Association between Pre-Treatment Biological Indicators and Compliance to Neoadjuvant/Perioperative Chemotherapy in Operable Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics
2.2. Endpoints
2.3. Anthropometric Measurements, Body Composition Measurements and Sarcopenia Assessment
2.4. Peripheral Venous Blood Sample
2.5. Treatment Protocols
2.6. Ethical Approval
2.7. Follow-Up
2.8. Statistical Analysis
3. Results
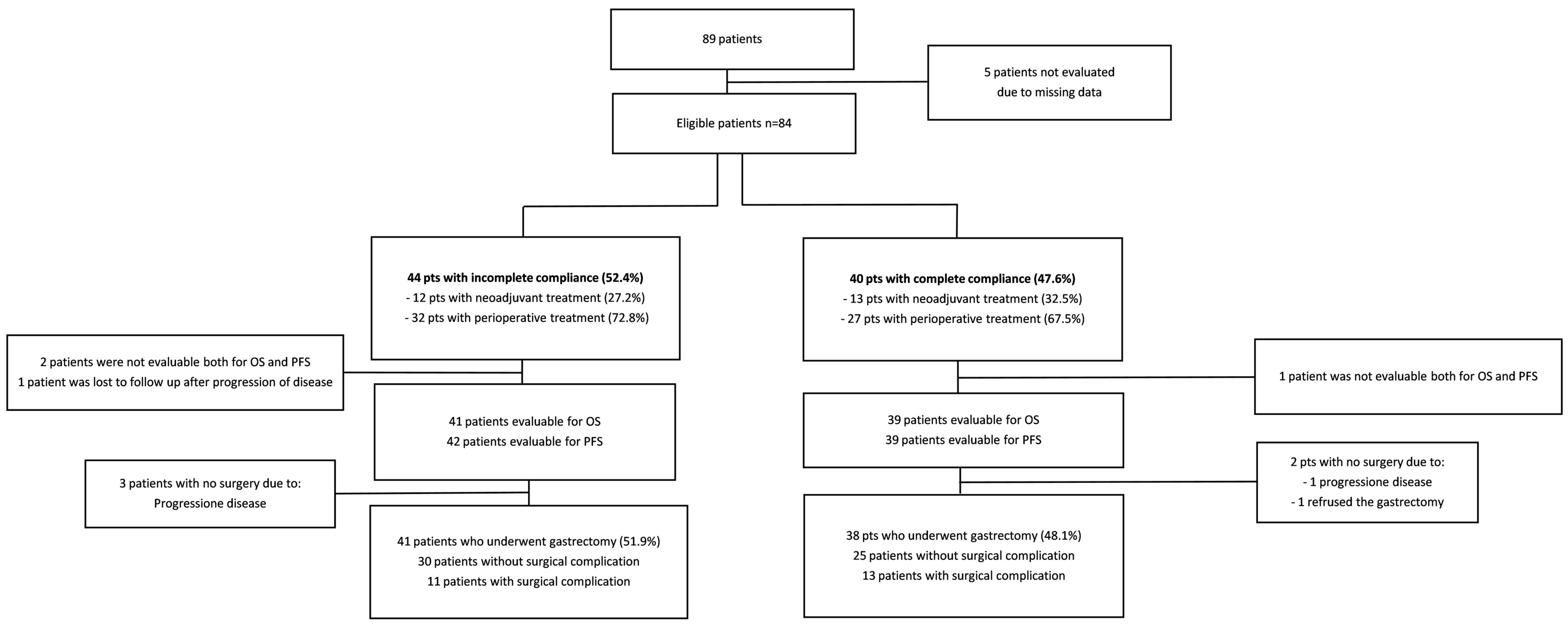
3.1. Demographic Characteristics
3.2. Characteristics of Drop Out
3.3. Relationship between Patient/Cancer/Chemotherapy and Compliance
3.4. Relationship between Patient/Cancer Characteristics/Chemotherapy and Reasons of Non-Adherence
3.5. Chemotherapies Toxicity and Surgical Complications as Cause of Discontinuation
3.6. Relationship between Pathological Regression, Sarcopenia and Compliance
3.7. Assessment of Potential Bias
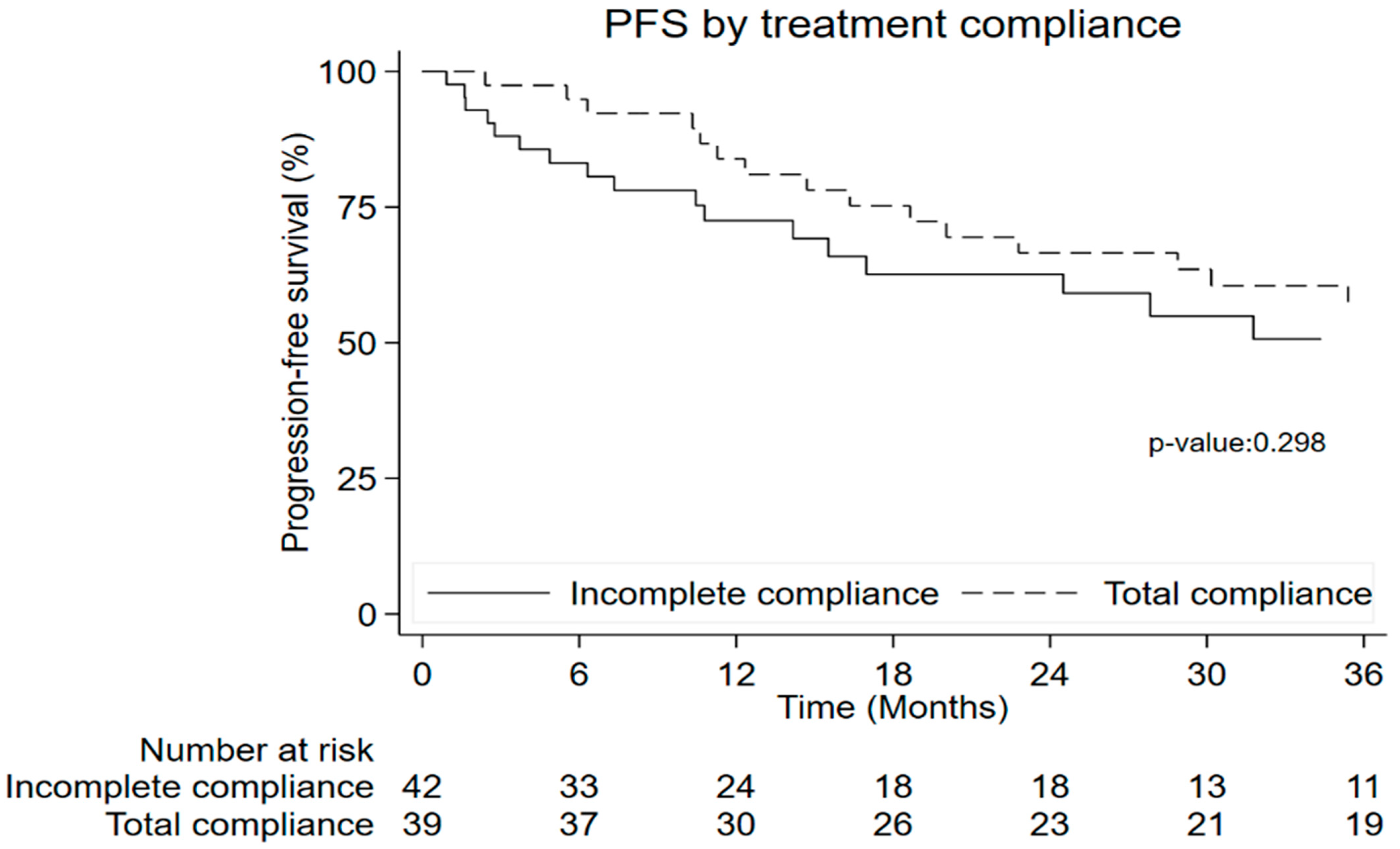
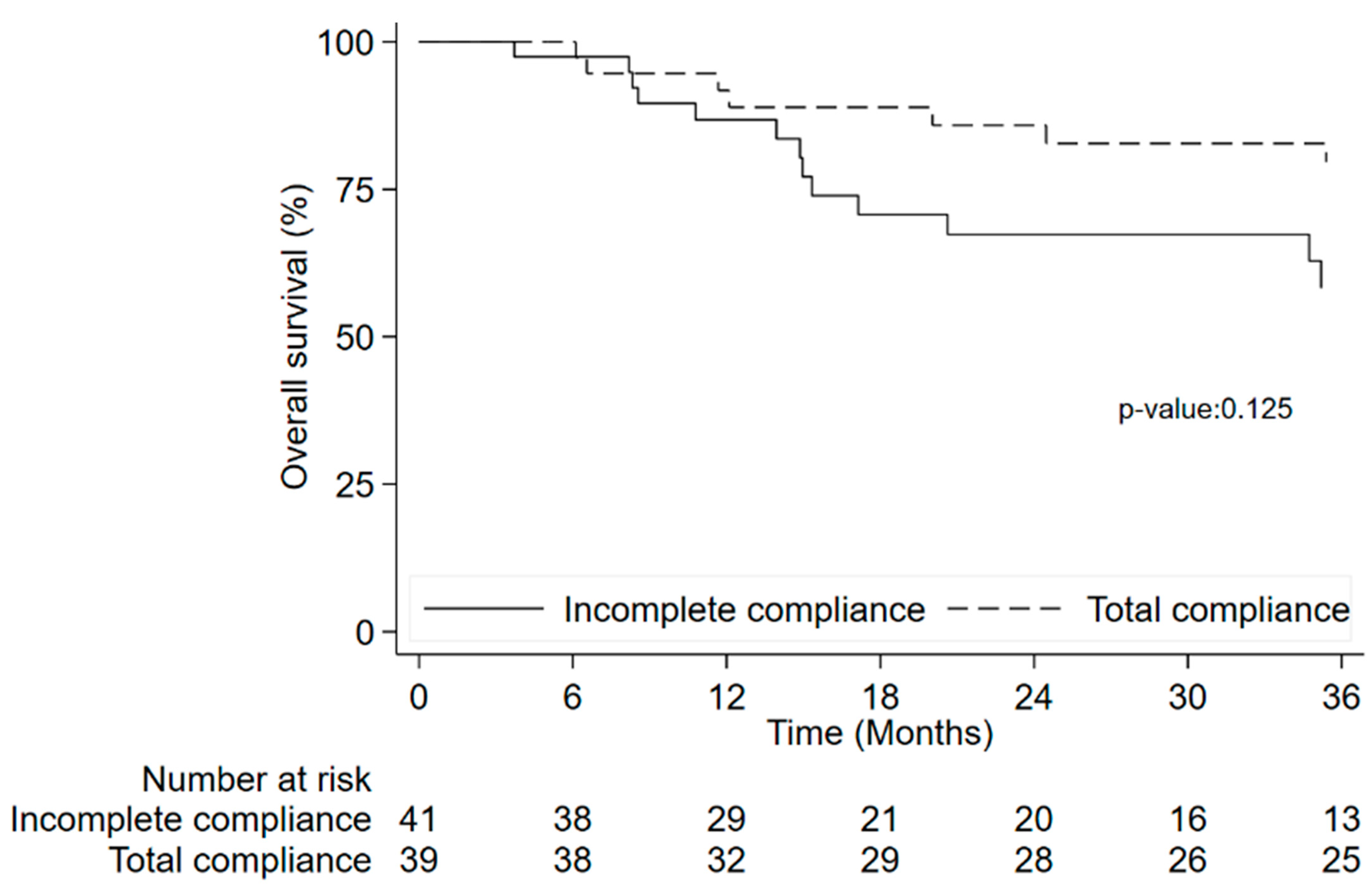
3.8. Time to Event Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Globocan. 2012. Available online: https://www.iarc.who.int/news-events/latest-world-cancer-statistics-globocan-2012-estimated-cancer-incidence-mortality-and-prevalence-worldwide-in-2012/ (accessed on 18 November 2022).
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel; oxaliplatin; fluorouracil; and leucovorin versus epirubicin; cisplatin; and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre; open-label; randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis; treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Tomasello, G.; Petrelli, F.; Ghidini, M.; Pezzica, E.; Passalacqua, R.; Steccanella, F.; Turati, L.; Sgroi, G.; Barni, S. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur. J. Surg. Oncol. 2017, 43, 1607–1616. [Google Scholar] [CrossRef]
- Shoji, F.; Kozuma, Y.; Toyokawa, G.; Yamazaki, K.; Takeo, S. Complete Blood Cell Count-Derived Inflammatory Biomarkers in Early-Stage Non-Small-Cell Lung Cancer. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 248–255. [Google Scholar] [CrossRef]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 483, 48–56. [Google Scholar] [CrossRef]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Halazun, K.J.; Aldoori, A.; Malik, H.Z.; Al-Mukhtar, A.; Prasad, K.R.; Toogood, G.J.; Lodge, J.P. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2008, 34, 55–60. [Google Scholar] [CrossRef]
- Sarraf, K.M.; Belcher, E.; Raevsky, E.; Nicholson, A.G.; Goldstraw, P.; Lim, E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2009, 137, 425–428. [Google Scholar] [CrossRef]
- Al Murri, A.M.; Wilson, C.; Lannigan, A.; Doughty, J.C.; Angerson, W.J.; McArdle, C.S.; McMillan, D.C. Evaluation of the relationship between the systemic inflammatory response and cancer-specific survival in patients with primary operable breast cancer. Br. J. Cancer 2007, 96, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, W.; Huang, Y.; Chen, W.; Wu, R.; Chen, X.; Lou, N.; Wang, P. Sarcopenia is associated with the neutrophil/lymphocyte and platelet/lymphocyte ratios in operable gastric cancer patients: A prospective study. Cancer Manag. Res. 2018, 10, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Morgagni, P.; Nanni, O.; Framarini, M.; Saragoni, L.; Marrelli, D.; Roviello, F.; Petrioli, R.; Fumagalli Romario, U.; Rimassa, L.; et al. Preoperative or Perioperative Docetaxel; Oxaliplatin; and Capecitabine (GASTRODOC Regimen) in Patients with Locally-Advanced Resectable Gastric Cancer: A Randomized Phase-II Trial. Cancers 2020, 12, 2790. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Handbook, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Siewert, J.R.; Feith, M.; Stein, H.J. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: Relevance of a topographic-anatomic subclassification. J. Surg. Oncol. 2005, 90, 139–146. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Sasako, M.; Yamamoto, S.; Sano, T.; Imamura, H.; Fujitani, K.; Oshita, H.; Ito, S.; Kawashima, Y.; Fukushima, N. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br. J. Surg. 2009, 96, 1015–1022. [Google Scholar] [CrossRef]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Böttcher, K.; Siewert, J.R.; Höfler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef]
- Palmela, C.; Velho, S.; Agostinho, L.; Branco, F.; Santos, M.; Santos, M.P.; Oliveira, M.H.; Strecht, J.; Maio, R.; Cravo, M.; et al. Body Composition as a Prognostic Factor of Neoadjuvant Chemotherapy Toxicity and Outcome in Patients with Locally Advanced Gastric Cancer. J. Gastric Cancer 2017, 17, 74–87. [Google Scholar] [CrossRef]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index; area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor; independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- James, M.T.; Hemmelgarn, B.R.; Wiebe, N.; Pannu, N.; Manns, B.J.; Klarenbach, S.W.; Tonelli, M.; Alberta Kidney Disease Network. Glomerular filtration rate; proteinuria; and the incidence and consequences of acute kidney injury: A cohort study. Lancet 2010, 376, 2096–2103. [Google Scholar] [CrossRef]
- Bindroo, S.; Quintanilla Rodriguez, B.S.; Challa, H.J. Renal Failure. StatPearls Publishing. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519012/ (accessed on 16 June 2023).
- Huang, X.Z.; Yang, Y.C.; Chen, Y.; Wu, C.C.; Lin, R.F.; Wang, Z.N.; Zhang, X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis. Markers 2019, 2019, 7606128. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, B.; Nie, Z.; Pan, Y.; Lin, K.; Peng, H.; Xu, T.; Chen, X.; Hu, X.; Wu, Z.; et al. A nomogram based on serum bilirubin and albumin levels predict survival in gastric cancer patients. Oncotarget 2017, 8, 41305–41318. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; He, B.; Liu, X.; Yue, J.; Ying, H.; Pan, Y.; Sun, H.; Chen, J.; Wang, F.; Gao, T.; et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J. Transl. Med. 2015, 13, 66. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Zhang, L.; Xue, Y.; Zhang, S.; Li, X.; Song, H. In Gastric Cancer Patients Receiving Neoadjuvant Chemotherapy Systemic Inflammation Response Index is a Useful Prognostic Indicator. Pathol. Oncol. Res. 2021, 27, 1609811. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, Y.; Cao, H.; Zhu, H.; Chen, B.; Ji, W. Nomogram Based on Systemic Immune-Inflammation Index to Predict Overall Survival in Gastric Cancer Patients. Dis. Markers 2018, 2018, 1787424. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.C.; Jia, Z.F.; Cao, D.H.; Wu, Y.H.; Jiang, J.; Wen, S.M.; Zhao, D.; Zhang, S.L.; Cao, X.Y. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine 2018, 97, e13896. [Google Scholar] [CrossRef]
- Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol. Ther. 2016, 157, 112–119. [Google Scholar] [CrossRef]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Song, G.M.; Tian, X.; Liang, H.; Yi, L.J.; Zhou, J.G.; Zeng, Z.; Shuai, T.; Ou, Y.X.; Zhang, L.; Wang, Y. Role of Enteral Immunonutrition in Patients Undergoing Surgery for Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2015, 94, e1311. [Google Scholar] [CrossRef]
- Kanda, M.; Mizuno, A.; Tanaka, C.; Kobayashi, D.; Fujiwara, M.; Iwata, N.; Hayashi, M.; Yamada, S.; Nakayama, G.; Fujii, T.; et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine 2016, 95, e3781. [Google Scholar] [CrossRef]
- Allen, S.K.; Brown, V.; White, D.; King, D.; Hunt, J.; Wainwright, J.; Emery, A.; Hodge, E.; Kehinde, A.; Prabhu, P.; et al. Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: Effect on cardiopulmonary exercise test performance; muscle mass and quality of life-a pilot randomized clinical trial. Ann. Surg. Oncol. 2022, 29, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Bagnacci, G.; Gentili, F.; Nigri, A.; Pelini, V.; Vindigni, C.; Mazzei, F.G.; Baiocchi, G.L.; Pittiani, F.; Morgagni, P.; et al. Gastric Cancer Maximum Tumour Diameter Reduction Rate at CT Examination as a Radiological Index for Predicting Histopathological Regression after Neoadjuvant Treatment: A Multicentre GIRCG Study. Gastroenterol. Res. Pract. 2018, 2018, 1794524. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; van Haaren, M.; Harlaar, J.J.; Park, H.C.; Bonjer, H.J.; Jeekel, J.; Zwaginga, J.J.; Schipperus, M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kanda, M.; Tanaka, C.; Kobayashi, D.; Mizuno, A.; Iwata, N.; Hayashi, M.; Niwa, Y.; Takami, H.; Yamada, S.; et al. Usefulness of preoperative estimated glomerular filtration rate to predict complications after curative gastrectomy in patients with clinical T2-4 gastric cancer. Gastric Cancer 2017, 20, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality; composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan. 2014, 3, 9. [Google Scholar] [CrossRef]
- Joglekar, S.; Asghar, A.; Mott, S.L.; Johnson, B.E.; Button, A.M.; Clark, E.; Mezhir, J.J. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J. Surg. Oncol. 2015, 111, 771–775. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef]
- Reisinger, K.W.; Bosmans, J.W.; Uittenbogaart, M.; Alsoumali, A.; Poeze, M.; Sosef, M.N.; Derikx, J.P. Loss of Skeletal Muscle Mass During Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann. Surg. Oncol. 2015, 22, 4445–4452. [Google Scholar] [CrossRef]
- Wagner, D.; DeMarco, M.M.; Amini, N.; Buttner, S.; Segev, D.; Gani, F.; Pawlik, T.M. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J. Gastrointest. Surg. 2016, 8, 27–40. [Google Scholar] [CrossRef]
- Huang, D.D.; Wang, S.L.; Zhuang, C.L.; Zheng, B.S.; Lu, J.X.; Chen, F.F.; Zhou, C.J.; Shen, X.; Yu, Z. Sarcopenia; as defined by low muscle mass; strength and physical performance; predicts complications after surgery for colorectal cancer. Colorectal. Dis. 2015, 17, O256–O264. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Tan, B.H.; Cui, H.; Bhalla, A.; Fearon, K.C.; Parsons, S.L.; Catton, J.A.; Lobo, D.N. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin. Nutr. 2012, 31, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef]
- Ali, R.; Baracos, V.E.; Sawyer, M.B.; Bianchi, L.; Roberts, S.; Assenat, E.; Mollevi, C.; Senesse, P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016, 5, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Damrauer, J.S.; Stadler, M.E.; Acharyya, S.; Baldwin, A.S.; Couch, M.E.; Guttridge, D.C. Chemotherapy-induced muscle wasting: Association with NF-κB and cancer cachexia. Eur. J. Transl. Myol. 2018, 28, 7590. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J.; Catton, J.A. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur. J. Surg. Oncol. 2015, 41, 333–338. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Liu, H.; Zhong, D.; Liu, W.; Shan, G.; Dong, F.; Gao, W.; Bai, C.; Li, X. A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br. J. Cancer 2016, 114, 1326–1333. [Google Scholar] [CrossRef]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. The value of neutrophil-to-lymphocyte ratio for response and prognostic effect of neoadjuvant chemotherapy in solid tumors: A systematic review and meta-analysis. J. Cancer 2018, 9, 861–871. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, X.; Yu, S.; Wang, Y.; Hou, J.; Li, Q.; Shen, Z.; Liu, T.; Cui, Y. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. Onco. Targets Ther. 2018, 11, 7061–7075. [Google Scholar] [CrossRef]
- Rinninela, E.; Strippoli, A.; Cintoni, M.; Raoul, P.; Vivolo, R.; Di Salvatore, M.; Genco, E.; Manfredi, R.; Bria, E.; Tortora, G.; et al. Body Composition Changes in Gastric Cancer Patients during Preoperative FLOT Therapy: Preliminary Results of an Italian Cohort Study. Nutrients 2021, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Motoori, M.; Fujitani, K.; Nishizawa, Y.; Komatsu, H.; Miyazaki, Y.; Miyazaki, S.; Tomokuni, A.; Komori, T.; Iwase, K. Correlation between Skeletal Muscle Mass and Adverse Events of Neoadjuvant Chemotherapy in Patients with Gastric Cancer. Oncology 2020, 98, 29–34. [Google Scholar] [CrossRef] [PubMed]

| Covariates | N° of Cases (%) | Univariable Models | Multivariable Model | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Treatment | |||||
| CF/FOLFOX4/6 | 21 (25.0) | 5.10 (1.02–25.5) | 0.047 | 6.15 (0.94–39.97) | 0.057 |
| DOC | 44 (52.4) | 1.00 (0.27–3.77) | 1.000 | 1.27 (0.26–6.19) | 0.770 |
| ECX/ECF/EOX | 8 (9.5) | 0.40 (0.05–2.93) | 0.367 | 0.40 (0.04–3.68) | 0.419 |
| FLOT | 11 (13.1) | 1.00 | 1.00 | ||
| PLR variation variation from baseline to 2nd * | |||||
| Both lower than cut off | 22 (26.8) | 1.00 | 1.00 | ||
| From lower to higher | 17 (20.7) | 2.99 (0.79–11.42) | 0.107 | 4.64 (1.02–21.02) | 0.047 |
| From higher to lower | 7 (8.5) | 1.99 (0.34–11.70) | 0.442 | 1.18 (0.13–10.52) | 0.881 |
| Both higher than cut off | 36 (44.0) | 5.33 (1.66–17.12) | 0.005 | 5.03 (1.34–18.89) | 0.017 |
| Monocyte variation from baseline to 2nd * | |||||
| Decrease | 24 (29.3) | 1.00 | 1.00 | ||
| Increase | 58 (70.7) | 0.24 (0.08–0.68) | 0.008 | 0.38 (0.09–1.51) | 0.171 |
| Adverse Event | No Cycle Completed n = 44 (%) | All Completed Cycles n = 40 (%) | ||
|---|---|---|---|---|
| G1-G2 | G3-G4 | G1-G2 | G3-G4 | |
| Neutropenia | 5 (10.2) | 15 (30.6) | 6 (17.1) | 11 (31.4) |
| Interruption due to toxicity | 3 | 3 | - | - |
| Febrile neutropenia | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| Interruption due to toxicity | 0 | 1 | - | - |
| Leukopenia | 1 (2.0) | 3 (6.1) | 1 (2.9) | 0 (0.0) |
| Interruption due to toxicity | 1 | 1 | - | - |
| Anemia | 4 (8.2) | 1 (2.0) | 2 (5.7) | 1 (2.9) |
| Interruption due to toxicity | 1 | 0 | - | - |
| Thrombocytopenia | 2 (4.1) | 0 (0.0) | 3 (8.6) | 0 (0.0) |
| Interruption due to toxicity | 2 | 0 | - | - |
| Asthenia | 13 (26.5) | 4 (8.2) | 13 (37.1) | 4 (11.4) |
| Interruption due to toxicity | 8 | 1 | - | - |
| Fever | 1 (2.0) | 0 (0.0) | 3 (8.6) | 0 (0.0) |
| Interruption due to toxicity | 0 | 0 | - | - |
| Nausea | 13 (26.5) | 1 (2.0) | 12 (34.3) | 2 (5.7) |
| Interruption due to toxicity | 5 | 0 | - | - |
| Vomiting | 6 (12.2) | 1 (2.0) | 8 (22.9) | 1 (2.9) |
| Interruption due to toxicity | 2 | 0 | - | - |
| Diarrhea | 16 (32.7) | 4 (8.2) | 16 (45.7) | 3 (8.6) |
| Interruption due to toxicity | 4 | 1 | - | - |
| Stomatitis | 9 (18.4) | 0 (0.0) | 7 (20.0) | 0 (0.0) |
| Interruption due to toxicity | 2 | 0 | - | - |
| Rash | 2 (4.1) | 0 (0.0) | 1 (2.9) | 0 (0.0) |
| Interruption due to toxicity | 0 | 0 | - | - |
| Pruritus | 1 (2.0) | 0 (0.0) | 1 (2.9) | 0 (0.0) |
| Interruption due to toxicity | 0 | 0 | - | - |
| Bronchospasm | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) |
| Interruption due to toxicity | 0 | 0 | - | - |
| Edema | 3 (6.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Interruption due to toxicity | 1 | 0 | - | - |
| Paresthesia | 5 (10.2) | 0 (0.0) | 5 (14.3) | 0 (0.0) |
| Interruption due to toxicity | 2 | 0 | - | - |
| Hand-foot syndrome | 3 (6.1) | 1 (2.0 | 4 (11.4) | 1 (2.9) |
| Interruption due to toxicity | 1 | 1 | - | - |
| Weight | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Interruption due to toxicity | 1 | 0 | - | - |
| Liver | 2 (4.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Interruption due to toxicity | 1 | 0 | - | - |
| Renal | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Interruption due to toxicity | 1 | 0 | - | - |
| Pain | 5 (10.2) | 0 (0.0) | 10 (28.6) | 0 (0.0) |
| Interruption due to toxicity | 1 | 0 | - | - |
| Variable | No Cycle Completed n = 39 (%) | All Completed Cycles n = 36 (%) | Overall n = 75 (%) | p-Value |
|---|---|---|---|---|
| SMI | ||||
| Reduced or equal | 31 (79.5) | 28 (77.8) | 59 (78.7) | 0.857 |
| Increased | 8 (20.5) | 8 (22.2) | 16 (21.3) | |
| VATI | ||||
| Reduced or equal | 22 (56.4) | 27 (75.0) | 49 (65.3) | 0.091 |
| Increased | 17 (43.6) | 9 (25.0) | 26 (34.7) | |
| SATI | ||||
| Reduced or equal | 24 (61.5) | 25 (69.4) | 49 (65.3) | 0.472 |
| Increased | 15 (38.5) | 11 (30.6) | 26 (34.7) | |
| IMATI | ||||
| Reduced or equal | 16 (41.0) | 14 (38.9) | 30 (40.0) | 0.850 |
| Increased | 23 (59.0) | 22 (61.1) | 45 (60.0) |
| Variable | Becker 1 n = 19 (%) | Becker 2 n = 23 (%) | Becker 3 n = 29 (%) | Overall * n = 71 (%) | p-Value |
|---|---|---|---|---|---|
| Treatment compliance | |||||
| Incomplete compliance | 8 (42.1) | 10 (43.5) | 18 (62.1) | 36 (50.7) | 0.281 |
| Total compliance | 11 (57.9) | 13 (56.5) | 11 (37.9) | 35 (49.3) | |
| Treatment adherence | |||||
| All completed cycles | 11 (57.9) | 13 (56.5) | 11 (38.0) | 35 (49.2) | 0.172 |
| Incomplete for toxicity | 4 (21.1) | 4 (17.4) | 3 (10.3) | 11 (15.5) | |
| Incomplete for patient decision | 3 (15.8) | 1 (4.4) | 2 (6.9) | 6 (8.5) | |
| Incomplete for investigator decision | 0 (0.0) | 3 (13.0) | 3 (10.3) | 6 (8.5) | |
| Incomplete for other reasons (miscellaneous) | 1 (5.2) | 2 (8.7) | 10 (34.5) | 13 (18.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, M.; Prochowski Iamurri, A.; Bianchini, D.; Gallio, C.; Esposito, L.; Montanari, D.; Ruscelli, S.; Molinari, C.; Foca, F.; Passardi, A.; et al. Association between Pre-Treatment Biological Indicators and Compliance to Neoadjuvant/Perioperative Chemotherapy in Operable Gastric Cancer. Nutrients 2023, 15, 3604. https://doi.org/10.3390/nu15163604
Monti M, Prochowski Iamurri A, Bianchini D, Gallio C, Esposito L, Montanari D, Ruscelli S, Molinari C, Foca F, Passardi A, et al. Association between Pre-Treatment Biological Indicators and Compliance to Neoadjuvant/Perioperative Chemotherapy in Operable Gastric Cancer. Nutrients. 2023; 15(16):3604. https://doi.org/10.3390/nu15163604
Chicago/Turabian StyleMonti, Manlio, Andrea Prochowski Iamurri, David Bianchini, Chiara Gallio, Luca Esposito, Daniela Montanari, Silvia Ruscelli, Chiara Molinari, Flavia Foca, Alessandro Passardi, and et al. 2023. "Association between Pre-Treatment Biological Indicators and Compliance to Neoadjuvant/Perioperative Chemotherapy in Operable Gastric Cancer" Nutrients 15, no. 16: 3604. https://doi.org/10.3390/nu15163604
APA StyleMonti, M., Prochowski Iamurri, A., Bianchini, D., Gallio, C., Esposito, L., Montanari, D., Ruscelli, S., Molinari, C., Foca, F., Passardi, A., Vittimberga, G., Morgagni, P., & Frassineti, G. L. (2023). Association between Pre-Treatment Biological Indicators and Compliance to Neoadjuvant/Perioperative Chemotherapy in Operable Gastric Cancer. Nutrients, 15(16), 3604. https://doi.org/10.3390/nu15163604








