Individual Postprandial Glycemic Responses to Meal Types by Different Carbohydrate Levels and Their Associations with Glycemic Variability Using Continuous Glucose Monitoring
Abstract
1. Introduction
2. Materials and Methods
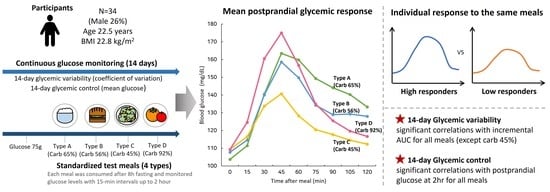
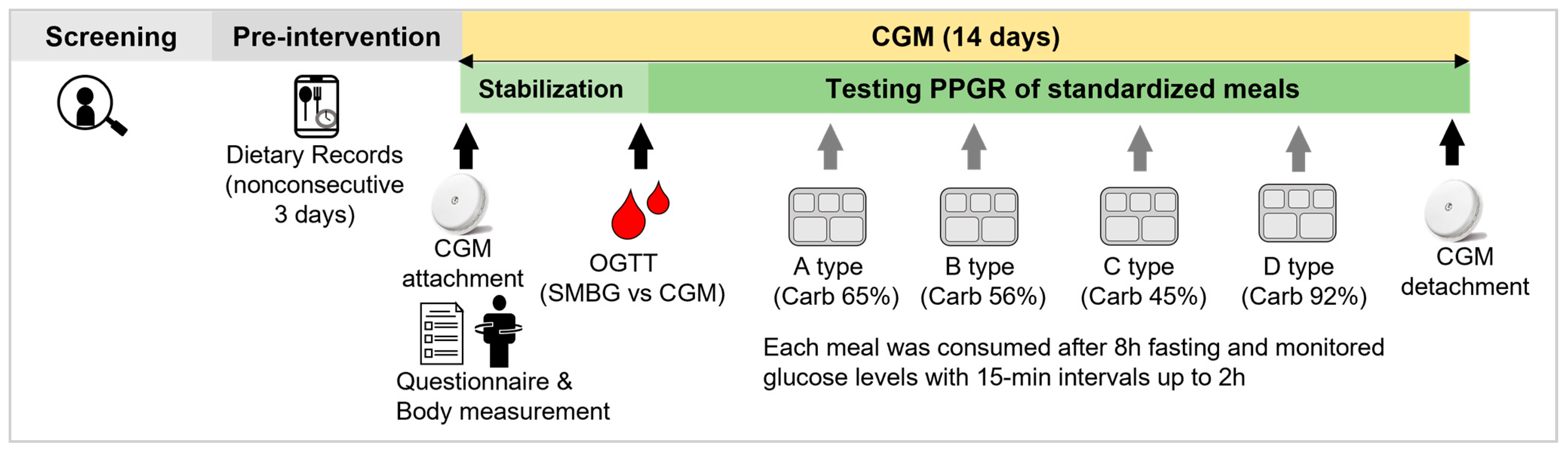
2.1. Study Design and Participants
2.2. Standardized Test Meals
2.3. Postprandial Glycemic Response and Grouping
2.4. CGM Measurement
2.5. Body Measurement
2.6. Dietary Assessment
2.7. Other Variables
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Mean Postprandial Glycemic Response According to Meal Type
3.3. Individual Postprandial Glycemic Responses According to Meal Type
3.4. High- vs. Low-Response Groups for Each Meal Type
3.5. Factors Associated with 14-Day Glycemic Variability and Control
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Keefe, J.H.; Bell, D.S.H. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Song, Y.; Ford, E.S.; Liu, S. Is Nondiabetic Hyperglycemia a Risk Factor for Cardiovascular Disease? Arch. Intern. Med. 2004, 164, 2147. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Ito, C.; Fujikawa, R.; Yamamoto, H.; Kihara, Y. Association between the Postprandial Glucose Levels and Arterial Stiffness Measured According to the Cardio-ankle Vascular Index in Non-diabetic Subjects. Intern. Med. 2015, 54, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Lim, S.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Cho, Y.M.; Park, K.S.; Jang, H.; Cho, N.H. One-hour postload plasma glucose concentration in people with normal glucose homeostasis predicts future diabetes mellitus: A 12-year community-based cohort study. Clin. Endocrinol. 2017, 86, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Dietary carbohydrate: Relationship to cardiovascular disease and disorders of carbohydrate metabolism. Eur. J. Clin. Nutr. 2007, 61, S100–S111. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.D.; Ismail, A.A.-S. Glycemic Responses of Patients with Type 2 Diabetes to Individual Carbohydrate-Rich Foods and Mixed Meals. Ann. Nutr. Metab. 2012, 60, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ballance, S.; Knutsen, S.H.; Fosvold, Ø.W.; Fernandez, A.S.; Monro, J. Predicting mixed-meal measured glycaemic index in healthy subjects. Eur. J. Nutr. 2019, 58, 2657–2667. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Chang, C.R.; Francois, M.E.; Little, J.P. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019, 109, 1302–1309. [Google Scholar] [CrossRef]
- Rasmussen, L.; Christensen, M.L.; Poulsen, C.W.; Rud, C.; Christensen, A.S.; Andersen, J.R.; Kampmann, U.; Ovesen, P.G. Effect of High Versus Low Carbohydrate Intake in the Morning on Glycemic Variability and Glycemic Control Measured by Continuous Blood Glucose Monitoring in Women with Gestational Diabetes Mellitus—A Randomized Crossover Study. Nutrients 2020, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, I.; Joung, H. A rice-based traditional dietary pattern is associated with obesity in Korean adults. J. Acad. Nutr. Diet. 2012, 112, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Camps, S.G.; Kaur, B.; Lim, J.; Loo, Y.T.; Pang, E.; Ng, T.; Henry, C.J. Improved Glycemic Control and Variability: Application of Healthy Ingredients in Asian Staples. Nutrients 2021, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Bergia, R.E.; Giacco, R.; Hjorth, T.; Biskup, I.; Zhu, W.; Costabile, G.; Vitale, M.; Campbell, W.W.; Landberg, R.; Riccardi, G. Differential Glycemic Effects of Low- versus High-Glycemic Index Mediterranean-Style Eating Patterns in Adults at Risk for Type 2 Diabetes: The MEDGI-Carb Randomized Controlled Trial. Nutrients 2022, 14, 706. [Google Scholar] [CrossRef]
- Kizirian, N.V.; Goletzke, J.; Brodie, S.; Atkinson, F.S.; Markovic, T.P.; Ross, G.P.; Buyken, A.; Brand-Miller, J.P. Lower glycemic load meals reduce diurnal glycemic oscillations in women with risk factors for gestational diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000351. [Google Scholar] [CrossRef] [PubMed]
- Tettamanzi, F.; Bagnardi, V.; Louca, P.; Nogal, A.; Monti, G.S.; Mambrini, S.P.; Lucchetti, E.; Maestrini, S.; Mazza, S.; Rodriguez-Mateos, A.; et al. A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study. Nutrients 2021, 13, 4380. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Kim, J.S.; Nam, K.; Chung, S.J. Effect of nutrient composition in a mixed meal on the postprandial glycemic response in healthy people: A preliminary study. Nutr. Res. Pract. 2019, 13, 126–133. [Google Scholar] [CrossRef]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and Reliability of Korean Version of International Physical Activity Questionnaire (IPAQ) Short Form. J. Korean Acad. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Song, Y.; Joung, H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Mondal, S. Clarke Error Grid Analysis on Graph Paper and Microsoft Excel. J. Diabetes Sci. Technol. 2020, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. Bmj 2012, 344, e1454. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.; Ben-Yacov, O.; Godneva, A.; Shilo, S.; Zmora, N.; Kolobkov, D.; Cohen-Dolev, N.; Wolf, B.-C.; Kosower, N.; Lotan-Pompan, M.; et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: A randomized dietary intervention pilot trial. BMC Med. 2022, 20, 56. [Google Scholar] [CrossRef]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Kolobkov, D.; Koren, N.; Cohen Dolev, N.; Travinsky Shmul, T.; Wolf, B.C.; Kosower, N.; et al. Personalized Postprandial Glucose Response-Targeting Diet Versus Mediterranean Diet for Glycemic Control in Prediabetes. Diabetes Care 2021, 44, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.E.M.; Evans, M.; O’Connell, S.M.; McElduff, P.; Lopez, P.E.; Jones, T.W.; Davis, E.A.; King, B.R. Both Dietary Protein and Fat Increase Postprandial Glucose Excursions in Children with Type 1 Diabetes, and the Effect Is Additive. Diabetes Care 2013, 36, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Fechner, E.; Op’t Eyndt, C.; Mulder, T.; Mensink, R.P. Diet-induced differences in estimated plasma glucose concentrations in healthy, non-diabetic adults are detected by continuous glucose monitoring—A randomized crossover trial. Nutr. Res. 2020, 80, 36–43. [Google Scholar] [CrossRef]
- Haldar, S.; Egli, L.; De Castro, C.A.; Tay, S.L.; Koh, M.X.N.; Darimont, C.; Mace, K.; Henry, C.J. High or low glycemic index (GI) meals at dinner results in greater postprandial glycemia compared with breakfast: A randomized controlled trial. BMJ Open Diabetes Res. Care 2020, 8, e001099. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Brighenti, F.; Benini, L.; Del Rio, D.; Casiraghi, C.; Pellegrini, N.; Scazzina, F.; Jenkins, D.J.; Vantini, I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 2006, 83, 817–822. [Google Scholar] [CrossRef]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L.; et al. Fresh Fruit Consumption and Major Cardiovascular Disease in China. N. Engl. J. Med. 2016, 374, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Ha, K.; Joung, H.; Song, Y. Frequency of Consumption of Whole Fruit, Not Fruit Juice, Is Associated with Reduced Prevalence of Obesity in Korean Adults. J. Acad. Nutr. Diet. 2019, 119, 1842–1851.e1842. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S68–S96. [Google Scholar] [CrossRef] [PubMed]



| Meal Type | Amount (g) | Glycemic Index | Energy (kcal) | Carb (g) | Sugars (g) | Fiber (g) | Protein (g) | Fat (g) | SFA (g) |
|---|---|---|---|---|---|---|---|---|---|
| Type A. Bibimbap | |||||||||
| Rice | 210.0 | 72.0 | 305.2 | 70.5 | 1.2 | 2.5 | 4.4 | 0.6 | 0.2 |
| Fried egg | 34.0 | 0.0 | 66.9 | 1.3 | 0.0 | 0.0 | 5.1 | 4.6 | 1.3 |
| Stir-fried beef | 17.0 | 43.0 | 22.7 | 0.7 | 0.0 | 0.0 | 2.0 | 1.3 | 0.6 |
| Seasoned vegetables | 68.0 | 42.0 | 78.5 | 6.0 | 1.3 | 3.4 | 2.4 | 5.0 | 0.7 |
| Stir-fried mushrooms | 23.0 | 31.0 | 30.7 | 2.3 | 0.0 | 2.1 | 0.7 | 2.1 | 0.1 |
| Red chili paste | 15.0 | 21.0 | 35.1 | 7.8 | 0.1 | 0.8 | 0.5 | 0.2 | 0.0 |
| Soybean paste soup | 168.0 | 14.0 | 19.3 | 2.2 | 0.0 | 1.0 | 1.3 | 0.6 | 0.1 |
| Sum | 535.0 | 63.7 | 558.4 | 90.8 | 2.5 | 9.8 | 16.4 | 14.4 | 3.0 |
| (65.0%) | (11.8%) | (23.1%) | (0.1%) | ||||||
| Type B. Sandwich | |||||||||
| Ciabatta | 120.0 | 61.0 | 358.2 | 65.8 | 0.0 | 2.7 | 12.4 | 5.0 | 0.7 |
| Ham | 60.0 | 0.0 | 72.3 | 1.2 | 0.0 | 0.0 | 12.4 | 2.0 | 0.8 |
| Cheese | 10.0 | 0.0 | 28.6 | 0.5 | 0.0 | 0.0 | 2.8 | 1.7 | 1.9 |
| Lettuce | 35.0 | 32.0 | 7.3 | 1.6 | 0.1 | 0.5 | 0.2 | 0.0 | 0.0 |
| Tomato | 50.0 | 23.0 | 11.4 | 2.1 | 0.6 | 1.3 | 0.5 | 0.1 | 0.0 |
| Mustard source | 10.0 | 32.0 | 31.5 | 4.0 | 0.0 | 0.7 | 0.6 | 1.5 | 0.1 |
| Soybean milk | 190.0 | 44.0 | 163.9 | 18.8 | 4.0 | 1.7 | 6.1 | 7.2 | 1.2 |
| Sum | 440.0 | 54.2 | 673.2 | 94.0 | 4.7 | 6.9 | 35.0 | 17.5 | 4.7 |
| (55.9%) | (20.8%) | (23.4%) | (0.1%) | ||||||
| Type C. Chicken salad | |||||||||
| Romaine | 48.0 | 32.0 | 13.6 | 2.2 | 0.4 | 1.8 | 0.9 | 0.2 | 0.0 |
| Asparagus | 18.0 | 32.0 | 3.8 | 0.5 | 0.1 | 0.5 | 0.4 | 0.0 | 0.0 |
| Tomato | 40.0 | 23.0 | 11.7 | 2.4 | 0.8 | 0.8 | 0.4 | 0.1 | 0.0 |
| Cranberry | 10.0 | 45.0 | 34.9 | 8.6 | 3.4 | 0.6 | 0.0 | 0.1 | 0.0 |
| Sweet pumpkin | 60.0 | 64.0 | 45.7 | 9.3 | 1.2 | 3.1 | 1.0 | 0.5 | 0.1 |
| Ricotta cheese | 55.0 | 27.0 | 87.3 | 3.7 | 0.0 | 0.0 | 3.9 | 6.4 | 4.0 |
| Chicken breast | 85.0 | 0.0 | 102.6 | 0.0 | 0.0 | 0.0 | 23.9 | 0.8 | 1.2 |
| Sweet potato, baked | 65.0 | 70.0 | 121.1 | 29.2 | 0.0 | 2.4 | 0.8 | 0.1 | 0.0 |
| Balsamic dressing | 30.0 | 50.0 | 104.0 | 3.4 | 1.5 | 0.0 | 0.1 | 10.0 | 0.4 |
| Sum | 411.0 | 58.0 | 524.8 | 59.3 | 7.4 | 9.2 | 31.4 | 18.2 | 5.7 |
| (45.1%) | (23.9%) | (30.9%) | (0.1%) | ||||||
| Type D. Fruit bowl | |||||||||
| Apple | 150.0 | 44.0 | 93.1 | 20.4 | 9.6 | 4.1 | 0.3 | 1.2 | 0.0 |
| Orange | 200.0 | 45.0 | 104.7 | 23.6 | 5.2 | 4.2 | 1.8 | 0.3 | 0.0 |
| Banana | 105.0 | 47.0 | 97.7 | 23.0 | 7.0 | 2.0 | 1.2 | 0.1 | 0.0 |
| Green grape | 70.0 | 54.0 | 50.3 | 12.2 | 5.6 | 1.2 | 0.2 | 0.0 | 0.0 |
| Sum | 525.0 | 46.7 | 345.8 | 79.2 | 27.4 | 11.5 | 3.5 | 1.6 | 0.1 |
| (91.7%) | (4.0%) | (4.2%) | (0.0%) |
| Total n = 34 | Men n = 9 | Women n = 25 | p Value 1 | |
|---|---|---|---|---|
| Basic characteristics | ||||
| Age (year) (mean ± SD) | 22.5 ± 3.0 | 24.0 ± 1.7 | 22.0 ± 3.2 | 0.0174 |
| Alcohol consumption, n (%) | 0.7362 | |||
| Yes | 25 (73.5) | 7 (20.6) | 18 (52.9) | |
| No | 9 (26.5) | 2 (5.9) | 7 (20.6) | |
| Current smoker, n (%) | 0.0033 | |||
| Yes | 5 (14.7) | 4 (11.8) | 1 (2.9) | |
| No | 29 (85.3) | 5 (14.7) | 24 (70.6) | |
| Physical activity, n (%) | 0.0464 | |||
| High | 9 (26.5) | 5 (14.7) | 4 (11.8) | |
| Moderate | 20 (58.8) | 4 (11.8) | 16 (47.1) | |
| Low | 5 (14.7) | 0 (0.0) | 5 (14.7) | |
| Body measurement | ||||
| Body mass index (kg/m2) | 22.8 ± 3.8 | 24.7 ± 4.0 | 22.1 ± 3.5 | 0.0725 |
| Muscle mass (kg) | 24.1 ± 6.4 | 33.0 ± 5.1 | 20.8 ± 2.8 | <0.0001 |
| Fat mass (kg) | 19.0 ± 8.3 | 16.9 ± 8.4 | 19.7 ± 8.2 | 0.5981 |
| Body fat (%) | 29.7 ± 8.9 | 21.3 ± 8.3 | 32.8 ± 7.1 | 0.0034 |
| Waist circumference (cm) | 74.8 ± 10.1 | 83.5 ± 9.5 | 71.6 ± 8.5 | 0.0025 |
| Nutrient intake | ||||
| Energy (kcal) | 1492.9 ± 533.5 | 1770 ± 758.4 | 1393.1 ± 400.6 | 0.2116 |
| Energy (% of EER) | 65 ± 20.3 | 62.2 ± 22.7 | 66 ± 19.7 | 0.4823 |
| Carbohydrate (%) | 51.1 ± 7.7 | 52.3 ± 12.3 | 50.6 ± 5.5 | 0.2919 |
| Protein (%) | 17.1 ± 3.1 | 17.6 ± 3.4 | 16.9 ± 3 | 0.7253 |
| Fat (%) | 32.4 ± 7.9 | 30.5 ± 12.5 | 33.1 ± 5.7 | 0.2416 |
| Sugars (%) | 11.7 ± 4.6 | 9.4 ± 3.5 | 12.5 ± 4.7 | 0.0610 |
| Saturated fat (%) | 11.7 ± 3.9 | 11.2 ± 6.1 | 11.9 ± 2.8 | 0.5069 |
| 14-Day Glycemic Variability | 14-Day Glycemic Control | |||
|---|---|---|---|---|
| Coefficient 2 | (p Value) | Coefficient 2 | (p Value) | |
| Basic and body measurement | ||||
| Age (year) | 0.2371 | (0.1991) | 0.2486 | (0.1775) |
| Body weight (kg) | −0.1919 | (0.3010) | −0.0285 | (0.8790) |
| Muscle mass (kg) | −0.0775 | (0.6785) | 0.1396 | (0.4539) |
| Body fat (%) | −0.1366 | (0.4637) | −0.1923 | (0.3001) |
| Postprandial glucose 2 h after test meals | ||||
| Glucose 75 g | 0.5547 | (0.0012) | 0.4969 | (0.0045) |
| Type A. Rice and soup | 0.3325 | (0.0676) | 0.6399 | (0.0001) |
| Type B. Sandwich and soymilk | 0.5039 | (0.0038) | 0.5445 | (0.0015) |
| Type C. Chicken salad | 0.0759 | (0.6847) | 0.4192 | (0.0189) |
| Type D. Fruit bowl | 0.5088 | (0.0035) | 0.3586 | (0.0476) |
| Incremental area under curve after test meals | ||||
| Glucose 75 g | 0.6363 | (0.0001) | 0.4057 | (0.0236) |
| Type A. Rice and soup | 0.5785 | (0.0007) | 0.4537 | (0.0104) |
| Type B. Sandwich and soymilk | 0.6842 | (<0.0001) | −0.0158 | (0.9327) |
| Type C. Chicken salad | 0.2428 | (0.1882) | 0.2322 | (0.2088) |
| Type D. Fruit bowl | 0.6023 | (0.0003) | 0.2204 | (0.2334) |
| Daily dietary intake from 3 days of dietary records | ||||
| Energy (kcal) | −0.0367 | (0.8448) | 0.0373 | (0.8423) |
| Carbohydrate (%) | 0.0314 | (0.8670) | 0.0019 | (0.9917) |
| Protein (%) | 0.1418 | (0.4466) | 0.0571 | (0.7602) |
| Fat (%) | −0.1514 | (0.4162) | −0.0223 | (0.9052) |
| Average fasting blood glucose 1 | 0.0942 | (0.6143) | 0.7568 | (<0.0001) |
| CGM mean glucose (mg/dL) | 0.2406 | (0.1922) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Oh, T.J.; Song, Y. Individual Postprandial Glycemic Responses to Meal Types by Different Carbohydrate Levels and Their Associations with Glycemic Variability Using Continuous Glucose Monitoring. Nutrients 2023, 15, 3571. https://doi.org/10.3390/nu15163571
Song J, Oh TJ, Song Y. Individual Postprandial Glycemic Responses to Meal Types by Different Carbohydrate Levels and Their Associations with Glycemic Variability Using Continuous Glucose Monitoring. Nutrients. 2023; 15(16):3571. https://doi.org/10.3390/nu15163571
Chicago/Turabian StyleSong, Jiwoo, Tae Jung Oh, and YoonJu Song. 2023. "Individual Postprandial Glycemic Responses to Meal Types by Different Carbohydrate Levels and Their Associations with Glycemic Variability Using Continuous Glucose Monitoring" Nutrients 15, no. 16: 3571. https://doi.org/10.3390/nu15163571
APA StyleSong, J., Oh, T. J., & Song, Y. (2023). Individual Postprandial Glycemic Responses to Meal Types by Different Carbohydrate Levels and Their Associations with Glycemic Variability Using Continuous Glucose Monitoring. Nutrients, 15(16), 3571. https://doi.org/10.3390/nu15163571