A Scoping Review of Nutritional Biomarkers Associated with Food Security
Abstract
1. Introduction
2. Materials and Methods
2.1. Conducting the Search
2.2. Study Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Analysis
3. Results
3.1. Study Characteristics
3.2. Associations between Food Insecurity and Biomarkers
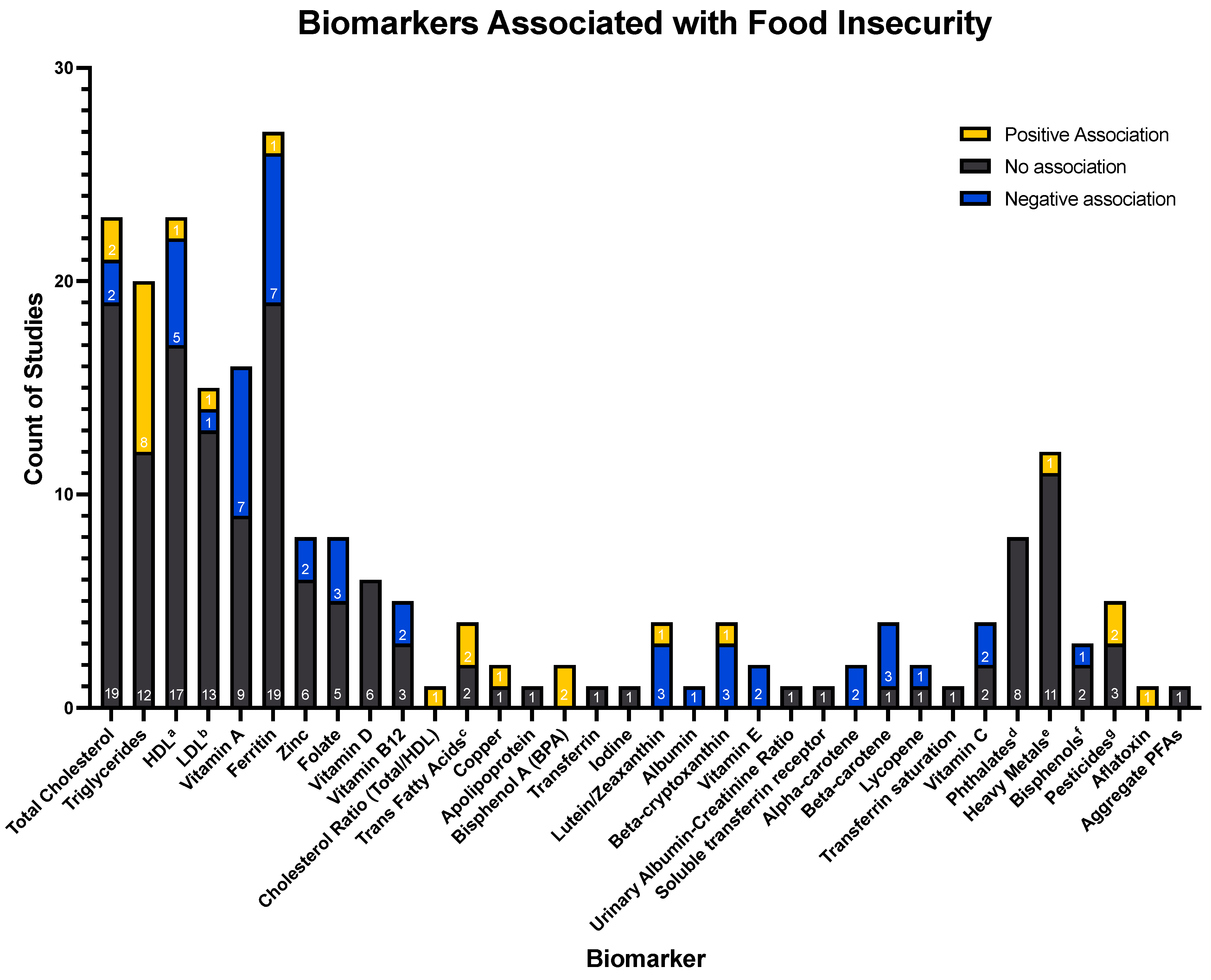
- Key:
- a = High-density lipoprotein;
- b = Low-density lipoprotein;
- c = Associations of trans fatty acids: Trans-9-hexadecenoic acid (0), Trans-11-octadecenoic acid (0), Trans-9-octadecenoic acid (+) and trans-9, Trans-12-octadecenoic acid (+);
- d = Associations of phthalates: Mono-2-ethylhexyl phthalate (0), Mono-(2-ethyl-5-oxohexyl) phthalate (0), Mono-(2-ethyl-5-hydroxyhexyl) phthalate (0), Mono-(2-ethyl-5-carboxypentyl) phthalate (0), Mono-n-butyl phthalate (0), Monoisobutyl phthalate (0), Mono-benzyl phthalate (0), Mono-ethyl phthalate (0);
- e = Associations of heavy metals: Antimony (+), Barium (0), Beryllium (0), Cadmium (0), Cesium (0), Molybdenum (0), Lead (0), Platinum (0), Thallium (0), Tungsten (0), Uranium (0);
- f = Associations of bisphenols: 4-tert-octylphenol (0), Benzophenone-3 (−), Triclosan (0);
- g = Associations of pesticides: 2,5-dichlorophenol (0), O-phenyl phenol (0), 2,4-dichlorophenol (0), 2,4,5-trichlorophenol (+), 2,4,6-trichlorophenol (+);
- h = Aggregate per- and poly-fluoroalkyl substances, not separated by category (perfluoronanoic acid (PFNA), perfluorooctanesulfonic (PFOS) acid, perfluorooctanoic acid (PFOA), methyl-perfluorooctane sulfonamide acetic acid (Me-PFOSA-AcOH), and perfluorohexanesulphonic acid (PFHxS).
4. Discussion
4.1. Lipid-Related Markers—Total Cholesterol, HDL, LDL, and Triglycerides
4.2. Zinc
4.3. Ferritin
4.4. Vitamin A
4.5. Folate
4.6. Vitamin D
4.7. Vitamin B12
4.8. General
4.9. Strengths and Limitations
4.10. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Author—Year | Country | Population Description | Population Size (N) | Population Age (SD) | % Female | Name of Food Security Assessment Tool | Source for Biomarker Assay | Fasting Status | Biomarker(s) Measured |
|---|---|---|---|---|---|---|---|---|---|
| Abdurahman—2021 [21] | Iran | Iranian adults with obesity | 300 | 43.4 (10.9) years | 84.1% | USDA a Household Food Security Status Questionnaire (modified for Iran) | Serum | Fasted | HDL b; Triglycerides |
| Akelo—2014 [22] | Kenya | Pregnant Kenyan women attending their first antenatal care visit | 505 | 24 years (median) | 100% | USAID c Food and Nutrition Technical Assistance Project (FANTA) Household Food Insecurity Assessment Scale (HFIAS) | Serum | Nonfasted | Ferritin; Transferrin; Retinol-binding protein |
| Arango—2021 [23] | Colombia | Pregnant individuals | 664 | 21 (7) years | 100% | La Escala Latinoamericana y Caribeña de Seguridad Alimentaria (Latin American and Caribbean Food Security Scale (ELCSA)) | Serum | Fasted | Ferritin |
| Baxter—2021 [24] | Pakistan | Unmarried and married, non-pregnant young women (15–23 years) living in rural Pakistan | 3461 | 17.2 (1.2) years | 100% | Household Food Insecurity Access Scale (HFIAS) | Serum | NR | Vitamin A; Ferritin; Vitamin D |
| Bayoumi—2020 [25] | Canada | Children aged 12–29 months | 1245 | 18.1 months | 47.8% | NutriSTEP questionnaire | Serum | NR | Ferritin |
| Carneiro—2020 [26] | Brazil | Representative sample of the population of children aged 6–59 months, treated at basic health units in the city of Rio de Janeiro, Brazil | 519 | 6–23 months: 31% of population 24–59 months: 69% of population | 49.8% | Brazilian Food Insecurity Scale | Serum | NR | Vitamin A |
| Chitekwe—2022 [27] | Nepal | Children aged 6–59 months | 1709 | NR | 46.3% | Household Food Insecurity (HFI) Scale (included in the Nepal National Micronutrient Status Survey) | Serum | Fasted | Vitamin A |
| Corona—2021 [28] | United States | Predominately low-income, urban Black women | 459 | 60.7 (12.93) years | 81.7% | Custom: 90 min structured interview that included questions about food access | Blood (not specified) | NR | HDL; Total cholesterol |
| Crook—2021 [29] | United States | Adults aged 20 years or older participating in NHANES d | 7607 | 20–39 years: 37.5% of population 40–59 years: 40.1% of population ≥60 years: 22.4% of population | 51.3% | Single question from USDA Household Food Security Survey Module | Serum | NR | Vitamin C |
| de Oliveira Campos—2016 [30] | Brazil | School children in Bahia, Brazil aged 6–14 | 1419 | 11 (2.83) years | 48.7% | Brazilian scale of food insecurity | Urine | NR | Iodine |
| Dixon—2001 [31] | United States | Adults aged 20 years or older participating in NHANES III who were interviewed at home and examined at the mobile examination center | 10,165 | NR | 52.1% | NHANES III Food Insufficiency Data | Serum | Fasted | Vitamin A; Vitamin B12; Vitamin B9; Vitamin C; HDL; LDL; e Triglycerides; Total cholesterol; Ferritin; Albumin, Alpha-carotene, Beta-carotene, Beta-cryptoxanthin, Lutein/zeaxanthin, Lycopene, Vitamin E |
| Dong—2021 [32] | United States | Urban American Indian/Alaska Native (AI/AN) youth | 142 | 14 years | 58.0% | Child Food Security Survey Module | Blood (not specified) | NR | Triglycerides; Total cholesterol |
| Duncan—2018 [33] | Canada | Non-pregnant women of reproductive age from Canadian Inuit communities | 249 | 29.1 (6.0) years | 100% | USDA Household Food Security Survey Module | Whole blood | Fasted | Vitamin B9 |
| Egeland—2011 [34] | Canada | Canadian Inuit populations | 2595 | 41 (14.7) years | 62.0% | USDA Household Food Security Survey Module | Serum | Fasted | Ferritin |
| Egeland—2011 [35] | Canada | Indigenous Inuit preschoolers in 16 of the 25 communities of Nunavut | 388 | NR | NR | USDA Household Food Security Survey module, modified by Indian and Northern Affairs Canada for Inuit communities | Plasma | NR | Ferritin |
| Eicher-Miller—2009 [36] | United States | Nonpregnant children and adolescents from 3 to 19 years old who successfully completed the household interview and participated in the MEC examination | 11,247 | NR | 45.1% | USDA Household Food Security Survey Module | Serum | NR | Ferritin |
| Eick—2022 [37] | United States | Pregnant individuals in the Chemicals In Our Bodies (CIOB) cohort | 497 | NR | 100% | Household Food Security Scale (short form) | Serum | NR | PFAS f (PFNA g, PFOS h acid, PFOA i, methyl-perfluorooctane sulfonamide acetic acid (Me-PFOSA-AcOH), and perfluorohexanesulphonic acid (PFHxS) |
| Faramarzi—2019 [38] | Iran | Subjects who had participated in Azar cohort study | 152 | 52.88 (9.08) years | NR | Household Food Insecurity Access Scale | Serum and whole blood | Fasted | Vitamin A; HDL; Triglycerides |
| Ford—2013 [39] | United States | Adults ≥ 20 years old participating in NHANES | 10,455 | 43.8 years (median) | 49.8% | USDA Household Food Security Survey Module | Whole blood | Fasted | HDL; Total cholesterol; urinary albumin-creatine ratio, non-HDL cholesterol |
| Fulay—2021 [40] | United States | Adolescents (12–17 years old) with household incomes at or below 300% federal poverty line | 2876 | NR | 49.1% | USDA Household Food Security Survey Module | Whole blood | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Ganpule-Rao—2020 [41] | India | 18-year-old children living in one of six rural villages near Pune, Maharashtra, India, born to mothers studied in the larger PMNSj study | 418 | 18 years | 46.7% | Number and type of food shops/1000 population, water availability, and distance from the highway | Plasma | Fasted | Vitamin B12 |
| Gebreegziabher—2017 [42] | Ethiopia | Non-pregnant women aged 18 and older in rural communities of Sidama, southern Ethiopia | 202 | 30.8 (7.8) years | 100% | Household Food Security Access Scale (HFIAS) | Plasma and whole blood | Fasted | Ferritin; Transferrin; Plasma iron, Soluble transferrin receptors |
| Gebreegziabher—2022 [43] | Ethiopia | School-aged children | 408 | 9 (1.8) years | 50.5% | Household Food Insecurity Access Scale (HFIAS) and Household Hunger Scale (HHS) | Urine | NR | Aflatoxin M1 |
| Gebremedhin—2011 [44] | Ethiopia | Pregnant women | 700 | 28.5 (5.5) years | 100% | Household Food Insecurity Access Scale (HFIAS) | Serum | Both fasted and nonfasted | Zinc |
| Gowda—2012 [45] | United States | Adults ≥ 18 years old participating in NHANES | 12,191 | NR | NR | USDA Household Food Insecurity Survey | Whole blood | Fasted | Vitamin B9 |
| Gubert—2016 [46] | Brazil | Brazilian children under 5 years old | 4064 | NR | NR | Brazilian Household Food Insecurity Measurement Scale | Whole blood | NR | Vitamin A |
| Habib—2016 [47] | Pakistan | Pakistani children aged 6–59 months | 7138 | NR | 48.3% | Household food insecurity access scale (HFIAS) developed by Food and Nutrition Technical Assistance (FANTA) project | Serum | NR | Vitamin A; Ferritin; Zinc |
| Habib—2018 [48] | Pakistan | Non-pregnant Pakistani women | 7491 | NR | 100% | Household food insecurity access scale (HFIAS) developed by Food and Nutrition Technical Assistance (FANTA) project | Serum | NR | Vitamin A; Ferritin; Zinc |
| Hamedi-Shahraki—2021 [49] | Iran | Women of reproductive age at health centers affiliated with Zabol University of Medical Sciences | 630 | 33.2 (7.8) years | 100% | Household Food Insecurity Access Scale (HFIAS) questionnaire validated in an Iranian population | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Hanson—2018 [50] | United States | Pregnant women recruited from the Labor and Delivery unit in a Midwestern United States Academic Medical Center at the time of delivery | 180 | 28.7 (5.6) years | 100% | US Household Food Security Survey Module (US HFSSM); USDA Economic Research Service national food desert database at the census-tract level | Serum | Nonfasted | Vitamin A; Vitamin E; Beta-cryptoxanthin; Lutein/zeaxanthin; Beta-carotene; Lycopene |
| Herran—2015 [51] | Colombia | Children and women (pregnant and non-pregnant) | 9024 | NR | 50.4% of kids, 100% of pregnant women | Community Childhood Hunger Identification Project (modified version) | Serum | NR | Vitamin B12 |
| Holben—2006 [52] | United States | Adults ≥ 18 years old from 6 rural counties in Ohio | 2580 | 44.7 (18) years | 66.1% | USDA Household Food Security Survey Module | Whole blood | Nonfasted | Total cholesterol |
| Jamieson—2011 [53] | Canada | Non-pregnant Inuk women ≥ 18 years old from Arctic Canada | 697 | 42 (15) years | 100% | USDA Household Food Security Survey Module | Serum | Fasted | Ferritin |
| Jamieson—2012 [54] | Canada | Inuit men | 880 | 42 (15) years | 0.0% | USDA Household Food Security Survey Module | Whole blood | Fasted | Ferritin |
| Jamieson—2013 [55] | Canada | Non-pregnant, self-identified Inuk women | 1550 | 42 (15) years | 100% | USDA Household Food Security Survey Module | Serum | Fasted | Ferritin |
| Janmohamed—2020 [56] | Mongolia | Mongolian children aged 6–23 months | 938 | NR | 50.9% | Household Food Insecurity Access Scale | Serum | NR | Vitamin A; Ferritin; Vitamin D |
| Jun—2021 [57] | United States | US children aged 1–18 years | 9147 | NR | 49.4% | USDA Household Food Security Survey Module | Whole Blood | Fasted | Vitamin B9; Ferritin; Zinc; Vitamin D |
| Kazemi—2020 [58] | Azerbaijan | Women of reproductive age | 266 | 40.93 (11.1) years | 100% | Household Food Security Scale (short form) | Serum | Fasted | Ferritin; Vitamin D |
| Kelli—2017 [59] | United States | Adults aged 20 to 79 years and residing in the Atlanta metropolitan area participating in the META-Health k study or the Predictive Health study | 1421 | 49.4 (10.2) years | 61.5% | USDA Food Access Research Atlas | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Kim—2020 [60] | United States | Adults 30–70 years old who self-identified as Black or African American from local communities of the Atlanta-Sandy Springs-Alpharetta, Georgia, metropolitan area | 394 | 52.8 (10.3) years | 61.0% | Neighborhood Health Questionnaire | Plasma | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Kobrosly—2012 [61] | United States | Women aged 20–39 years participating in NHANES | 1182 | 29 years (median) | 100% | USDA Household Food Security Survey Module | Urine | Adjusted for fasting time | Phthalates: four metabolites of DEHP:l mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP); two metabolites of DBP: m mono-n-butyl phthalate (MnBP), and monoisobutyl phthalate (MiBP); one metabolite of BzBP: n mono-benzyl phthalate (MBzP); one metabolite of DEP: o mono-ethyl phthalate (MEP) |
| Landry—2019 [62] | United States | Hispanic/Latino children in elementary schools that offered the after-school program “LA’s Best” | 218 | Food-secure population: 9.3 (0.9) years Food-insecure population: 8.9 (0.8) years | 50.9% | Child Food Security Assessment (CFSA) | Blood (not specified) | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Leyna—2010 [63] | Tanzania | Adults in Oria Village | 1014 | NR | 69.1% | Radimer/Cornell food insecurity measure (validated in rural Kilimanjaro, Tanzania) | Plasma | NR | Vitamin A; Ferritin |
| Li—2017 [64] | Colombia | Colombian children | 14,559 had ferritin levels analyzed, 4279 had zinc levels analyzed, 3844 had vitamin A levels analyzed | NR | 61.1% (ferritin) | Community Childhood Hunger Identification Project (adapted for and validated in a Colombian population) | Plasma and serum | NR | Vitamin A; Ferritin; Zinc |
| Mahfuz—2019 [65] | Bangladesh | Children with low socioeconomic status and limited sanitary conditions in a slum settlement in Bauniabadh in the Mirpur area of Dhaka city | 153 had ferritin levels analyzed, 154 had zinc levels analyzed, 155 had retinol levels analyzed | 7 months | 52.4% | Household Food Insecurity Access Scale (HFIAS) developed by the Food and Nutrition Technical Assistance (FANTA) project | Plasma | NR | Vitamin A; Ferritin; Zinc |
| Maldonado—2022 [66] | United States | Hispanic/Latino youth aged 8 to 16 years | 1325 | High food security population: 12.2 (2.6) years Marginal food security population: 11.7 (2.7) years Low food security population: 12.4 (2.5) years Very low food security population: 12.3 (2.6) years | 50% | USDA Household Food Security Survey Module | Serum | NR | HDL; Triglycerides |
| Marín—2021 [67] | Colombia | Bogotá school cohort formed in February 2006 by randomly recruiting 3202 children from 5 to 12 years who studied in the public schools of Bogotá and belonged to the middle and low socioeconomic strata | 2660 | 8.7 (1.8) years | 49.6% | Household Food Security Survey Module (HFSSM) validated in Spanish | Serum | NR | Vitamin A; Vitamin B12; Vitamin B9; Ferritin; Zinc |
| Mazidi—2018 [68] | United States | Adults ≥ 18 years old | 3876 | 48.1 years | 51.4% | USDA Household Food Security Survey Module | Plasma | NR | Trans-9-hexadecenoic acid; Trans-11-octadecenoic acid; trans-9-octadecenoic acid; trans-9-, trans-12-octadecenoic acid |
| Mohajeri—2022 [69] | Iran | Adults aged 35–65 years selected by random sampling from the participants of the Persian Cohort Study | 500 | 45 years | 62.4% | USDA Household Food Security Survey Module | Serum | Fasted | LDL; Triglycerides; Total cholesterol |
| Mohamadpour—2012 [70] | Malaysia | Indian women working on palm plantations | 147 | NR | 100% | Radimer/Cornell Hunger and Food Insecurity Instrument | Serum | Fasted | HDL; LDL; Total cholesterol |
| Murillo-Castillo—2018 [71] | Mexico | Mothers of children attending elementary school (grades 1–5) in Kino Bay, a fishing community located in Northwest Mexico | 116 | 36.4 (8.9) years | 100% | Mexican Scale of Food Security | Serum | Fasted | HDL; Total cholesterol |
| Nguyen—2014 [72] | Vietnam | Women of reproductive age living in the mountainous region of Northeast Vietnam | 4986 | 26.2 (4.6) years | 100% | Household Food Insecurity Access Scale | Serum | NR | Ferritin |
| Nikniaz—2018 [73] | Iran | Individuals from districts of East Azerbaijan | 253 | Men: 43.56 (14.15) years Women: 41.14 (11.16) years | 55.7% | Household Food Security Scale (short form) | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Nur Atiqah—2015 [74] | Malaysia | Students from all departments of Universiti Teknologi MARA Puncak Alam | 124 | NR | 87.9% | US Adult Food Security Survey Module | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Pak—2021 [75] | United States | Individuals aged 50–100 years in the 2006–2014 Health and Retirement Study cohort | 14,394 | 60 (IQR 56–69) years | 56.6% | Two Item Household Food Insecurity Screener | Whole blood | NR | HDL; Total cholesterol |
| Park—2014 [76] | United States | Pregnant women | 1045 | NR | 100% | USDA Food Security Survey Module | Serum | NR | Ferritin; Transferrin |
| Park—2020 [77] | United States | Female adults | 4249 | NR | 100% | USDA Household Food Security Scale | Serum | Fasted | HDL; Triglycerides |
| Pasricha—2010 [78] | India | Children aged 12 to 23 months in 2 rural districts of Karnataka, India | 401 | 17.2 months (IQR 16.8–17.5) | 49.7% | Household Food Insecurity Access Scale | Serum | NR | Vitamin A; Vitamin B12; Vitamin B9; Ferritin |
| Pasricha—2011 [79] | India | Children 12–23 months old in rural India | 405 | 17.2 months (IQR 16.8–17.5) | 49.6% | Household Food Insecurity Access Scale | Serum | NR | Ferritin |
| Pedraza—2014 [80] | Brazil | Preschool children attending public children’s gardens of the government of the State of Paraíba, Brazil | 193 | NR | NR | Brazilian Food Insecurity Scale | Serum | NR | Vitamin A; Zinc |
| Pinzón-Rondón—2019 [81] | Colombia | Columbian children 12–59 months | 4275 | 2.66 (1.09) years | 46.8% | Latin-American and Caribbean household food security scale (ECLA) | Serum | Nonfasted | Zinc |
| Pirkle—2014 [82] | Canada | Inuit children from Nunavik whose mothers participated in either the Cord Blood Monitoring Program or Environmental Contaminants and Infant Development Study | 294 | 11.3 (0.8) years | 50.3% | USDA Household Food Security Survey Module | Serum | NR | Ferritin |
| Pobee—2020 [83] | Ghana | Ghanian women planning to become pregnant | 95 | NR | 100% | USDA Household Food Security Survey Module | Serum and plasma | NR | Vitamin A; Ferritin; Transferrin; Zinc; Vitamin D; Copper |
| Sahyoun—2000 [84] | United States | Adults aged 65 years and older participating in NHANES | 3855 | 72.3 years | 52.0% | Custom: One-question about food sufficiency | Serum | NR | Vitamin B9; Vitamin C; Lutein/zeaxanthin; beta-cryptoxanthin; beta-carotene; Vitamin E |
| Salarkia—2015 [85] | Iran | Children of Iranian mother-child pairs | 423 | 15.1 (5.7) months | 46.1% | Household Food Insecurity Access Scale | Serum | Nonfasted | Ferritin |
| Sattler—2016 [86] | United States | Adults aged 20 and older participating in NHANES | 7439 | 44.12 (12.97) years | 48.0% | USDA Household Food Security Survey Module | Serum | NR | Total cholesterol |
| Shariff—2014 [87] | Malaysia | Malay and Indian, non-pregnant, non-lactating women aged 19–49 years from rural and urban low-income households | 625 | 38 years | 100% | Radimer/Cornell Hunger and Food Insecurity Instrument | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Shin—2015 [88] | United States | Healthy adults from the 2008–2011 Survey of the Health of Wisconsin | 1663 | NR | 48.0% | Custom: Two-question food insecurity screener | Serum | Nonfasted | HDL; Total cholesterol |
| Shiue—2016 [89] | United States | Adults ≥ 20 years old | 4979 | NR | 52.1% | USDA Household Food Security Survey Module | Serum, urine | Fasted | Vitamin B9; Vitamin C; HDL; Total cholesterol; Phthalates, Vitamin D, apolipoprotein, heavy metals (barium, beryllium, cadmium, cobalt, cesium, molybdenum, lead, platinum, antimony, thallium, tungsten, uranium), bisphenols (4-tert-octylphenol, benzophenone-3, bisphenol A, triclosan), pesticides (2,5-dichlorophenol, O-phenyl phenol, 2,4-dichlorophenol, 2,4,5-trichlorophenol, 2,4,6-trichlorophenol) |
| Smalls—2020 [90] | United States | Grandparents residing in Appalachia, Kentucky, who were the primary caretakers for their grandchildren | 65 | 59.4 (7.4) years | 98.5% | USDA Household Food Security Survey Module | Whole blood | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Suarez—2015 [91] | United States | Adults aged 20 and older participating in NHANES | 22,173 | 46.7 (14.5) years | 51.9% | Food Desert US Census Tract Data | Serum | NR | Alpha-carotene; beta-carotene; beta-cryptoxanthin; Lutein/zeaxanthin |
| Tayie—2009 [92] | United States | Adults aged 18–50 participating in NHANES | 5549 | NR | 50.3% | USDA Adult Food Security Survey Module | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Tayie—2019 [93] | United States | Adults aged 18–65 participating in NHANES | 2780 | 41.6 (0.4) years | 51.5% | USDA Household Food Security Survey Module | Serum | NR | Copper |
| Tester—2016 [94] | United States | Low-income adolescents | 1072 | Food-secure population: 15 (14.9–15.1) Marginal food security population: 14.7 (14.5–14.9) Low food security population: 14.3 (14.1–14.5) Very low food security population: 14.8 (14.6–15.0) | 47.9% | USDA Household Food Security Survey Module | Serum | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| van Woerden—2019 [95] | United States | Adults aged 18 and older participating in NHANES | 9886 | NR | 50.0% | USDA Adult or Household Food Security Survey Module | Urine | NR | Bisphenol A |
| Weigel—2007 [96] | United States | Migrant and seasonal farmworkers | 100 | NR | 43.0% | USDA Household Food Security Survey Module | Whole blood | Fasted | HDL; Triglycerides; Total cholesterol |
| Weigel—2016 [97] | Ecuador | Adult female heads of households and their minor children aged 6–12 years | 794 | 34 (10.6) | 100% | Quito Household Food Security Survey Module (adapted from USDA HFSSM) | Whole blood | Fasted | HDL; LDL; Triglycerides; Total cholesterol |
| Yeudall—2007 [98] | Uganda | Children 2 to 5 years old | 296 | NR | NR | Custom: novel questionnaire developed from prior research in this region | Whole blood | Nonfasted | Vitamin A |
References
- Palakshappa, D.; Speiser, J.L.; Rosenthal, G.E.; Vitolins, M.Z. Food Insecurity Is Associated with an Increased Prevalence of Comorbid Medical Conditions in Obese Adults: NHANES 2007–2014. J. Gen. Intern. Med. 2019, 34, 1486–1493. [Google Scholar] [CrossRef]
- Cafiero, C.; Viviani, S.; Nord, M. Food security measurement in a global context: The food insecurity experience scale. Measurement 2018, 116, 146–152. [Google Scholar] [CrossRef]
- Myers, C.A.; Mire, E.F.; Katzmarzyk, P.T. Trends in Adiposity and Food Insecurity Among US Adults. JAMA Netw. Open 2020, 3, e2012767. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Rong, S.; Du, Y.; Xu, G.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Food Insecurity Is Associated With Cardiovascular and All-Cause Mortality Among Adults in the United States. J. Am. Heart Assoc. 2020, 9, e014629. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.A.; Baggett, T.P.; Wexler, D.J.; Huskey, K.W.; Wee, C.C. Food insecurity and metabolic control among U.S. Adults with diabetes. Diabetes Care 2013, 36, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Radak, T.; Khubchandani, J.; Dunn, P. Food Insecurity and Mortality in American Adults: Results From the NHANES-Linked Mortality Study. Health Promot. Pract. 2021, 22, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.T.; Frank, D.A.; Levenson, S.M.; Neault, N.B.; Heeren, T.C.; Black, M.M.; Berkowitz, C.; Casey, P.H.; Meyers, A.F.; Cutts, D.B.; et al. Child Food Insecurity Increases Risks Posed by Household Food Insecurity to Young Children’s Health. J. Nutr. 2006, 136, 1073–1076. [Google Scholar] [CrossRef]
- Petersen, C.L.; Brooks, J.M.; Titus, A.J.; Vasquez, E.; Batsis, J.A. Relationship Between Food Insecurity and Functional Limitations in Older Adults from 2005-2014 NHANES. J. Nutr. Gerontol. Geriatr. 2019, 38, 231–246. [Google Scholar] [CrossRef]
- Lauren, B.N.; Silver, E.R.; Faye, A.S.; Rogers, A.M.; Woo-Baidal, J.A.; Ozanne, E.M.; Hur, C. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public. Health Nutr. 2021, 24, 3929–3936. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fleischhacker, S.; Andrés, J.R. Prioritizing Nutrition Security in the US. JAMA 2021, 325, 1605–1606. [Google Scholar] [CrossRef]
- Leung, C.W.; Epel, E.S.; Ritchie, L.D.; Crawford, P.B.; Laraia, B.A. Food insecurity is inversely associated with diet quality of lower-income adults. J. Acad. Nutr. Diet. 2014, 114, 1943–1953.e2. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture (Ed.) USDA Actions on Nutrition Security; US Department of Agriculture: Washington, DC, USA, 2022.
- Byker Shanks, C.; Calloway, E.E.; Parks, C.A.; Yaroch, A.L. Scaling up measurement to confront food insecurity in the USA. Transl. Behav. Med. 2020, 10, 1382–1389. [Google Scholar] [CrossRef]
- Brennan, L.; Hu, F.B.; Sun, Q. Metabolomics Meets Nutritional Epidemiology: Harnessing the Potential in Metabolomics Data. Metabolites 2021, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.A.; Gao, X.; Tucker, K.L. Food-insecure dietary patterns are associated with poor longitudinal glycemic control in diabetes: Results from the boston puerto rican health study. Diabetes Care 2014, 37, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.E.; Berkowitz, S.A. The Relationship between Food Insecurity, Dietary Patterns, and Obesity. Curr. Nutr. Rep. 2016, 5, 54–60. [Google Scholar] [CrossRef]
- Rafiq, T.; Azab, S.M.; Teo, K.K.; Thabane, L.; Anand, S.S.; Morrison, K.M.; de Souza, R.J.; Britz-McKibbin, P. Nutritional Metabolomics and the Classification of Dietary Biomarker Candidates: A Critical Review. Adv. Nutr. 2021, 12, 2333–2357. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The Relationship between Dietary Polyphenol Intakes and Urinary Polyphenol Concentrations in Adults Prescribed a High Vegetable and Fruit Diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software. Available online: https://www.covidence.org/ (accessed on 15 October 2022).
- Abdurahman, A.; Bule, M.; Fallahyekt, M.; Abshirini, M.; Azadbakht, L.; Qorbani, M.; Dorosty, A. Association of diet quality and food insecurity with metabolic syndrome in obese adults. Int. J. Prev. Med. 2021, 12, 138. [Google Scholar] [CrossRef]
- Akelo, V.; Grant, F.; Okuku, H.S.; Wanjala, R.; Low, J.; Cole, D.; Levin, C.; Girard, A.W. Determinants of vitamin A status among pregnant women participating in the Mama SASHA Cohort Study of Vitamin A in Western Kenya: Preliminary findings (624.9). FASEB J. 2014, 28, 624–629. [Google Scholar] [CrossRef]
- Arango, C.M.; Molina, C.F.; Mejía, C.M. Factors associated with inadequate iron stores in women in the first trimester of pregnancy. Rev. Chil. De. Nutr. 2021, 48, 595–608. [Google Scholar] [CrossRef]
- Baxter, J.-A.B.; Wasan, Y.; Hussain, A.; Soofi, S.B.; Ahmed, I.; Bhutta, Z.A. Characterizing Micronutrient Status and Risk Factors among Late Adolescent and Young Women in Rural Pakistan: A Cross-Sectional Assessment of the MaPPS Trial. Nutrients 2021, 13, 1237. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, I.; Parkin, P.C.; Birken, C.S.; Maguire, J.L.; Borkhoff, C.M. Association of Family Income and Risk of Food Insecurity With Iron Status in Young Children. JAMA Netw. Open 2020, 3, e208603. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.B.V.; Castro, I.R.R.D.; Juvanhol, L.L.; Gomes, F.D.S.; Cardoso, L.D.O. Association between food insecurity and hemoglobin and retinol levels in children treated in the Brazilian Unified National Health System in the city of Rio de Janeiro, Brazil. Cad. Saude Publica 2019, 36, e00243418. [Google Scholar] [CrossRef]
- Chitekwe, S.; Parajuli, K.R.; Paudyal, N.; Haag, K.C.; Renzaho, A.; Issaka, A.; Agho, K. Individual, household and national factors associated with iron, vitamin A and zinc deficiencies among children aged 6–59 months in Nepal. Matern. Child. Nutr. 2022, 18, e13305. [Google Scholar] [CrossRef]
- Corona, G.; Dubowitz, T.; Troxel, W.M.; Ghosh-Dastidar, M.; Rockette-Wagner, B.; Gary-Webb, T.L. Neighborhood food environment associated with cardiometabolic health among predominately low-income, urban, black women. Ethn. Dis. 2021, 31, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Horgas, A.; Yoon, S.J.; Grundmann, O.; Johnson-Mallard, V. Insufficient vitamin c levels among adults in the united states: Results from the nhanes surveys, 2003–2006. Nutrients 2021, 13, 3910. [Google Scholar] [CrossRef]
- De Oliveira Campos, R.; Reboucas, S.C.L.; Beck, R.; De Jesus, L.R.M.; Ramos, Y.R.; Dos Santos Barreto, I.; Marques, T.X.; Cerqueira, T.L.O.; Santos, W.A.; Oliveira, C.A.; et al. Iodine Nutritional Status in Schoolchildren from Public Schools in Brazil: A Cross-Sectional Study Exposes Association with Socioeconomic Factors and Food Insecurity. Thyroid 2016, 26, 972–979. [Google Scholar] [CrossRef]
- Dixon, L.B.; Winkleby, M.A.; Radimer, K.L. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2001, 131, 1232–1246. [Google Scholar] [CrossRef]
- Dong, L.; D'Amico, E.; Dickerson, D.; Brown, R.; Palimaru, A.; Johnson, C.; Troxel, W. Food insecurity and cardiometabolic risks in urban american indian/alaska native (AI/AN) youth: The role of sleep health. Sleep. 2021, 44, A68. [Google Scholar] [CrossRef]
- Duncan, K.; Erickson, A.C.; Egeland, G.M.; Weiler, H.; Arbour, L.T. Red blood cell folate levels in Canadian Inuit women of childbearing years: Influence of food security, body mass index, smoking, education, and vitamin use. Can. J. Public. Health 2018, 109, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Egeland, G.M.; Johnson-Down, L.; Cao, Z.R.; Sheikh, N.; Weiler, H. Food insecurity and nutrition transition combine to affect nutrient intakes in canadian arctic communities. J. Nutr. 2011, 141, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Egeland, G.M.; Williamson-Bathory, L.; Johnson-Down, L.; Sobol, I. Traditional food and monetary access to market-food: Correlates of food insecurity among inuit preschoolers. Int. J. Circumpolar Health 2011, 70, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Eicher-Miller, H.A.; Mason, A.C.; Weaver, C.M.; McCabe, G.P.; Boushey, C.J. Food insecurity is associated with iron deficiency anemia in US adolescents. Am. J. Clin. Nutr. 2009, 90, 1358–1371. [Google Scholar] [CrossRef]
- Eick, S.M.; Goin, D.E.; Cushing, L.; DeMicco, E.; Smith, S.; Park, J.S.; Padula, A.M.; Woodruff, T.J.; Morello-Frosch, R. Joint effects of prenatal exposure to per- and poly-fluoroalkyl substances and psychosocial stressors on corticotropin-releasing hormone during pregnancy. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 27–36. [Google Scholar] [CrossRef]
- Faramarzi, E.; Somi, M.; Ostadrahimi, A.; Dastgiri, S.; Ghayour Nahand, M.; Asgari Jafarabadi, M.; Sanaie, S. Association between food insecurity and metabolic syndrome in North West of Iran: Azar Cohort study. J. Cardiovasc. Thorac. Res. 2019, 11, 196–202. [Google Scholar] [CrossRef]
- Ford, E.S. Food security and cardiovascular disease risk among adults in the United States: Findings from the National Health and Nutrition Examination Survey, 2003–2008. Prev. Chronic. Dis. 2013, 10, E202. [Google Scholar] [CrossRef]
- Fulay, A.P.; Vercammen, K.A.; Moran, A.J.; Rimm, E.B.; Leung, C.W. Household and child food insecurity and CVD risk factors in lower-income adolescents aged 12–17 years from the National Health and Nutrition Examination Survey (NHANES) 2007–2016. Public. Health Nutr. 2022, 25, 922–929. [Google Scholar] [CrossRef]
- Ganpule-Rao, A.V.; Roy, D.; Karandikar, B.A.; Yajnik, C.S.; Rush, E.C. Food Access and Nutritional Status of Rural Adolescents in India: Pune Maternal Nutrition Study. Am. J. Prev. Med. 2020, 58, 728–735. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Stoecker, B.J. Iron deficiency was not the major cause of anemia in rural women of reproductive age in Sidama zone, southern Ethiopia: A cross-sectional study. PLoS ONE 2017, 12, e0184742. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Dean, M.; Elias, E.; Tsegaye, W.; Stoecker, B.J. Urinary Aflatoxin M1 Concentration and Its Determinants in School-Age Children in Southern Ethiopia. Nutrients 2022, 14, 2580. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, S.; Enquselassie, F.; Umeta, M. Prevalence of prenatal zinc deficiency and its association with socio-demographic, dietary and health care related factors in rural Sidama, Southern Ethiopia: A cross-sectional study. BMC Public. Health 2011, 11, 898. [Google Scholar]
- Gowda, C.; Hadley, C.; Aiello, A.E. The Association Between Food Insecurity and Inflammation in the US Adult Population. Am. J. Public. Health 2012, 102, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Gubert, M.B.; Spaniol, A.M.; Bortolini, G.A.; Pérez-Escamilla, R. Household food insecurity, nutritional status and morbidity in Brazilian children. Public. Health Nutr. 2016, 19, 2240–2245. [Google Scholar] [CrossRef]
- Habib, M.A.; Black, K.; Soofi, S.B.; Hussain, I.; Bhatti, Z.; Bhutta, Z.A.; Raynes-Greenow, C. Prevalence and Predictors of Iron Deficiency Anemia in Children under Five Years of Age in Pakistan, A Secondary Analysis of National Nutrition Survey Data 2011–2012. PLoS ONE 2016, 11, e0155051. [Google Scholar] [CrossRef]
- Habib, M.A.; Raynes-Greenow, C.; Soofi, S.B.; Ali, N.; Nausheen, S.; Ahmed, I.; Bhutta, Z.A.; Black, K.I. Prevalence and determinants of iron deficiency anemia among non-pregnant women of reproductive age in Pakistan. Asia Pac. J. Clin. Nutr. 2018, 27, 195–203. [Google Scholar] [CrossRef]
- Hamedi-Shahraki, S.; Mir, F.; Amirkhizi, F. Food Insecurity and Cardiovascular Risk Factors among Iranian Women. Ecol. Food Nutr. 2021, 60, 163–181. [Google Scholar] [CrossRef]
- Hanson, C.; Schumacher, M.V.; Lyden, E.; Su, D.; Furtado, J.; Cammack, R.; Bereitschaft, B.; Van Ormer, M.; Needelman, H.; McGinn, E.; et al. Fat-soluble vitamins A and E and health disparities in a cohort of pregnant women at delivery. J. Nutr. Sci. 2018, 7, e14. [Google Scholar] [CrossRef]
- Herrán, O.F.; Ward, J.B.; Villamor, E. Vitamin B12 serostatus in Colombian children and adult women: Results from a nationally representative survey. Public. Health Nutr. 2015, 18, 836–843. [Google Scholar] [CrossRef]
- Holben, D.H.; Pheley, A.M. Diabetes risk and obesity in food-insecure households in rural Appalachian Ohio. Prev. Chronic Dis. 2006, 3, A82. [Google Scholar]
- Jamieson, J.A.; Weiler, H.; Kuhnlein, H.; Egeland, G. Prevalence and determinants of iron depletion and anemia among Canadian Inuit. FASEB J. 2011, 25, lb244. [Google Scholar] [CrossRef]
- Jamieson, J.A.; Weiler, H.A.; Kuhnlein, H.V.; Egeland, G.M. Traditional food intake is correlated with iron stores in Canadian Inuit men. J. Nutr. 2012, 142, 764–770. [Google Scholar] [CrossRef]
- Jamieson, J.A.; Kuhnlein, H.V.; Weiler, H.A.; Egeland, G.M. Higher n3-fatty acid status is associated with lower risk of iron depletion among food insecure Canadian Inuit women. BMC Public. Health 2013, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Janmohamed, A.; Luvsanjamba, M.; Norov, B.; Batsaikhan, E.; Jamiyan, B.; Blankenship, J.L. Complementary feeding practices and associated factors among Mongolian children 6–23 months of age. Matern. Child. Nutr. 2020, 16, e12838. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Cowan, A.E.; Dodd, K.W.; Tooze, J.A.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; et al. Association of food insecurity with dietary intakes and nutritional biomarkers among US children, National Health and Nutrition Examination Survey (NHANES) 2011–2016. Am. J. Clin. Nutr. 2021, 114, 1059–1069. [Google Scholar] [CrossRef]
- Kazemi, A.; Ghaemmaghami Hezaveh, S.J.; Nikniaz, L.; Nikniaz, Z. Is Food Insecurity Associated With Iron Deficiency Anemia and Vitamin D Deficiency Among Women of Reproductive Age? Top. Clin. Nutr. 2020, 35, 240–247. [Google Scholar] [CrossRef]
- Kelli, H.M.; Hammadah, M.; Ahmed, H.; Ko, Y.A.; Topel, M.; Samman-Tahhan, A.; Awad, M.; Patel, K.; Mohammed, K.; Sperling, L.S.; et al. Association between living in food deserts and cardiovascular risk. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003532. [Google Scholar] [CrossRef]
- Kim, J.H.; Baltrus, P.; Topel, M.L.; Liu, C.; Ko, Y.A.; Mujahid, M.; Vaccarino, V.; Sims, M.; Mubasher, M.; Khan, A.; et al. Better Neighborhood characteristics are associated with ideal cardiovascular health among blacks: Results from the morehouse-emory cardiovascular (MECA) center for health equity. Circulation 2019, 139, A015. [Google Scholar] [CrossRef]
- Kobrosly, R.W.; Parlett, L.E.; Stahlhut, R.W.; Barrett, E.S.; Swan, S.H. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ. Res. 2012, 115, 11–17. [Google Scholar] [CrossRef]
- Landry, M.J.; Khazaee, E.; Markowitz, A.K.; Vandyousefi, S.; Ghaddar, R.; Pilles, K.; Asigbee, F.M.; Gatto, N.M.; Davis, J.N. Impact of food security on glycemic control among low-income primarily Hispanic/Latino children in Los Angeles, California: A cross-sectional study. J. Hunger. Environ. Nutr. 2019, 14, 709–724. [Google Scholar] [CrossRef]
- Leyna, G.H.; Mmbaga, E.J.; Mnyika, K.S.; Hussain, A.; Klepp, K.-I. Food insecurity is associated with food consumption patterns and anthropometric measures but not serum micronutrient levels in adults in rural Tanzania. Public. Health Nutr. 2010, 13, 1438–1444. [Google Scholar] [CrossRef]
- Li, W.; Herrán, O.F.; Villamor, E. Trends in Iron, Zinc, and Vitamin A Status Biomarkers Among Colombian Children: Results From 2 Nationally Representative Surveys. Food Nutr. Bull. 2017, 38, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, M.; Murray-Kolb, L.E.; Hasan, S.M.T.; Das, S.; Fahim, S.M.; Alam, M.A.; Caulfield, L.; Ahmed, T. Why Do Children in Slums Suffer from Anemia, Iron, Zinc, and Vitamin A Deficiency? Results from a Birth Cohort Study in Dhaka. Nutrients 2019, 11, 3025. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.E.; Sotres-Alvarez, D.; Mattei, J.; Perreira, K.M.; McClain, A.C.; Gallo, L.C.; Isasi, C.R.; Albrecht, S.S. Food Insecurity and Cardiometabolic Markers: Results From the Study of Latino Youth. Pediatrics 2022, 149, e2021053781. [Google Scholar] [CrossRef] [PubMed]
- Marín, C.; Oliveros, H.; Villamor, E.; Mora, M. Food Insecurity and micronutrient status biomarkers in school-age Colombian children. Biomedica 2021, 41, 458–471. [Google Scholar] [CrossRef]
- Mazidi, M.; Vatanparast, H. Serum trans-fatty acids level are positively associated with lower food security among american adults. Nutr. Diabetes 2018, 8, 17. [Google Scholar] [CrossRef]
- Mohajeri, M.; Mohajery, R. Food security association with some risk factors of obesity-related diseases in Ardabil-Iran population. Mediterr. J. Nutr. Metab. 2022, 15, 229–237. [Google Scholar] [CrossRef]
- Mohamadpour, M.; Mohd Sharif, Z.; Avakh Keysami, M. Food Insecurity, Health and nutritional status among sample of Palm-Plantation households in Malaysia. J. Health Popul. Nutr. 2012, 30, 291–302. [Google Scholar] [CrossRef]
- López-Teros, V.; Haby, M.M.; Frongillo, E.A.; Murillo Castillo, K.D.; Corella-Madueño, M.A.; Díaz-Zavala, R.G.; Quizán-Plata, T. Food insecurity was associated with low quality diet and low HDL level in mothers of Northwest Mexico relying on fisheries for livelihood. Nutr. Hosp. 2018, 35, 1379–1386. [Google Scholar] [CrossRef]
- Nguyen, P.; Gonzalez-Casanova, I.; Nguyen, H.; Pham, H.; Truong, T.; Nguyen, S.; Martorell, R.; Ramakrishnan, U. Multi-causal determinants of anemia among women of reproductive age in Vietnam. FASEB J. 2014, 28, 804–807. [Google Scholar] [CrossRef]
- Nikniaz, L.; Tabrizi, J.S.; Sadeghi-Bazargani, H.; Farahbakhsh, M.; Nikniaz, Z. Is Food Insecurity Associated With Lipid Profile and Atherogenic Indices in Iranian Adults? A Population-Based Study. Top. Clin. Nutr. 2018, 33, 23–30. [Google Scholar] [CrossRef]
- Nur Atiqah, A.; Norazmir, M.N.; Khairil Anuar, M.I.; Mohd Fahmi, M.; Norazlanshah, H. Food security status: It's association with inflammatory marker and lipid profile among young adult. Int. Food Res. J. 2015, 22, 1855–1863. [Google Scholar]
- Pak, T.Y.; Kim, G. Association of Food Insecurity with Allostatic Load among Older Adults in the US. JAMA Netw. Open 2021, 4, e2137503. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Eicher-Miller, H.A. Iron deficiency is associated with food insecurity in pregnant females in the United States: National Health and Nutrition Examination Survey 1999–2010. J. Acad. Nutr. Diet. 2014, 114, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Strauss, S.M. Food insecurity as a predictor of metabolic syndrome in U.S. female adults. Public. Health Nurs. 2020, 37, 663–670. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Black, J.; Muthayya, S.; Shet, A.; Bhat, V.; Nagaraj, S.; Prashanth, N.S.; Sudarshan, H.; Biggs, B.A.; Shet, A.S. Determinants of anemia among young children in rural India. Pediatrics 2010, 126, e140–e149. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Shet, A.S.; Black, J.F.; Sudarshan, H.; Prashanth, N.S.; Biggs, B.-A. Vitamin B-12, folate, iron, and vitamin A concentrations in rural Indian children are associated with continued breastfeeding, complementary diet, and maternal nutrition. Am. J. Clin. Nutr. 2011, 94, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, D.F.; Queiroz, D.d.; Paiva, A.d.A.; Cunha, M.A.L.d.; Lima, Z.N. Food security, growth and vitamin A, hemoglobin and zinc levels of preschool children in the northeast of Brazil. Cien Saude Colet. 2014, 19, 641–650. [Google Scholar] [CrossRef]
- Pinzón-Rondón, Á.M.; Hoyos-Martínez, A.; Parra-Correa, D.; Pedraza-Flechas, A.M.; Ruiz-Sternberg, Á.M. Association of nutritional support programs with zinc deficiency in Colombian children: A cross-sectional study. BMC Nutr. 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, C.M.; Lucas, M.; Dallaire, R.; Ayotte, P.; Jacobson, J.L.; Jacobson, S.W.; Dewailly, E.; Muckle, G. Food insecurity and nutritional biomarkers in relation to stature in Inuit children from Nunavik. Can. J. Public. Health 2014, 105, e233–e238. [Google Scholar] [CrossRef]
- Pobee, R.A.; Aguree, S.; Colecraft, E.K.; Gernand, A.D.; Murray-Kolb, L.E. Food Insecurity and Micronutrient Status among Ghanaian Women Planning to Become Pregnant. Nutrients 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, N.; Basiotis, P. Food insufficiency and the nutritional status of the elderly population. Nutr. Insight 2000, 13, 58–60. [Google Scholar]
- Salarkia, N.; Neyestani, T.R.; Omidvar, N.; Zayeri, F. Household food insecurity, mother’s feeding practices, and the early childhood’s iron status. Int. J. Prev. Med. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Sattler, E.L.; Bhargava, V. Food insecurity is associated with increased cardiovascular risk in us adults. Circulation 2016, 133, 24. [Google Scholar] [CrossRef]
- Shariff, Z.M.; Sulaiman, N.; Jalil, R.A.; Yen, W.C.; Yaw, Y.H.; Taib, M.N.; Kandiah, M.; Lin, K.G. Food insecurity and the metabolic syndrome among women from low income communities in Malaysia. Asia Pac. J. Clin. Nutr. 2014, 23, 138–147. [Google Scholar] [CrossRef]
- Shin, J.I.; Bautista, L.E.; Walsh, M.C.; Malecki, K.C.; Nieto, F.J. Food insecurity and dyslipidemia in a representative population-based sample in the US. Prev. Med. 2015, 77, 186–190. [Google Scholar] [CrossRef]
- Shiue, I. People with diabetes, respiratory, liver or mental disorders, higher urinary antimony, bisphenol A, or pesticides had higher food insecurity: USA NHANES, 2005–2006. Env. Sci. Pollut. Res. Int. 2016, 23, 198–205. [Google Scholar] [CrossRef]
- Smalls, B.L.; Adegboyega, A.; Contreras, O.A.; Palmer, K.; Hatcher, J. Assessing Diabetes Risk Factors in Rural Dwelling Grandparent Caregivers. Gerontol. Geriatr. Med. 2020, 6, 2333721420924986. [Google Scholar] [CrossRef]
- Suarez, J.J.; Isakova, T.; Anderson, C.A.M.; Boulware, L.E.; Wolf, M.; Scialla, J.J. Food Access, Chronic Kidney Disease, and Hypertension in the U.S. Am. J. Prev. Med. 2015, 49, 912–920. [Google Scholar] [CrossRef]
- Tayie, F.A.; Zizza, C.A. Food insecurity and dyslipidemia among adults in the United States. Prev. Med. 2009, 48, 480–485. [Google Scholar] [CrossRef]
- Tayie, F.; Xu, B.; Timlin, M.; Dumars, A.; Jackson, J. Food insecurity is associated with higher-than-normal blood serum copper level. Public. Health Nutr. 2019, 22, 2339–2345. [Google Scholar] [CrossRef]
- Tester, J.M.; Laraia, B.A.; Leung, C.W.; Mietus-Snyder, M.L. Dyslipidemia and Food Security in Low-Income US Adolescents: National Health and Nutrition Examination Survey, 2003–2010. Prev. Chronic. Dis. 2016, 13, E22. [Google Scholar] [CrossRef]
- van Woerden, I.; Bruening, M.; Montresor-López, J.; Payne-Sturges, D.C. Trends and disparities in urinary BPA concentrations among U.S. emerging adults. Environ. Res. 2019, 176, 108515. [Google Scholar] [CrossRef] [PubMed]
- Weigel, M.M.; Armijos, R.X.; Hall, Y.P.; Ramirez, Y.; Orozco, R. The household food insecurity and health outcomes of U.S. Mexico border migrant and seasonal farmworkers. J. Immigr. Minor. Health 2007, 9, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Weigel, M.M.; Armijos, R.X.; Racines, M.; Cevallos, W.; Castro, N.P. Association of Household Food Insecurity with the Mental and Physical Health of Low-Income Urban Ecuadorian Women with Children. J. Environ. Public. Health 2016, 2016, 5256084. [Google Scholar] [CrossRef] [PubMed]
- Yeudall, F.; Sebastian, R.; Cole, D.C.; Ibrahim, S.; Lubowa, A.; Kikafunda, J. Food and nutritional security of children of urban farmers in Kampala, Uganda. Food Nutr. Bull. 2007, 28, S237–S246. [Google Scholar] [CrossRef]
- Hanson, K.L.; Connor, L.M. Food insecurity and dietary quality in US adults and children: A systematic review1,2,3. Am. J. Clin. Nutr. 2014, 100, 684–692. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Tarasuk, V. Food Insecurity Is Associated with Nutrient Inadequacies among Canadian Adults and Adolescents. J. Nutr. 2008, 138, 604–612. [Google Scholar] [CrossRef]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Djoumbou-Feunang, Y.; Dorrestein, P.C.; Dragsted, L.O.; Draper, J.; et al. Perspective: Dietary Biomarkers of Intake and Exposure-Exploration with Omics Approaches. Adv. Nutr. 2020, 11, 200–215. [Google Scholar] [CrossRef]
- Arenas, D.J.; Beltrán, S.; Montgomery, C.; Pharel, M.; Lopez-Hinojosa, I.; Vilá-Arroyo, G.; DeLisser, H.M. A Systematic Review and Meta-Analysis of Food Insecurity and Dyslipidemia. J. Am. Board. Fam. Med. 2022, 35, 656–667. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Gooding, H.C.; Walls, C.E.; Richmond, T.K. Food insecurity and increased BMI in young adult women. Obesity 2012, 20, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.S.; Peerson, J.; Love, B.; Achterberg, C.; Murphy, S.P. Food insecurity is positively related to overweight in women. J. Nutr. 2001, 131, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Nagata, J.M.; Palar, K.; Gooding, H.C.; Garber, A.K.; Bibbins-Domingo, K.; Weiser, S.D. Food Insecurity and Chronic Disease in US Young Adults: Findings from the National Longitudinal Study of Adolescent to Adult Health. J. Gen. Intern. Med. 2019, 34, 2756–2762. [Google Scholar] [CrossRef]
- Ito, F.; Ito, T. High-Density Lipoprotein (HDL) Triglyceride and Oxidized HDL: New Lipid Biomarkers of Lipoprotein-Related Atherosclerotic Cardiovascular Disease. Antioxidants 2020, 9, 362. [Google Scholar] [CrossRef]
- Egeland, G.M. IPY Inuit Health Survey speaks to need to address inadequate housing, food insecurity and nutrition transition. Int. J. Circumpolar Health 2011, 70, 444–446. [Google Scholar] [CrossRef][Green Version]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Vitamin A Review1234. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef]
- Wilkinson, A.; Pedersen, S.; Urassa, M.; Mkwashapi, D.; Andreasen, A.; Kinunghi, S.; Todd, J.; Changalucha, J.; McDermid, J. Mild HIV-induced maternal cachexia predicts adverse birth outcomes in rural Tanzania. FASEB J. 2014, 28, 620–625. [Google Scholar]
- Kuhnlein, H.V.; Receveur, O.; Soueida, R.; Egeland, G.M. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J. Nutr. 2004, 134, 1447–1453. [Google Scholar] [CrossRef]
- Tarasuk, V.; Fitzpatrick, S.; Ward, H. Nutrition inequities in Canada. Appl. Physiol. Nutr. Metab. 2010, 35, 172–179. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; West, K.A.; Bittremieux, W.; Williams, C.L.; Weldon, K.C.; Panitchpakdi, M.; Di Ottavio, F.; Aceves, C.M.; Brown, E.; Sikora, N.C.; et al. Enhancing untargeted metabolomics using metadata-based source annotation. Nat. Biotechnol. 2022, 40, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Schmid, R.; Gauglitz, J.M.; Wang, M.; Dorrestein, P.C. foodMASST a mass spectrometry search tool for foods and beverages. NPJ Sci. Food 2022, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef]
- Witkowski, M.; Nemet, I.; Alamri, H.; Wilcox, J.; Gupta, N.; Nimer, N.; Haghikia, A.; Li, X.S.; Wu, Y.; Saha, P.P.; et al. The artificial sweetener erythritol and cardiovascular event risk. Nat. Med. 2023, 29, 710–718. [Google Scholar] [CrossRef]
- Chen, F.; Du, M.; Blumberg, J.B.; Chui, K.K.H.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association Among Dietary Supplement Use, Nutrient Intake, and Mortality Among U.S. Adults. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important Considerations for Sample Collection in Metabolomics Studies with a Special Focus on Applications to Liver Functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.C.; Pagoto, S.L.; Hurley, T.G.; Magner, R.P.; Ockene, I.S.; Schneider, K.L.; Merriam, P.A.; Hébert, J.R. Number of 24-h diet recalls needed to estimate energy intake. Ann. Epidemiol. 2009, 19, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Gonzalez, F.J. Challenges and opportunities of metabolomics. J. Cell Physiol. 2012, 227, 2975–2981. [Google Scholar] [CrossRef]
- Han, W.; Li, L. Evaluating and minimizing batch effects in metabolomics. Mass. Spectrom. Rev. 2022, 41, 421–442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnovsky, L.; Crowley, A.P.; Naeem, F.; Wang, L.S.; Wu, G.D.; Chao, A.M. A Scoping Review of Nutritional Biomarkers Associated with Food Security. Nutrients 2023, 15, 3576. https://doi.org/10.3390/nu15163576
Krasnovsky L, Crowley AP, Naeem F, Wang LS, Wu GD, Chao AM. A Scoping Review of Nutritional Biomarkers Associated with Food Security. Nutrients. 2023; 15(16):3576. https://doi.org/10.3390/nu15163576
Chicago/Turabian StyleKrasnovsky, Lev, Aidan P. Crowley, Fawaz Naeem, Lucy S. Wang, Gary D. Wu, and Ariana M. Chao. 2023. "A Scoping Review of Nutritional Biomarkers Associated with Food Security" Nutrients 15, no. 16: 3576. https://doi.org/10.3390/nu15163576
APA StyleKrasnovsky, L., Crowley, A. P., Naeem, F., Wang, L. S., Wu, G. D., & Chao, A. M. (2023). A Scoping Review of Nutritional Biomarkers Associated with Food Security. Nutrients, 15(16), 3576. https://doi.org/10.3390/nu15163576






