Abstract
Urinary tract infections represent a common and significant health concern worldwide. The high rate of recurrence and the increasing antibiotic resistance of uropathogens are further worsening the current scenario. Nevertheless, novel key ingredients such as D-mannose, chondroitin sulphate, hyaluronic acid, and N-acetylcysteine could represent an important alternative or adjuvant to the prevention and treatment strategies of urinary tract infections. Several studies have indeed evaluated the efficacy and the potential use of these compounds in urinary tract health. In this review, we aimed to summarize the characteristics, the role, and the application of the previously reported compounds, alone and in combination, in urinary tract health, focusing on their potential role in urinary tract infections.
1. Introduction
Urinary tract infections (UTIs) are a common and significant health concern worldwide, affecting over 150 million individuals each year [1]. While both men and women can develop UTIs, with a prevalence which increases with age, the condition is more prevalent in women due to anatomical and behavioral factors such as a shorter urethra, which provides easier access for bacteria to reach the bladder, and the tendency to delay micturition. Specifically, a spike in the prevalence of UTIs is associated with young women aged 14–24 years old, while the prevalence in women over 65 years of age is approximately 20% versus 11% of the overall population [2]. UTIs occur when pathogenic microorganisms, primarily bacteria, invade the urinary tract, leading to inflammation and infection. UTIs can affect different parts of the urinary tract, including the urethra, bladder, ureters, and kidneys, albeit the most common type of UTI is a lower urinary tract infection, which primarily involves the bladder, provoking cystitis. Nevertheless, if left untreated or if the infection spreads, it can progress to an upper urinary tract infection, affecting the kidneys and thus developing pyelonephritis. UTIs can cause various symptoms, including frequent and painful urination, a persistent urge to urinate, cloudy or bloody urine, and lower abdominal discomfort. As previously described, the reason for an increased prevalence of UTIs in women is mostly related to anatomical and hormonal reasons [3]. In addition to the short length of the female urethra, which could easily allow the migration of bacteria from the anogenital area to the urethra and, therefore, to the bladder, other risk factors leading to the insurgence of UTIs are sexual activity as well as menopause [4,5]. In the first case, sexual intercourse could alter bacterial flora and vaginal pH, which, added to a lack of postcoital urination, vaginal douches and poor hygiene of the male partner could increase the risk of UTIs. In the second case, postmenopausal women report, among other common risk factors (i.e., delayed micturition, low fluid intake, and even genetic predisposition), vulvovaginal atrophy as a main causative factor which is associated with the decreased colonization of Lactobacilli [6]. Albeit the primary causative agent of UTIs is the bacterium Escherichia coli, which normally resides in the intestinal tract but can migrate to the urinary tract and cause infection, other bacteria, such as Klebsiella pneumoniae, Proteus mirabilis, and Staphylococcus saprophyticus, can also contribute to UTIs. In some cases, UTIs can be caused by fungal or viral pathogens, although they are less common [7,8]. In addition to previously described risk factors, urinary catheterization, urinary tract abnormalities, compromised immune function, hormonal changes, and poor hygiene practices could increase the risk of UTIs while certain populations, such as pregnant women, individuals with diabetes and the elderly, are also at higher risk of developing UTIs [6,9].
The consequences of UTIs can range from discomfort and inconvenience to more severe complications. If left untreated, UTIs can lead to recurrent infections, kidney damage, and potentially life-threatening bloodstream infections. The impact of UTIs extends beyond individual health, as they contribute to a significant economic burden through healthcare costs and productivity losses. It has been indeed estimated, in the United States, that UTIs represent over 7 million office visits and 1 million emergency department visits for a total of over 100,000 hospitalizations and an annual cost of USD 1.6 billion [10]. Commonly, the conventional treatment for UTIs involves antibiotics, which are prescribed based on the identified pathogen and its susceptibility to specific antibiotics. However, the overuse and misuse of antibiotics have led to the emergence of antibiotic-resistant bacteria, making the treatment of UTIs more challenging. As a result, there is growing interest in alternative approaches, including natural compounds and preventive strategies, to support urinary tract health and reduce the incidence of UTIs [11].
In this paper, we will focus on highlighting the potential benefits of specific natural compounds, including D-mannose, chondroitin sulphate, N-acetylcysteine (NAC), and hyaluronic acid, in promoting urinary tract health and preventing UTIs via the action of the urothelial barrier (Figure 1). By understanding the underlying mechanisms and evidence supporting these natural compounds, we aim to contribute to the development of effective strategies for maintaining urinary tract health and reducing the burden of UTIs.
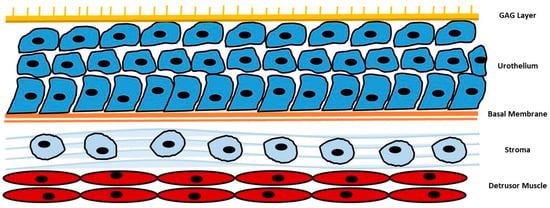
Figure 1.
Summarization of urothelial barrier. GAG: glycosaminoglycans.
2. D-mannose
D-mannose is a monosaccharide which is naturally found in several plants, fruits, and berries; albeit, it is also synthesized in the human body from glucose, where it contributes to glycoprotein synthesis and the glycosylation of proteins. The interest towards this compound in the treatment of UTIs dates back to the 1970s when the first episodes of the emergence of antibiotic-resistant uropathogens prompted the use of alternative methods, when feasible, to antibiotic treatment [12,13]. D-mannose has demonstrated in vitro no effects on uropathogens metabolism or growth nor interfere with antibiotic activity while, instead, could inhibit the adherence of E. coli to urothelial cells occupying the FimH fimbriae of the bacteria. The adhesion of pathogens to the urothelial cells prevents, indeed, their removal or washing off and represents the first step for urinary bladder colonization. The FimH fimbriae of E. coli interact with peptides and glycosylated residues on urothelial cells and extracellular matrix, permitting the bacteria to bind onto bladder epithelial cells. The attachments are made at the level of uroplakin 1a, which is a glycosylated protein composed of mannose molecules in the terminal units. Exogenously delivered D-mannose competitively inhibits the adhesion of bacteria to this terminal part of uroplakin 1a, saturating FimH adhesins [14,15,16] (Figure 2). Several in vivo studies have investigated the effects of D-mannose in the treatment and prevention of UTIs. Domenici et al., reported a lower recurrence rate of uncomplicated UTIs in 43 women, in the absence of side effects [17]. Similarly, Kranjcec et al. reported a significantly decreased risk of recurrent UTIs in 308 women treated with D-mannose or nitrofurantoin daily. In particular, the efficacy in decreasing the recurrent episodes of UTIs was similar among the groups treated with nitrofurantoin or D-mannose [18]. The efficacy of D-mannose was also reported in men, as described by Palleschi et al., which used a combination of NAC and D-mannose, compared to prulifloxacin, in patients undergoing urodynamic examinations. The association of D-mannose and NAC yielded similar results compared to antibiotics, in preventing UTIs in patients undergoing urodynamic examination [19]. Additionally, the use of D-mannose permitted a decrease in the perception of lower urinary tract symptoms as well as the incidence of UTIs in women undergoing prolapse surgery, as reported by Russo et al., in a recent study [20]. Analogous results were obtained in other studies utilizing D-mannose alone or in combination with other nutraceutical products or therapies [21,22,23,24]. Lastly, Lenger et al., published, in 2020, a systematic review and meta-analysis regarding the role of D-mannose in reducing UTI recurrence in adult women with recurrent UTIs compared with other prevention agents. The comparison of D-mannose with preventive antibiotics yielded a pooled relative risk for recurrent UTIs of 0.39 compared to 0.23 of the pooled relative risk of D-mannose only [25].
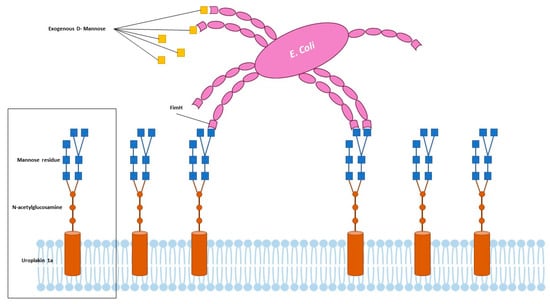
Figure 2.
Adherence of uropathogens and role of D-Mannose.
3. Chondroitin Sulphate
In recent years, chondroitin sulphate has emerged as a potential candidate for UTI prevention [26,27,28]. Chondroitin sulphate is a naturally occurring compound found in connective tissues, including those lining the urinary tract. The urothelium of the bladder is indeed coated by a thick layer of glycosaminoglycans (GAGs), which acts as a non-specific anti-adherence factor and defense mechanism against infections and irritants. Two types of GAGs are reported in bladder urothelium: non-sulphated GAGs as the hyaluronic acid and sulphated GAGs as heparan sulphate, chondroitin sulphate and dermatan sulphate. Chondroitin sulphate is known for its ability to maintain tissue integrity and provide structural support [29,30]. By supplementing with chondroitin sulphate, it is hypothesized that the protective effects on the urinary tract lining can help prevent the attachment and colonization of uropathogenic bacteria, reducing the risk of UTIs. Similar to D-mannose, chondroitin sulphate has been shown to interfere with bacterial adhesion [26]. Uropathogenic bacteria, such as E. coli, utilize adhesins to attach to the urinary tract epithelium [31]. Chondroitin sulphate can act as a decoy molecule, competing with these adhesins for binding sites and reducing bacterial attachment. This inhibition of bacterial adhesion can impede the initial steps of UTI development. Additionally, chondroitin sulphate has been found to have immunomodulatory properties, which can influence the immune response within the urinary tract. By regulating immune cell activity and cytokine production, chondroitin sulphate may contribute to a balanced immune response, helping to control inflammation and prevent the progression of UTIs [32]. Chondroitin sulphate supplementation has shown promise in reducing the frequency of UTI recurrences, both via intravesical and oral administration. Damiano et al. reported, in a prospective, randomized double-blinded placebo-controlled study including 57 women, a decreased UTI rate per patient per year (−86.6% versus −9.6%) and mean time of UTI recurrence (185.2 versus 52.7 days) in patients treated with intravesical instillation of hyaluronic acid plus chondroitin sulphate [33]. Similarly, Rahnnama’i et al., reported, in a retrospective study involving a total of 151 patients with recurrent UTI undergoing intravesical therapy with chondroitin sulphate only, a statistically significant reduction of the number of infections from 7.10 to 0.45 infection episodes per year as well as a reduction in the number of visits to the urologist from 7.46 to 1.28 episodes per year. Interestingly, these findings were comparable to patients treated with low-dose long-term antibiotics [28]. In a smaller study performed by De Vita et al. on 28 women followed for one year, the authors also demonstrated how intravesical instillation of hyaluronic acid plus chondroitin sulphate for 4 weeks and then once every 2 weeks, compared to long-term antibiotic prophylaxis using sulfamethoxazole and trimethoprim, reported similar results in terms of UTI recurrence, pain symptoms, sexual function, and quality of life [34]. Lastly, in regards to concerns surrounding the intravesical administration of chondroitin sulphate, an older meta-analysis involving four studies, for a total of 143 patients, reported a significant decrease in UTI rate per patient per year (−3.41, 95% confidence interval (CI) −4.33 to −2.49, p < 0.00001) as well as a longer mean UTI recurrence time (187.35 days, 95% CI 94.33–280.37, p < 0.0001) in patients treated with chondroitin sulphate compared to placebo [35]. More recently, Torella et al., reported in a prospective study involving 145 postmenopausal women, the efficacy of chondroitin sulphate, in combination with hyaluronic acid, curcumin, and quercetin, in the prevention of recurrent UTIs, with a significant improvement of related symptoms at a 12-month follow up [36]. Similarly, Schiavi et al. reported, in a study involving 98 consecutive patients of reproductive age affected by UTIs and treated with a combination of hyaluronic acid, chondroitin sulphate, curcumin, and quercetin administered orally, a significant decrease of dysuria episodes after 6 months of treatment. During the treatment, only 7.1% of patients experienced UTI recurrence, confirmed by a positive urine culture, proposing the oral combination of hyaluronic acid, chondroitin sulphate, curcumin, and quercetin as a valid and well-tolerated non-antibiotic treatment in the prevention of UTIs [37]. According to the previously reported findings, chondroitin sulphate may be a valuable addition to preventive strategies for recurrent UTIs. Additionally, combining chondroitin sulphate with other compounds, such as mannose and N-acetylcysteine, has been explored for their potential synergistic effects in UTI prevention [38,39]. The combination of chondroitin sulphate with other agents may therefore enhance the overall efficacy by targeting multiple steps in the pathogenesis of UTIs, including bacterial adhesion, biofilm formation, and immune modulation [40,41].
4. Hyaluronic Acid
Hyaluronic acid is a non-sulphated glycosaminoglycan and a major mucopolysaccharide widely found in connective, epithelial, and neural tissue, representing the main components of the extracellular matrix [42,43]. While its role in urinary tract infections (UTIs) is still being investigated, hyaluronic acid is thought to play a crucial role in maintaining the health and integrity of the urinary tract epithelium due to its protective properties, constituting a barrier against infections and irritants [44,45]. Similar to chondroitin sulphate, by supporting the health of the urinary tract epithelium and modulating the immune response, hyaluronic acid may contribute to reducing susceptibility to UTIs. Additionally, hyaluronic acid exhibits anti-inflammatory properties and could control the inflammatory cascade triggered by UTIs, reducing tissue damage and promoting healing. These characteristics, associated with the inhibition of adherence of immune complexes as well as cells, inhibition of leukocyte migration/aggregation, regulation of endothelial and fibroblast proliferation and, lastly, regulation of connective tissue healing, make this compound a key factor in the urothelial barrier [46,47,48]. Hyaluronic acid represents, indeed, a protective compound for the urothelial lining which, when it becomes permeable, allows the penetration of toxic substances and irritants into the bladder wall, producing a cascade of inflammatory response which results in additional pain and damage to the urothelium [49]. Clinical studies evaluating the efficacy of hyaluronic acid in UTIs are still limited, but initial findings are promising. Intravesical instillation of hyaluronic acid has been explored as a therapeutic approach in patients with recurrent UTIs and interstitial cystitis, reporting remarkable improvements in urinary symptoms, decreased UTI recurrence rates, and enhanced quality of life in treated individuals. One of the first studies to evaluate the efficacy of hyaluronic acid against recurrent UTIs was performed by Lipovac et al. on 20 women with a history of UTIs treated with nine intravesical instillations of hyaluronic acid over 6 months. The total number of UTIs were, before and after treatment, 67 and 10, respectively (p < 0.001), with 13 patients free of recurrences until the end of the study (mean follow of up 47.6 weeks). In the remaining seven women who had UTI recurrences, the mean time to recurrence was increased from 76.7 days to 178.3 days for a mean number of infections per year reduced from 4.99 to 0.56 (p < 0.001) [50]. Similar studies were conducted by Damiano et al., De Vita et al., and Torella et al., which involved the use of intravesical hyaluronic acid plus chondroitin sulphate and were already previously described [33,35,36]. In 2019, Scarneciu et al., conducted a clinical trial on 30 patients with UTIs, evaluating the efficacy of intravesical instillation of hyaluronic acid in reducing and relieving lower urinary tract irritation symptoms. A significant improvement in urinary bladder pain, day-time urinary frequency, and quality of life, assessed via questionnaires, was reported, in a mean follow-up of 15 months [51]. Lastly, a systematic review and meta-analysis by Goddard and Janssen related to the use of intravesical hyaluronic acid and chondroitin sulphate for recurrent UTIs reported, in a total of eight studies (two randomized and six non-randomized), a decreased UTI rate per patient per year, with a pooled mean difference of −2.56 (95% confidence interval [CI] –3.86, −1.26; p < 0.001) and an increased time to first UTI recurrence, with a pooled mean difference of 130.05 days (95% CI 5.84, 254.26; p = 0.04) [52]. While the evidence supporting the role of hyaluronic acid in UTIs is growing, more research is needed to establish standardized treatment protocols, determine optimal dosing regimens, and evaluate long-term safety. Additionally, the specific formulations and delivery methods of hyaluronic acid need to be considered to ensure efficient delivery and sustained effects in the urinary tract. Indeed, it has to be considered that oral hyaluronic acid supplementation, which could further improve the compliance of treatment, has to be evaluated in terms of bioavailability and absorption considering that the size of the molecule and its breakdown during digestion which could affect its therapeutic potential [53,54].
5. N-acetylcysteine
NAC has gained recognition for its potential role in the management of UTIs, particularly in the context of recurrent UTIs. NAC is a precursor of the antioxidant glutathione and possesses several mechanisms that make it a promising adjunctive therapy for recurrent UTIs. In particular, NAC acts as a potent antibiofilm agent both alone and in combination with antibiotics against E. coli, Enterococcus faecalis, and other species associated with the production of bacterial biofilms [46,47,48]. Additionally, it seems that NAC penetrates the biofilm matrix and it is able to kill the bacteria within, via the dissociation and acidification of bacterial cytoplasm, leaving an empty gel-like matrix [55]. A concentration of 2 g per liter of NAC is able to inhibit E. coli biofilm formation by 20–40%, rising to 90% at a concentration of 4 g per liter [56,57]. The disruption of the bacterial biofilm, which represents a protective mechanism used by bacteria to resist the host immune response and antibiotic treatment, enhances the susceptibility of bacteria to conventional antibiotics, making them more effective in eradicating the infection [58,59]. A study by Manoharan et al. performed on bladder epithelial cells involved the use of bacteria which were added to the cell culture in the presence of NAC, allowing the bacterial invasion. NAC completely inhibited the invasion of bladder epithelial cells both in the presence of E. coli and E. faecalis, in a dose-dependent manner, displaying no cytotoxicity against the cells. Additionally, NAC damaged bacterial membranes and prevented biofilm formation. The effects were amplified when an antibiotic, in addition to NAC, was administered [60]. Other favorable effects of NAC are correlated to its immunomodulating activity as well as oxidative stress reduction, permitting an ability to maintain and improve the bladder mucosal protection. Although no studies have been conducted regarding the effects of NAC on bladder urothelial cells in terms of immunomodulation and reduction of oxidative stress, its effects on pulmonary and bronchial epithelium are well-known [61,62,63]. In particular, regarding concerns surrounding immunomodulation activity, UTIs involve complex interactions between the pathogen and the host immune system. The immunomodulatory properties of NAC could regulate the immune response in the urinary tract, enhancing the activity of immune cells, such as neutrophils and macrophages, which play crucial roles in eliminating bacteria [64,65]. Additionally, NAC can reduce the production of pro-inflammatory cytokines and promote an anti-inflammatory environment, thereby minimizing tissue damage and inflammation associated with UTIs [66,67]. The effects of NAC on oxidative stress reduction are, similarly, well-known. Considering that UTIs are characterized by increased oxidative stress in the urinary tract, which can contribute to tissue damage and exacerbate the inflammatory response, NAC could act as a potent antioxidant by replenishing intracellular glutathione levels and scavenging reactive oxygen species. The reduction of oxidative stress could therefore alleviate the damage caused by UTIs and support the healing process [68,69,70]. Lastly, the urinary tract’s mucosal lining acts as a physical barrier against bacterial colonization. Disruption of this barrier can increase the susceptibility to UTIs. NAC has been shown to enhance the integrity of the mucosal lining, thereby potentially reducing the adhesion of bacteria to the urinary tract epithelium. This protective effect could help prevent the initial attachment and colonization of uropathogenic bacteria, reducing the likelihood of recurrent UTIs [71,72]. All these effects of NAC, in combination with other agents such as mannose and chondroitin sulphate, could be furtherly enhanced, providing synergistic effects by targeting different aspects of UTI pathogenesis, including bacterial adhesion, biofilm formation, and immune modulation. Although, further studies are required in order to fully evaluate the role of NAC on the urothelial epithelium.
6. Cranberry
The role of cranberry (Vaccinium macrocarpon) in the prevention and management of UTIs has garnered substantial attention within the scientific community due to the unique composition of bioactive compounds, mostly proanthocyanidins, anthocyanidins, and flavonols, which have been shown to interfere with the adhesion of uropathogens to the urothelial cells, a crucial step in the initiation of infection [73,74]. In addition, cranberry consumption has been associated with a decrease in UTI-related symptoms via the suppression of inflammatory cascades [75]. Nevertheless, despite its historical association with urinary tract health, the mechanism implied in the preventive effects of cranberry consumption against UTIs is still not completely understood. It is however verified that in ex vivo studies, cranberry products have shown an antiadhesive activity as reported by Liu et al., which showed how the urine samples collected from healthy subjects consuming cranberry products reported antiadhesive properties compared to those collected from a placebo group [76]. Similarly, Baron et al. showed a significant reduction in the adherence and biofilm formation of Candida albicans strains in urine collected after the intake of cranberry products [77]. Concerning the effects of cranberry products in vivo, several recent studies have reported interesting results. Maki et al., in a randomized, double-blind, placebo-controlled multicenter clinical trial involving 373 women with a recent history of UTIs consuming 240 mL of cranberry beverage per day, reported a 40% reduction in the incidence of UTIs compared to the placebo with one clinical UTI event prevented for every 3.2 women [78]. In an analogous study by Koradia et al. involving 81 women followed up for 26 weeks, the supplementation of cranberry products significantly lowered the number of UTIs (with 90% of patients in the intervention group which did not report a UTI event compared to 67% of the control group) and increased the time to first UTI (174 days versus 90 days) [79]. Lastly, a meta-analysis by Fu et al., which considered seven randomized controlled trials for a total of 1498 healthy women, reported a reduced risk of UTIs by 26% in subjects consuming cranberry products. However, due to the limited size of the studies analyzed, with only two studies involving over 300 participants, the findings obtained should be confirmed by better and larger studies [80]. Another more recent meta-analysis by Xia et al., analyzing 23 trials for a total of 3979 participants, found that cranberry-based products could significantly reduce the incidence of UTIs in susceptible populations, especially in those assuming cranberry juice compared to capsules or tablets (with a relative risk reduction of 35%). Nevertheless, considering the limitations of the studies involved (insufficient information included, differences and inconsistencies in dosages and compositions of cranberry products, and limited sample sizes), the authors suggested a certain caution in the interpretation of the results [81]. Indeed, the inconsistency in meta-analysis methodologies and methodological heterogeneity could have led to varying results and interpretations, contributing to enriching the uncertainty regarding the efficacy of cranberry in UTI management and prophylaxis [82]. Also due to these reasons, other ongoing studies are currently investigating the role of cranberry in UTIs [83,84].
7. The Use of Combined Compounds in UTIs: A Synergistic Approach Targeting the Bladder
The combined use of previously described compounds permits targeting multiple aspects of UTI pathogenesis, combining the individual properties of every compound in a synergistic approach aimed to provide a comprehensive and multifaceted approach to UTI prevention and management. To summarize, mannose could interfere with bacterial adhesion to the urinary tract epithelium, reducing the initial step of UTI development; chondroitin sulphate and hyaluronic acid protect the urinary tract lining, inhibit bacterial adhesion, and modulate the immune response while NAC exhibits antimicrobial effects, modulates the immune response, reduces oxidative stress, and may have a role in recurrent UTI prevention [28,37,60,85]. Therefore, when used in combination, these compounds could act synergistically, targeting various stages of UTI pathogenesis. According to these premises, the role of an oral supplement such as Uroial PLUS®, which include all the previously reported compounds, could represent a novelty in the urological panorama due to the high concentrations of D-mannose (3000 mg), chondroitin sulphate (500 mg), NAC (600 mg), and hyaluronic acid (35 mg). By targeting multiple mechanisms, the combined approach may enhance the overall effectiveness of UTI prevention and management. It is important to note that the optimal dosages, treatment durations, and specific combinations of these compounds require further research and clinical investigation. Additionally, individual variations, underlying health conditions, and UTI risk factors should be considered when determining the most suitable combination therapy for each patient. Further compounds and nutraceuticals could be further added and integrated into a multifaceted approach to UTI [86].
8. The Use of Combined Compounds in UTIs: A Synergistic Approach Targeting the Gut
Recently, a new intestinal mucosal protective effect was described as a consequence of the interaction of two of these compounds, specifically cranberry and chondroitin sulphate in a specific mass ratio [26]. The precipitate formed can act as a barrier agent with a mucosal protective effect by improving the preservation of the physiological intestinal barrier and the tight junctions of epithelial cells thus reducing the risk of bacterial translocation from the gut to the urological tract. In particular, by forming a covering layer on the mucosa, the mucosal barrier prevents the adhesion of E. coli (mediated by the fimbriae) and consequently the invasion of the epithelium. In fact, in the face of excessive intestinal colonization by uropathogens (which by their nature tend to adhere), the presence of the protective film prevents their nesting at the epithelial level, favoring their elimination. This second part of the mechanism of action is as important as the first, as an accumulation of uropathogens in the intestine with the formation of a bacterial reservoir is an important risk factor for urinary colonization, according to the fecal-perineal-urethral hypothesis, according to which the proximity of the urethra to the terminal tract of the colon is responsible for the external migration of uropathogens and for the contamination of the urinary tract [87,88,89].
Furthermore, when cranberry and chondroitin sulphate are in combination with other compounds, specifically D-mannose, hyaluronic acid (HA), and NAC, the biological effects applied to UTI pathogens virulence showed a significant reduction of biofilm production, especially on antibiotic-resistant strains of uropathogens [26,90,91]. Based on these effects, new mucosal protective agents such as compounds are herein assessed to represent a new potential approach against recurrent cystitis and UTIs that originate from intestinal dysfunction (Table 1).

Table 1.
Main effects of compounds reported on the urothelial barrier.
9. Conclusions
The role of D-mannose in UTIs is well-established and known, but other interesting and widely used compounds such as hyaluronic acid, chondroitin sulphate, and NAC, are starting to quickly deliver interesting findings considering that their combination may represent a novel therapeutic approach. In fact, the combination of these compounds targets both the bladder and the gut, offering a novel mechanism of action for the prevention and management of UTIs. The combined approach, i.e., the use of multiple compounds synergically, could offer an even more comprehensive and effective approach to urinary health and UTI prevention. Future research should focus on well-designed clinical trials to establish the safety, efficacy, and optimal combinations of these compounds, alone and in combination, additionally understanding the potential interactions and compatibility of the combined compounds to provide the best therapeutic effectiveness.
Author Contributions
Conceptualization: F.C. (Felice Crocetto) and B.B.; methodology: U.A., L.D.L., A.F. and B.F.M.; software: G.G., G.F., and F.C. (Federico Capone); validation: F.M., D.V. and E.S.; investigation: F.C. (Felice Crocetto), B.B., G.P., A.L. and F.L.; resources: U.A., L.D.L., G.G., G.F., F.C. (Federico Capone), F.M., D.V., E.S. and G.P.; data curation: A.L., R.B., and F.L.; writing—original draft preparation: F.C. (Felice Crocetto), B.B. and F.D.G.; writing—review and editing: B.B., G.L. and M.F.; visualization: G.M.B. and C.I.; supervision: G.M.B., M.F. and C.I.; project administration, B.B. and F.C. (Felice Crocetto). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, K.; Broś-Konopielko, M.; Teliga-Czajkowska, J. Urinary Tract Infection in Women. Prz. Menopauzalny 2021, 20, 40–47. [Google Scholar] [CrossRef]
- Moore, E.E.; Hawes, S.E.; Scholes, D.; Boyko, E.J.; Hughes, J.P.; Fihn, S.D. Sexual Intercourse and Risk of Symptomatic Urinary Tract Infection in Post-Menopausal Women. J. Gen. Intern. Med. 2008, 23, 595–599. [Google Scholar] [CrossRef]
- Raz, R. Urinary Tract Infection in Postmenopausal Women. Korean J. Urol. 2011, 52, 801–808. [Google Scholar] [CrossRef]
- Storme, O.; Tirán Saucedo, J.; Garcia-Mora, A.; Dehesa-Dávila, M.; Naber, K.G. Risk Factors and Predisposing Conditions for Urinary Tract Infection. Ther. Adv. Urol. 2019, 11, 1756287218814382. [Google Scholar] [CrossRef]
- Abou Heidar, N.F.; Degheili, J.A.; Yacoubian, A.A.; Khauli, R.B. Management of Urinary Tract Infection in Women: A Practical Approach for Everyday Practice. Urol. Ann. 2019, 11, 339–346. [Google Scholar] [CrossRef] [PubMed]
- McLellan, L.K.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Abdelkarim, S.; Zenida, M.; Baiti, M.A.H.; Alhazmi, A.A.Y.; Alfaifi, B.A.H.; Majrabi, R.Q.M.; Khormi, N.Q.M.; Hakami, A.A.A.; Alqaari, R.A.M.; et al. Prevalence and Associated Risk Factors of Urinary Tract Infection among Diabetic Patients: A Cross-Sectional Study. Healthcare 2023, 11, 861. [Google Scholar] [CrossRef]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. Open Forum. Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef]
- Chardavoyne, P.C.; Kasmire, K.E. Appropriateness of Antibiotic Prescriptions for Urinary Tract Infections. West. J. Emerg. Med. 2020, 21, 633–639. [Google Scholar] [CrossRef]
- Spencer, J.F.; Gorin, P.A. Mannose-Containing Polysaccharides of Yeasts. Biotechnol. Bioeng. 1973, 15, 1–12. [Google Scholar] [CrossRef]
- Ballou, C.E.; Lipke, P.N.; Raschke, W.C. Structure and Immunochemistry of the Cell Wall Mannans from Saccharomyces Chevalieri, Saccharomyces Italicus, Saccharomyces Diastaticus, and Saccharomyces Carlsbergensis. J. Bacteriol. 1974, 117, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; Sun, D.; Dale, J.B.; Beachey, E.H. Conservation of the D-Mannose-Adhesion Protein among Type 1 Fimbriated Members of the Family Enterobacteriaceae. Nature 1988, 336, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-Bond Mechanism of the Bacterial Adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia Is the Urothelial Receptor for Uropathogenic Escherichia Coli: Evidence from in Vitro FimH Binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Domenici, L.; Monti, M.; Bracchi, C.; Giorgini, M.; Colagiovanni, V.; Muzii, L.; Benedetti Panici, P. D-Mannose: A Promising Support for Acute Urinary Tract Infections in Women. A Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2920–2925. [Google Scholar]
- Kranjčec, B.; Papeš, D.; Altarac, S. D-Mannose Powder for Prophylaxis of Recurrent Urinary Tract Infections in Women: A Randomized Clinical Trial. World J. Urol. 2014, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Palleschi, G.; Carbone, A.; Zanello, P.P.; Mele, R.; Leto, A.; Fuschi, A.; Al Salhi, Y.; Velotti, G.; Al Rawashdah, S.; Coppola, G.; et al. Prospective Study to Compare Antibiosis versus the Association of N-Acetylcysteine, D-Mannose and Morinda Citrifolia Fruit Extract in Preventing Urinary Tract Infections in Patients Submitted to Urodynamic Investigation. Arch. Ital. Urol. Androl. 2017, 89, 45–50. [Google Scholar] [CrossRef][Green Version]
- Russo, E.; Montt Guevara, M.; Giannini, A.; Mannella, P.; Palla, G.; Caretto, M.; Pancetti, F.; Genazzani, A.D.; Simoncini, T. Cranberry, D-Mannose and Anti-Inflammatory Agents Prevent Lower Urinary Tract Symptoms in Women Undergoing Prolapse Surgery. Climacteric 2020, 23, 201–205. [Google Scholar] [CrossRef]
- Genovese, C.; Davinelli, S.; Mangano, K.; Tempera, G.; Nicolosi, D.; Corsello, S.; Vergalito, F.; Tartaglia, E.; Scapagnini, G.; Di Marco, R. Effects of a New Combination of Plant Extracts plus D-Mannose for the Management of Uncomplicated Recurrent Urinary Tract Infections. J. Chemother. 2018, 30, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F. Effectiveness of an Association of a Cranberry Dry Extract, D-Mannose, and the Two Microorganisms Lactobacillus Plantarum LP01 and Lactobacillus Paracasei LPC09 in Women Affected by Cystitis: A Pilot Study. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Lenger, S.M.; Chu, C.M.; Ghetti, C.; Durkin, M.J.; Jennings, Z.; Wan, F.; Sutcliffe, S.; Lowder, J.L. D-Mannose for Recurrent Urinary Tract Infection Prevention in Postmenopausal Women Using Vaginal Estrogen: A Randomized Controlled Trial. Urogynecology 2023, 29, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Casado, J.; Méndez-Rubio, S.; Esteban-Fuertes, M.; Gómez-Rodríguez, A.; Vírseda-Chamorro, M.; Luján-Galán, M.; Iglesias-García, C.; Rituman, G. Large Study (283 Women) on the Effectiveness of Manosar®: 2 g of d-Mannose + 140 Mg of Proanthocyanidins (PAC), of Prolonged Release. Arch. Esp. Urol. 2020, 73, 491–498. [Google Scholar]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-Mannose vs Other Agents for Recurrent Urinary Tract Infection Prevention in Adult Women: A Systematic Review and Meta-Analysis. Am. J. Obs. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef] [PubMed]
- Caglioti, C.; Iannitti, R.; Ceccarelli, G.; Selan, L.; Artini, M.; Papa, R.; Malvasi, A.; Gentile, R.; Del Bianco, D.; Apone, F.; et al. Cranberry/Chondroitin Sulfate Co-Precipitate as a New Method for Controlling Urinary Tract Infections. Antibiotics 2023, 12, 1053. [Google Scholar] [CrossRef]
- Dinh, A.; Duran, C.; Hamami, K.; Afif, M.; Bonnet, F.; Donay, J.-L.; Lafaurie, M.; Chartier-Kastler, E. Hyaluronic Acid and Chondroitin Sulphate Treatment for Recurrent Severe Urinary Tract Infections Due to Multidrug-Resistant Gram-Negative Bacilli in a Patient With Multiple Sclerosis: Case Report and Literature Review. Open Forum Infect. Dis. 2022, 9, ofac245. [Google Scholar] [CrossRef]
- Rahnama’i, M.S.; Javan Balegh Marand, A.; Röschmann-Doose, K.; Steffens, L.; Arendsen, H.J. The Efficacy and Safety of Intravesical Chondroitin Sulphate Solution in Recurrent Urinary Tract Infections. BMC Urol. 2022, 22, 188. [Google Scholar] [CrossRef]
- Klingler, C.H. Glycosaminoglycans: How Much Do We Know about Their Role in the Bladder? Urologia 2016, 83 (Suppl. S1), 11–14. [Google Scholar] [CrossRef]
- Janssen, D.A.W.; van Wijk, X.M.R.; Jansen, K.C.F.J.; van Kuppevelt, T.H.; Heesakkers, J.P.F.A.; Schalken, J.A. The Distribution and Function of Chondroitin Sulfate and Other Sulfated Glycosaminoglycans in the Human Bladder and Their Contribution to the Protective Bladder Barrier. J. Urol. 2013, 189, 336–342. [Google Scholar] [CrossRef]
- Kallas, P.; Haugen, H.J.; Gadegaard, N.; Stormonth-Darling, J.; Hulander, M.; Andersson, M.; Valen, H. Adhesion of Escherichia Coli to Nanostructured Surfaces and the Role of Type 1 Fimbriae. Nanomaterials 2020, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- du Souich, P.; García, A.G.; Vergés, J.; Montell, E. Immunomodulatory and Anti-Inflammatory Effects of Chondroitin Sulphate. J. Cell Mol. Med. 2009, 13, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Damiano, R.; Quarto, G.; Bava, I.; Ucciero, G.; De Domenico, R.; Palumbo, M.I.; Autorino, R. Prevention of Recurrent Urinary Tract Infections by Intravesical Administration of Hyaluronic Acid and Chondroitin Sulphate: A Placebo-Controlled Randomised Trial. Eur. Urol. 2011, 59, 645–651. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Giordano, S. Effectiveness of Intravesical Hyaluronic Acid/Chondroitin Sulfate in Recurrent Bacterial Cystitis: A Randomized Study. Int. Urogynecol. J. 2012, 23, 1707–1713. [Google Scholar] [CrossRef]
- De Vita, D.; Antell, H.; Giordano, S. Effectiveness of Intravesical Hyaluronic Acid with or without Chondroitin Sulfate for Recurrent Bacterial Cystitis in Adult Women: A Meta-Analysis. Int. Urogynecol. J. 2013, 24, 545–552. [Google Scholar] [CrossRef]
- Torella, M.; Del Deo, F.; Grimaldi, A.; Iervolino, S.A.; Pezzella, M.; Tammaro, C.; Gallo, P.; Rappa, C.; De Franciscis, P.; Colacurci, N. Efficacy of an Orally Administered Combination of Hyaluronic Acid, Chondroitin Sulfate, Curcumin and Quercetin for the Prevention of Recurrent Urinary Tract Infections in Postmenopausal Women. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 207, 125–128. [Google Scholar] [CrossRef]
- Schiavi, M.C.; Porpora, M.G.; Vena, F.; Prata, G.; Sciuga, V.; D’Oria, O.; Di Tucci, C.; Savone, D.; Aleksa, N.; Giannini, A.; et al. Orally Administered Combination of Hyaluronic Acid, Chondroitin Sulfate, Curcumin, and Quercetin in the Prevention of Postcoital Recurrent Urinary Tract Infections: Analysis of 98 Women in Reproductive Age After 6 Months of Treatment. Female Pelvic Med. Reconstr. Surg. 2019, 25, 309–312. [Google Scholar] [CrossRef]
- Ala-Jaakkola, R.; Laitila, A.; Ouwehand, A.C.; Lehtoranta, L. Role of D-Mannose in Urinary Tract Infections—A Narrative Review. Nutr. J. 2022, 21, 18. [Google Scholar] [CrossRef]
- Marchiori, D.; Zanello, P.P. Efficacy of N-Acetylcysteine, D-Mannose and Morinda Citrifolia to Treat Recurrent Cystitis in Breast Cancer Survivals. In Vivo 2017, 31, 931–936. [Google Scholar] [CrossRef][Green Version]
- Lila, A.S.A.; Rajab, A.A.H.; Abdallah, M.H.; Rizvi, S.M.D.; Moin, A.; Khafagy, E.-S.; Tabrez, S.; Hegazy, W.A.H. Biofilm Lifestyle in Recurrent Urinary Tract Infections. Life 2023, 13, 148. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Lüthje, P.; Chromek, M.; Peters, V.; Wang, X.; Holm, Å.; Kádas, L.; Hedlund, K.-O.; Johansson, J.; Chapman, M.R.; et al. Uropathogenic Escherichia Coli Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37. PLOS Pathog. 2010, 6, e1001010. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Iavazzo, C.; Athanasiou, S.; Pitsouni, E.; Falagas, M.E. Hyaluronic Acid: An Effective Alternative Treatment of Interstitial Cystitis, Recurrent Urinary Tract Infections, and Hemorrhagic Cystitis? Eur. Urol. 2007, 51, 1534–1540; discussion 1540–1541. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Pirozzi, A.V.A.; Diana, P.; Reale, S.; Vassallo, V.; Fusco, A.; Donnarumma, G.; Rosa, M.D.; Schiraldi, C. Hyaluronic Acid and Chondroitin Sulfate, Alone or in Combination, Efficiently Counteract Induced Bladder Cell Damage and Inflammation. PLoS ONE 2019, 14, e0218475. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, D.; Noble, P.W. Hyaluronan as a Therapeutic Target in Human Diseases. Adv. Drug Deliv. Rev. 2016, 97, 186–203. [Google Scholar] [CrossRef]
- NOBLE, P.W.; LIANG, J.; JIANG, D. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Cervigni, M.; Natale, F.; Nasta, L.; Mako, A. Intravesical Hyaluronic Acid and Chondroitin Sulphate for Bladder Pain Syndrome/Interstitial Cystitis: Long-Term Treatment Results. Int. Urogynecol. J. 2012, 23, 1187–1192. [Google Scholar] [CrossRef]
- Lipovac, M.; Kurz, C.; Reithmayr, F.; Verhoeven, H.C.; Huber, J.C.; Imhof, M. Prevention of Recurrent Bacterial Urinary Tract Infections by Intravesical Instillation of Hyaluronic Acid. Int. J. Gynaecol. Obs. 2007, 96, 192–195. [Google Scholar] [CrossRef]
- Scarneciu, I.; Bungau, S.; Lupu, A.-M.; Scarneciu, C.C.; Bratu, O.G.; Martha, O.; Tit, D.M.; Aleya, L.; Lupu, S. Efficacy of Instillation Treatment with Hyaluronic Acid in Relieving Symptoms in Patients with BPS/IC and Uncomplicated Recurrent Urinary Tract Infections—Long-Term Results of a Multicenter Study. Eur. J. Pharm. Sci. 2019, 139, 105067. [Google Scholar] [CrossRef]
- Goddard, J.C.; Janssen, D.A.W. Intravesical Hyaluronic Acid and Chondroitin Sulfate for Recurrent Urinary Tract Infections: Systematic Review and Meta-Analysis. Int. Urogynecol. J. 2018, 29, 933–942. [Google Scholar] [CrossRef]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Absorption of Orally Administered Hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef]
- Šimek, M.; Turková, K.; Schwarzer, M.; Nešporová, K.; Kubala, L.; Hermannová, M.; Foglová, T.; Šafránková, B.; Šindelář, M.; Šrůtková, D.; et al. Molecular Weight and Gut Microbiota Determine the Bioavailability of Orally Administered Hyaluronic Acid. Carbohydr. Polym. 2023, 313, 120880. [Google Scholar] [CrossRef]
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic Action of Weak Acid Drugs on Biofilms. Sci. Rep. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-Acetylcysteine-Functionalized Coating Avoids Bacterial Adhesion and Biofilm Formation. Sci. Rep. 2017, 7, 17374. [Google Scholar] [CrossRef]
- Barone, B.; Mirto, B.F.; Falcone, A.; Del Giudice, F.; Aveta, A.; Napolitano, L.; Del Biondo, D.; Ferro, M.; Busetto, G.M.; Manfredi, C.; et al. The Efficacy of Flogofilm® in the Treatment of Chronic Bacterial Prostatitis as an Adjuvant to Antibiotic Therapy: A Randomized Prospective Trial. J. Clin. Med. 2023, 12, 2784. [Google Scholar] [CrossRef]
- Dinicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-Acetylcysteine as Powerful Molecule to Destroy Bacterial Biofilms. A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar]
- Cai, T.; Gallelli, L.; Meacci, F.; Brugnolli, A.; Prosperi, L.; Roberta, S.; Eccher, C.; Mazzoli, S.; Lanzafame, P.; Caciagli, P.; et al. The Efficacy of Umbelliferone, Arbutin, and N-Acetylcysteine to Prevent Microbial Colonization and Biofilm Development on Urinary Catheter Surface: Results from a Preliminary Study. J. Pathog. 2016, 2016, e1590952. [Google Scholar] [CrossRef][Green Version]
- Manoharan, A.; Ognenovska, S.; Paino, D.; Whiteley, G.; Glasbey, T.; Kriel, F.H.; Farrell, J.; Moore, K.H.; Manos, J.; Das, T. N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens. Antibiotics 2021, 10, 900. [Google Scholar] [CrossRef]
- Limansubroto, N.; Chung, W.O.; Johnson, J.D.; Paranjpe, A. Immunomodulatory Effects of N-Acetyl Cysteine Treated SCAP. J. Endod. 2022, 48, 1055–1062. [Google Scholar] [CrossRef]
- Omara, F.O.; Blakley, B.R.; Bernier, J.; Fournier, M. Immunomodulatory and Protective Effects of N-Acetylcysteine in Mitogen-Activated Murine Splenocytes in Vitro. Toxicology 1997, 116, 219–226. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, F.; Kang, X.; Zhou, J.; Yao, Q.; Lin, Y.; Zhang, W. The Antioxidant N-Acetylcysteine Promotes Immune Response and Inhibits Epithelial-Mesenchymal Transition to Alleviate Pulmonary Fibrosis in Chronic Obstructive Pulmonary Disease by Suppressing the VWF/P38 MAPK Axis. Mol. Med. 2021, 27, 97. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-y-Keenoy, B.; Vertongen, T.; Schippers, G.; Radomska-Lesniewska, D.; Heytens, E.; De Backer, W.A. Effect of N-Acetylcysteine on Neutrophil Activation Markers in Healthy Volunteers: In Vivo and in Vitro Study. Pharmacol. Res. 2006, 53, 216–225. [Google Scholar] [CrossRef]
- Palacio, J.R.; Markert, U.R.; Martínez, P. Anti-Inflammatory Properties of N-Acetylcysteine on Lipopolysaccharide-Activated Macrophages. Inflamm. Res. 2011, 60, 695–704. [Google Scholar] [CrossRef]
- Al-Shukaili, A.; Al-Abri, S.; Al-Ansari, A.; Monteil, M.A. Effect of N-Acetyl-L-Cysteine on Cytokine Production by Human Peripheral Blood Mononuclear Cells. Sultan Qaboos Univ. Med. J. 2009, 9, 70–74. [Google Scholar]
- Kaufman, G.; Skrtic, D. N-Acetyl Cysteine Modulates the Inflammatory and Oxidative Stress Responses of Rescued Growth-Arrested Dental Pulp Microtissues Exposed to TEGDMA in ECM. Int. J. Mol. Sci. 2020, 21, 7318. [Google Scholar] [CrossRef]
- Belge Kurutas, E.; Ciragil, P.; Gul, M.; Kilinc, M. The Effects of Oxidative Stress in Urinary Tract Infection. Mediat. Inflamm. 2005, 2005, 242–244. [Google Scholar] [CrossRef]
- Khosla, L.; Gong, S.; Weiss, J.P.; Birder, L.A. Oxidative Stress Biomarkers in Age-Related Lower Urinary Tract Disorders: A Systematic Review. Int. Neurourol. J. 2022, 26, 3–19. [Google Scholar] [CrossRef]
- Xu, Z.; Elrashidy, R.A.; Li, B.; Liu, G. Oxidative Stress: A Putative Link Between Lower Urinary Tract Symptoms and Aging and Major Chronic Diseases. Front. Med. 2022, 9, 812967. [Google Scholar] [CrossRef]
- Qin, X.; Sheth, S.U.; Sharpe, S.M.; Dong, W.; Lu, Q.; Xu, D.; Deitch, E.A. The Mucus Layer Is Critical in Protecting against Ischemia/Reperfusion-Mediated Gut Injury and in the Restitution of Gut Barrier Function. Shock 2011, 35, 275–281. [Google Scholar] [CrossRef]
- Farinati, F.; Cardin, R.; della Libera, G.; Pallotta, T.; Rugge, M.; Colantoni, A.; Gurrieri, G. Effects of N-Acetyl-l-Cysteine in Patients with Chronic Atrophic Gastritis and Nonulcer Dyspepsia: A Phase III Pilot Study. Curr. Ther. Res. 1997, 58, 724–733. [Google Scholar] [CrossRef]
- González de Llano, D.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef]
- Gonzalez de Llano, D.; Liu, H.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomé, B. Some New Findings Regarding the Antiadhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Bacteria. J. Agric. Food Chem. 2019, 67, 2166–2174. [Google Scholar] [CrossRef]
- Colletti, A.; Sangiorgio, L.; Martelli, A.; Testai, L.; Cicero, A.F.G.; Cravotto, G. Highly Active Cranberry’s Polyphenolic Fraction: New Advances in Processing and Clinical Applications. Nutrients 2021, 13, 2546. [Google Scholar] [CrossRef]
- Liu, H.; Howell, A.B.; Zhang, D.J.; Khoo, C. A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Assess Bacterial Anti-Adhesive Activity in Human Urine Following Consumption of a Cranberry Supplement. Food Funct. 2019, 10, 7645–7652. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Borghi, E.; Ottaviano, E.; Del Bo, C.; Riso, P.; Allegrini, P.; et al. Profiling Vaccinium Macrocarpon Components and Metabolites in Human Urine and the Urine Ex-Vivo Effect on Candida Albicans Adhesion and Biofilm-Formation. Biochem. Pharmacol. 2020, 173, 113726. [Google Scholar] [CrossRef]
- Maki, K.C.; Kaspar, K.L.; Khoo, C.; Derrig, L.H.; Schild, A.L.; Gupta, K. Consumption of a Cranberry Juice Beverage Lowered the Number of Clinical Urinary Tract Infection Episodes in Women with a Recent History of Urinary Tract Infection. Am. J. Clin. Nutr. 2016, 103, 1434–1442. [Google Scholar] [CrossRef]
- Koradia, P.; Kapadia, S.; Trivedi, Y.; Chanchu, G.; Harper, A. Probiotic and Cranberry Supplementation for Preventing Recurrent Uncomplicated Urinary Tract Infections in Premenopausal Women: A Controlled Pilot Study. Expert. Rev. Anti Infect. Ther. 2019, 17, 733–740. [Google Scholar] [CrossRef]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef]
- Xia, J.-Y.; Yang, C.; Xu, D.-F.; Xia, H.; Yang, L.-G.; Sun, G.-J. Consumption of Cranberry as Adjuvant Therapy for Urinary Tract Infections in Susceptible Populations: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. PLoS ONE 2021, 16, e0256992. [Google Scholar] [CrossRef]
- Liska, D.J.; Kern, H.J.; Maki, K.C. Cranberries and Urinary Tract Infections: How Can the Same Evidence Lead to Conflicting Advice? Adv. Nutr. 2016, 7, 498–506. [Google Scholar] [CrossRef]
- Gbinigie, O.; Allen, J.; Williams, N.; Moore, M.; Hay, A.D.; Heneghan, C.; Boylan, A.-M.; Butler, C.C. Does Cranberry Extract Reduce Antibiotic Use for Symptoms of Acute Uncomplicated Urinary Tract Infections (CUTI)? A Feasibility Randomised Trial. BMJ Open 2021, 11, e046791. [Google Scholar] [CrossRef]
- Asma, B.; Vicky, L.; Stephanie, D.; Yves, D.; Amy, H.; Sylvie, D. Standardised High Dose versus Low Dose Cranberry Proanthocyanidin Extracts for the Prevention of Recurrent Urinary Tract Infection in Healthy Women [PACCANN]: A Double Blind Randomised Controlled Trial Protocol. BMC Urol. 2018, 18, 29. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Lorenz, H.; Ewald, O.; Gerke, P. Why D-Mannose May Be as Efficient as Antibiotics in the Treatment of Acute Uncomplicated Lower Urinary Tract Infections—Preliminary Considerations and Conclusions from a Non-Interventional Study. Antibiotics 2022, 11, 314. [Google Scholar] [CrossRef]
- Das, S. Natural Therapeutics for Urinary Tract Infections—A Review. Future J. Pharm. Sci. 2020, 6, 64. [Google Scholar] [CrossRef]
- Sklan, D.; Hurwitz, S. Movement and Absorption of Major Minerals and Water in Ovine Gastrointestinal Tract. J. Dairy. Sci. 1985, 68, 1659–1666. [Google Scholar] [CrossRef]
- Worby, C.J.; Olson, B.S.; Dodson, K.W.; Earl, A.M.; Hultgren, S.J. Establishing the Role of the Gut Microbiota in Susceptibility to Recurrent Urinary Tract Infections. J. Clin. Investig. 2022, 132, e158497. [Google Scholar] [CrossRef]
- Priadko, K.; Romano, L.; Olivieri, S.; Romeo, M.; Barone, B.; Sciorio, C.; Spirito, L.; Morelli, M.; Crocetto, F.; Arcaniolo, D.; et al. Intestinal Microbiota, Intestinal Permeability and the Urogenital Tract: Is There a Pathophysiological Link? J. Physiol. Pharmacol. 2022, 73, 575–585. [Google Scholar] [CrossRef]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic Prevention and Management of Recurrent Urinary Tract Infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef]
- Tsuji, T.; Yoon, J.; Kitano, N.; Okura, T.; Tanaka, K. Effects of N-Acetyl Glucosamine and Chondroitin Sulfate Supplementation on Knee Pain and Self-Reported Knee Function in Middle-Aged and Older Japanese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Aging Clin. Exp. Res. 2016, 28, 197–205. [Google Scholar] [CrossRef]
- Scaglione, F.; Musazzi, U.M.; Minghetti, P. Considerations on D-Mannose Mechanism of Action and Consequent Classification of Marketed Healthcare Products. Front. Pharmacol. 2021, 12, 636377. [Google Scholar] [CrossRef]
- Rozenberg, B.B.; Janssen, D.A.W.; Jansen, C.F.J.; Schalken, J.A.; Heesakkers, J.P.F.A. Improving the Barrier Function of Damaged Cultured Urothelium Using Chondroitin Sulfate. Neurourol. Urodyn. 2020, 39, 558–564. [Google Scholar] [CrossRef]
- Manoharan, A.; Das, T.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; Manos, J. The Effect of N-Acetylcysteine in a Combined Antibiofilm Treatment against Antibiotic-Resistant Staphylococcus Aureus. J. Antimicrob. Chemother. 2020, 75, 1787–1798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).