementation with Bovine Colostrum Effectively Reduces URTIs Symptoms Frequency and Gravity for Up to 20 Weeks in Pre-School Children. Nutrients 2023, 15, 3626. https://doi.org/10.3390/nu15163626
Hałasa M, Skonieczna-Żydecka K, Machaliński B, Bühner L, Baśkiewicz-Hałasa M. Six Weeks of Supplementation with Bovine Colostrum Effectively Reduces URTIs Symptoms Frequency and Gravity for Up to 20 Weeks in Pre-School Children. Nutrients. 2023; 15(16):3626. https://doi.org/10.3390/nu15163626
Chicago/Turabian StyleHałasa, Maciej, Karolina Skonieczna-Żydecka, Bogusław Machaliński, Leonard Bühner, and Magdalena Baśkiewicz-Hałasa. 2023. "Six Weeks of Supplementation with Bovine Colostrum Effectively Reduces URTIs Symptoms Frequency and Gravity for Up to 20 Weeks in Pre-School Children" Nutrients 15, no. 16: 3626. https://doi.org/10.3390/nu15163626
APA StyleHałasa, M., Skonieczna-Żydecka, K., Machaliński, B., Bühner, L., & Baśkiewicz-Hałasa, M. (2023). Six Weeks of Supplementation with Bovine Colostrum Effectively Reduces URTIs Symptoms Frequency and Gravity for Up to 20 Weeks in Pre-School Children. Nutrients, 15(16), 3626. https://doi.org/10.3390/nu15163626