Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications
Abstract
1. Introduction
2. Developmental Processes of the Placenta
2.1. Placental Cell Types in the Human and Mouse Placenta
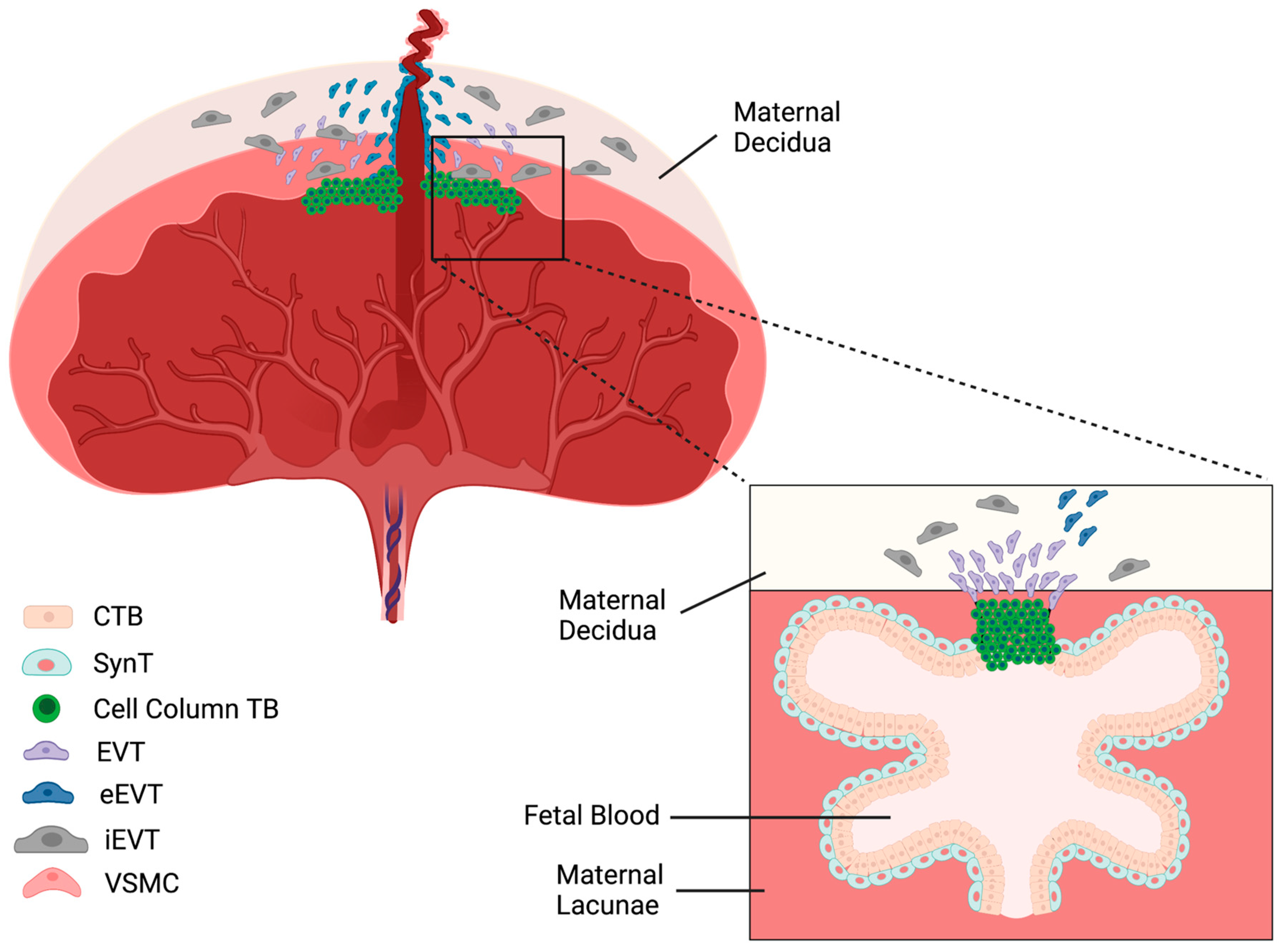
2.1.1. Distinct Types of Trophoblast Cells in the Human Placenta
2.1.2. Distinct Types of Trophoblast Cells in the Mouse Placenta
2.1.3. Comparative Roles of Cell Types in the Mouse and Human Placentas
2.2. Differentiation of Trophoblast Cells
2.2.1. The Developmental Stages of Human Trophoblast Differentiation
2.2.2. The Developmental Stages of Mouse Trophoblast Differentiation
3. Important Cell Signaling in Trophoblast Differentiation
3.1. FGF4 Signaling Pathway
3.1.1. FGF4
3.1.2. Cross-Talk between FGF4 and Other Signaling Pathways
3.2. IGF-1 Pathway
3.2.1. IGF-1
3.2.2. Cross-Talk between IGF-1 and Other Signaling Pathways
3.3. TGF-β Signaling
3.3.1. TGF-β
3.3.2. Nodal
3.3.3. Activin
3.4. Hypoxia and HIF-1
3.5. Retinoic Acid Signaling Pathway
4. Epigenetic Regulation of Trophoblast Differentiation
5. Biomarkers of Trophoblast Cells
5.1. Extraembryonic Ectoderm, Ectoplacental Cone, and Chorion
5.2. Spongiotrophoblast Cells
5.3. Glycogen Trophoblast Cells
5.4. Trophoblast Giant Cells
5.5. Syncytiotrophoblast Cells
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, T.; Chen, S. Risk of abnormal fetal growth in women with early-and late-onset preeclampsia. Pregnancy Hypertens. 2018, 12, 201–206. [Google Scholar] [CrossRef] [PubMed]
- McKinney, D.; Boyd, H.; Langager, A.; Oswald, M.; Pfister, A.; Warshak, C.R. The impact of fetal growth restriction on latency in the setting of expectant management of preeclampsia. Obstet. Gynecol. 2016, 214, 395.e1–395.e7. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, M.T.; Hickman, A.F.; Maser, B.; Pudwell, J.; Smith, G.N.; Brien, D.; Stroman, P.W.; Adams, M.A.; Reynolds, J.N.; Croy, B.A.; et al. Impact of preeclampsia on cognitive function in the offspring. Behav. Brain Res. 2016, 302, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, M.T.; Paolozza, A.; Hickman, A.F.; Maser, B.; Kay, V.R.; Mohammad, S.; Pudwell, J.; Smith, G.N.; Brien, D.; Stroman, P.W.; et al. Brain structural and vascular anatomy is altered in offspring of pre-eclamptic pregnancies: A pilot study. Am. J. Neuroradiol. 2016, 37, 939–945. [Google Scholar] [CrossRef]
- Wardinger, J.E.; Ambati, S. Placental insufficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Garovic, V.D.; Bailey, K.R.; Boerwinkle, E.; Hunt, S.C.; Weder, A.B.; Curb, D.; Mosley, T.H., Jr.; Wiste, H.J.; Turner, S.T. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J. Hypertens. 2010, 28, 826–833. [Google Scholar] [CrossRef]
- Mann, J.I.; Doll, R.; Thorogood, M.; Vessey, M.P.; Waters, W.E. Risk factors for myocardial infarction in young women. J. Epidemiol. Community Health 1976, 30, 94–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnadottir, G.A.; Geirsson, R.T.; Arngrimsson, R.; Jonsdottir, L.S.; Olafsson, Ö. Cardiovascular death in women who had hypertension in pregnancy: A case–control study. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 286–292. [Google Scholar] [CrossRef]
- Wu, C.; Chen, S.; Ho, C.; Liang, F.; Chu, C.; Wang, H.; Lu, Y. End-stage renal disease after hypertensive disorders in pregnancy. Obstet. Gynecol. 2014, 210, 147.e1–147.e8. [Google Scholar] [CrossRef]
- Wang, I.; Muo, C.; Chang, Y.; Liang, C.; Chang, C.; Lin, S.; Yen, T.; Chuang, F.; Chen, P.; Huang, C. Association between hypertensive disorders during pregnancy and end-stage renal disease: A population-based study. CMAJ 2013, 185, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; de Oliveira, A.C.M.; Goulart, M.O.F. Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxid. Med. Cell. Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef] [PubMed]
- Berzan, E.; Doyle, R.; Brown, C.M. Treatment of preeclampsia: Current approach and future perspectives. Curr. Hypertens. Rep. 2014, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Varberg, K.M.; Soares, M.J. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021, 113, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.C.; Vong, J.S.; Ji, L.; Poon, L.C.; Jiang, P.; Lui, K.O.; Ni, Y.; To, K.F.; Cheng, Y.K.; Chiu, R.W.; et al. Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, E7786–E7795. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Liu, Y.; Dang, Y.; Jiang, X.; Xu, H.; Huang, X.; Wang, Y.; Wang, H.; Zhu, C.; Xue, L.; et al. PLAC8, a new marker for human interstitial extravillous trophoblast cells, promotes their invasion and migration. Development 2018, 145, dev148932. [Google Scholar] [CrossRef]
- Kreis, N.; Friemel, A.; Ritter, A.; Roth, S.; Rolle, U.; Louwen, F.; Yuan, J. Function of p21 (Cip1/Waf1/CDKN1A) in migration and invasion of cancer and trophoblastic cells. Cancers 2019, 11, 989. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, L.; Liang, X.; Wang, H. Progress in deciphering trophoblast cell differentiation during human placentation. Curr. Opin. Cell Biol. 2020, 67, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Brkić, J.; Dunk, C.; O’Brien, J.; Fu, G.; Nadeem, L.; Wang, Y.; Rosman, D.; Salem, M.; Shynlova, O.; Yougbaré, I. MicroRNA-218-5p promotes endovascular trophoblast differentiation and spiral artery remodeling. Mol. Ther. 2018, 26, 2189–2205. [Google Scholar] [CrossRef]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef]
- Soares, M.J.; Varberg, K.M.; Iqbal, K. Hemochorial placentation: Development, function, and adaptations. Biol. Reprod. 2018, 99, 196–211. [Google Scholar] [CrossRef]
- Barreto, R.S.N.; Romagnolli, P.; Cereta, A.D.; Coimbra-Campos, L.M.C.; Birbrair, A.; Miglino, M.A. Pericytes in the Placenta: Role in Placental Development and Homeostasis. Adv. Exp. Med. Biol. 2019, 1122, 125–151. [Google Scholar]
- Brosens, I.; Robertson, W.B.; Dixon, H.G. The physiological response of the vessels of the placental bed to normal pregnancy. J. Pathol. Bacteriol. 1967, 93, 569–579. [Google Scholar] [CrossRef]
- Whitley, G.S.J.; Cartwright, J.E. Cellular and molecular regulation of spiral artery remodelling: Lessons from the cardiovascular field. Placenta 2010, 31, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kolahi, K.S.; Valent, A.M.; Thornburg, K.L. Cytotrophoblast, not syncytiotrophoblast, dominates glycolysis and oxidative phosphorylation in human term placenta. Sci. Rep. 2017, 7, 42941. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Gamage, T.K.; Chamley, L.W.; James, J.L. Stem cell insights into human trophoblast lineage differentiation. Hum. Reprod. Update 2017, 23, 77–103. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Wang, R.; Lu, X.; Dang, Y.; Wang, H.; Lin, H.; Zhu, C.; Ge, H.; Cross, J.C.; et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 2018, 28, 819–832. [Google Scholar] [CrossRef]
- Benirschke, K.; Kaufman, P.; Qumsiyeh, M.B. Pathology of the human placenta. Int. J. Gynecol. Pathol. 1998, 17, 93. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What is the placenta? Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef]
- Fisher, S.J.; Damsky, C.H. Human cytotrophoblast invasion. Semin. Cell Biol. 1993, 4, 183–188. [Google Scholar] [CrossRef]
- Hu, D.; Cross, J.C. Development and function of trophoblast giant cells in the rodent placenta. Int. J. Dev. Biol. 2009, 54, 341–354. [Google Scholar] [CrossRef]
- Robson, A.; Harris, L.K.; Innes, B.A.; Lash, G.E.; Aljunaidy, M.M.; Aplin, J.D.; Baker, P.N.; Robson, S.C.; Bulmer, J.N. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012, 26, 4876–4885. [Google Scholar] [CrossRef]
- Chen, J.; Khalil, R.A. Matrix metalloproteinases in normal pregnancy and preeclampsia. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–165. [Google Scholar]
- Plaks, V.; Rinkenberger, J.; Dai, J.; Flannery, M.; Sund, M.; Kanasaki, K.; Ni, W.; Kalluri, R.; Werb, Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc. Natl. Acad. Sci. USA 2013, 110, 11109–11114. [Google Scholar] [CrossRef] [PubMed]
- Soncin, F.; Natale, D.; Parast, M.M. Signaling pathways in mouse and human trophoblast differentiation: A comparative review. Cell. Mol. Life Sci. 2015, 72, 1291–1302. [Google Scholar] [CrossRef]
- Cross, J.C.; Hemberger, M.; Lu, Y.; Nozaki, T.; Whiteley, K.; Masutani, M.; Adamson, S.L. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol. Cell. Endocrinol. 2002, 187, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.G.; Fortier, A.L.; Cross, J.C. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev. Biol. 2007, 304, 567–578. [Google Scholar] [CrossRef]
- Coan, P.M.; Conroy, N.; Burton, G.J.; Ferguson-Smith, A.C. Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn. 2006, 235, 3280–3294. [Google Scholar] [CrossRef]
- Simmons, D.G.; Cross, J.C. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev. Biol. 2005, 284, 12–24. [Google Scholar] [CrossRef]
- Zhu, D.; Gong, X.; Miao, L.; Fang, J.; Zhang, J. Efficient induction of syncytiotrophoblast layer II cells from trophoblast stem cells by canonical Wnt signaling activation. Stem Cell Rep. 2017, 9, 2034–2049. [Google Scholar] [CrossRef]
- Ochiai, Y.; Suzuki, C.; Segawa, K.; Uchiyama, Y.; Nagata, S. Inefficient development of syncytiotrophoblasts in the Atp11a-deficient mouse placenta. Proc. Natl. Acad. Sci. USA 2022, 119, e2200582119. [Google Scholar] [CrossRef] [PubMed]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of human trophoblast stem cells. Cell Stem Cell 2018, 22, 50–63.e6. [Google Scholar] [CrossRef]
- Lee, C.Q.; Gardner, L.; Turco, M.; Zhao, N.; Murray, M.J.; Coleman, N.; Rossant, J.; Hemberger, M.; Moffett, A. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 2016, 6, 257–272. [Google Scholar] [CrossRef]
- Li, Y.; Moretto-Zita, M.; Leon-Garcia, S.; Parast, M.M. p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am. J. Pathol. 2014, 184, 3332–3343. [Google Scholar] [CrossRef]
- James, J.L.; Carter, A.M.; Chamley, L.W. Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation? Placenta 2012, 33, 327–334. [Google Scholar] [CrossRef]
- Boss, A.L.; Chamley, L.W.; James, J.L. Placental formation in early pregnancy: How is the centre of the placenta made? Hum. Reprod. Update 2018, 24, 750–760. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Dixon, G.; Robertson, W.B.; Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Haider, S.; Lackner, A.I.; Dietrich, B.; Kunihs, V.; Haslinger, P.; Meinhardt, G.; Maxian, T.; Saleh, L.; Fiala, C.; Pollheimer, J.; et al. Transforming growth factor-β signaling governs the differentiation program of extravillous trophoblasts in the developing human placenta. Proc. Natl. Acad. Sci. USA 2022, 119, e2120667119. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. The influence of the intrauterine environment on human placental development. J. Reprod. Immunol. 2010, 2, 81–82. [Google Scholar] [CrossRef]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef]
- Gardner, R.L.; Papaioannou, V.E.; Barton, S.C. Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. Development 1973, 30, 561–572. [Google Scholar] [CrossRef]
- Tanaka, S.; Kunath, T.; Hadjantonakis, A.; Nagy, A.; Rossant, J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998, 282, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, F.; Nagy, A.; Auerbach, A.; Rossant, J.; Joyner, A.L. Essential role of Mash-2 in extraembryonic development. Nature 1994, 371, 333–336. [Google Scholar] [CrossRef]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of placental development and its impact on fetal growth—New insights from mouse models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cross, J.C. Ablation of Tpbpa-positive trophoblast precursors leads to defects in maternal spiral artery remodeling in the mouse placenta. Dev. Biol. 2011, 358, 231–239. [Google Scholar] [CrossRef]
- Hemberger, M.; Hughes, M.; Cross, J.C. Trophoblast stem cells differentiate in vitro into invasive trophoblast giant cells. Dev. Biol. 2004, 271, 362–371. [Google Scholar] [CrossRef]
- Scott, I.C.; Anson-Cartwright, L.; Riley, P.; Reda, D.; Cross, J.C. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol. Cell. Biol. 2000, 20, 530–541. [Google Scholar] [CrossRef]
- Riley, P.; Anaon-Cartwight, L.; Cross, J.C. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 1998, 18, 271–275. [Google Scholar] [CrossRef]
- Courtney, J.A.; Wilson, R.L.; Cnota, J.; Jones, H.N. Conditional mutation of Hand1 in the mouse placenta disrupts placental vascular development resulting in fetal loss in both early and late pregnancy. Int. J. Mol. Sci. 2021, 22, 9532. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.; Li, X. FGF signaling pathway: A key regulator of stem cell pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Hui, Q.; Jin, Z.; Li, X.; Liu, C.; Wang, X. FGF family: From drug development to clinical application. Int. J. Mol. Sci. 2018, 19, 1875. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.; Shin, U.S.; Kim, H. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef]
- Rossant, J.; Gardner, R.L.; Alexandre, H.L. Investigation of the potency of cells from the postimplantation mouse embryo by blastocyst injection: A preliminary report. J. Embryol. Exp. Morphol. 1978, 48, 239–247. [Google Scholar] [CrossRef]
- Johnson, M.H.; Rossant, J. Molecular studies on cells of the trophectodermal lineage of the postimplantation mouse embryo. J. Embryol. Exp. Morphol. 1981, 61, 103–116. [Google Scholar] [CrossRef]
- Carney, E.W.; Prideaux, V.; Lye, S.J.; Rossant, J. Progressive expression of trophoblast-specific genes during formation of mouse trophoblast giant cells in vitro. Mol. Reprod. Dev. 1993, 34, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Ayala, M.; Ben-Haim, N.; Beck, S.; Constam, D.B. Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. Proc. Natl. Acad. Sci. USA 2004, 101, 15656–15660. [Google Scholar] [CrossRef]
- Senner, C.E.; Hemberger, M. Regulation of early trophoblast differentiation–lessons from the mouse. Placenta 2010, 31, 944–950. [Google Scholar] [CrossRef]
- Feng, G. Shp-2 tyrosine phosphatase: Signaling one cell or many. Exp. Cell Res. 1999, 253, 47–54. [Google Scholar] [CrossRef]
- Yang, W.; Klaman, L.D.; Chen, B.; Araki, T.; Harada, H.; Thomas, S.M.; George, E.L.; Neel, B.G. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell 2006, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.N.; Granger, D.A.; Johnson, N.L.; Vincent-Jordan, N.; Dibble, C.F.; Johnson, G.L. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol. Cell. Biol. 2009, 29, 2748–2761. [Google Scholar] [CrossRef][Green Version]
- Jeong, W.; Lee, J.; Bazer, F.W.; Song, G.; Kim, J. Fibroblast growth factor 4-induced migration of porcine trophectoderm cells is mediated via the AKT cell signaling pathway. Mol. Cell. Endocrinol. 2016, 419, 208–216. [Google Scholar] [CrossRef]
- Natale, D.R.; Hemberger, M.; Hughes, M.; Cross, J.C. Activin promotes differentiation of cultured mouse trophoblast stem cells towards a labyrinth cell fate. Dev. Biol. 2009, 335, 120–131. [Google Scholar] [CrossRef]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.J.; Pine-Twaddell, E.; Sima, D.I.; Miller, R.S.; He, L.; Wondisford, F.; Radovick, S. Insulin-like growth factor 1 mediates negative feedback to somatotroph GH expression via POU1F1/CREB binding protein interactions. Mol. Cell. Biol. 2012, 32, 4258–4269. [Google Scholar] [CrossRef]
- Yakar, S.; Liu, J.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; LeRoith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef]
- Han, V.K.; Bassett, N.; Walton, J.; Challis, J.R. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: Evidence for IGF-IGFBP interactions at the feto-maternal interface. J. Clin. Endocrinol. Metab. 1996, 81, 2680–2693. [Google Scholar] [PubMed]
- Kamei, T.; Jones, S.R.; Chapman, B.M.; McGonigle, K.L.; Dai, G.; Soares, M.J. The phosphatidylinositol 3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol. Endocrinol. 2002, 16, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhu, T.; Tan, N.; Jing, G.; Dang, Y.; Li, Z. In missed abortion the decrease of IGF-1 down-regulates PI3K/AKT signaling pathway reducing the secretion of progesterone and β-hCG. Growth Horm. IGF Res. 2022, 65, 101479. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A. Biological functions of hCG and hCG-related molecules. Reprod. Biol. Endocrinol. 2010, 8, 102. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Schlupf, J.; Steinbeisser, H. IGF antagonizes the Wnt/β-Catenin pathway and promotes differentiation of extra-embryonic endoderm. Differentiation 2014, 87, 209–219. [Google Scholar] [CrossRef]
- Forbes, K.; Westwood, M.; Baker, P.N.; Aplin, J.D. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am. J. Physiol.-Cell Physiol. 2008, 294, C1313–C1322. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knöfler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell. Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Heldin, C.; Moustakas, A. Signaling receptors for TGF-β family members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef] [PubMed]
- Adu-Gyamfi, E.A.; Ding, Y.; Wang, Y. Regulation of placentation by the transforming growth factor beta superfamily. Biol. Reprod. 2020, 102, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Ray, P.D.; Smeester, L.; Grace, M.R.; Boggess, K.; Fry, R.C. Epigenetics and preeclampsia: Defining functional epimutations in the preeclamptic placenta related to the TGF-β pathway. PLoS ONE 2015, 10, e0141294. [Google Scholar] [CrossRef]
- Brkić, J.; Dunk, C.; Shan, Y.; O’Brien, J.A.; Lye, P.; Qayyum, S.; Yang, P.; Matthews, S.G.; Lye, S.J.; Peng, C. Differential role of Smad2 and Smad3 in the acquisition of an endovascular trophoblast-like phenotype and preeclampsia. Front. Endocrinol. 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Lysiak, J.J.; McCrae, K.R.; Lala, P.K. Localization of transforming growth factor-β at the human fetal-maternal interface: Role in trophoblast growth and differentiation. Biol. Reprod. 1992, 46, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Albers, R.E.; Selesniemi, K.; Natale, D.R.; Brown, T.L. TGF-β induces Smad2 phosphorylation, ARE induction, and trophoblast differentiation. Int. J. Stem Cells 2018, 11, 111–120. [Google Scholar] [CrossRef]
- Caniggia, I.; Lye, S.J.; Cross, J.C. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 1997, 138, 3976–3986. [Google Scholar] [CrossRef]
- Pathirage, N.A.; Cocquebert, M.; Sadovsky, Y.; Abumaree, M.; Manuelpillai, U.; Borg, A.; Keogh, R.J.; Brennecke, S.P.; Evain-Brion, D.; Fournier, T. Homeobox gene transforming growth factor β-induced factor-1 (TGIF-1) is a regulator of villous trophoblast differentiation and its expression is increased in human idiopathic fetal growth restriction. Mol. Hum. Reprod. 2013, 19, 665–675. [Google Scholar] [CrossRef]
- Ma, G.T.; Soloveva, V.; Tzeng, S.; Lowe, L.A.; Pfendler, K.C.; Iannaccone, P.M.; Kuehn, M.R.; Linzer, D.I. Nodal regulates trophoblast differentiation and placental development. Dev. Biol. 2001, 236, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Randall, S.M.; Collier, T.S.; Nero, A.; Russell, T.A.; Muddiman, D.C.; Rao, B.M. Activin/nodal signaling switches the terminal fate of human embryonic stem cell-derived trophoblasts. J. Biol. Chem. 2015, 290, 8834–8848. [Google Scholar] [CrossRef]
- Visser, A.; Beijer, M.; Oudejans, C.B.; van Dijk, M. The effect of maternal NODAL on STOX1 expression in extravillous trophoblasts is mediated by IGF1. PLoS ONE 2018, 13, e0202190. [Google Scholar] [CrossRef]
- Li, Y.; Klausen, C.; Zhu, H.; Leung, P.C. Activin A increases human trophoblast invasion by inducing SNAIL-mediated MMP2 up-regulation through ALK4. J. Clin. Endocrinol. Metab. 2015, 100, E1415–E1427. [Google Scholar] [CrossRef]
- Nishi, H.; Nakada, T.; Hokamura, M.; Osakabe, Y.; Itokazu, O.; Huang, L.E.; Isaka, K. Hypoxia-inducible factor-1 transactivates transforming growth factor-β3 in trophoblast. Endocrinology 2004, 145, 4113–4118. [Google Scholar] [CrossRef] [PubMed]
- Wakeland, A.K.; Soncin, F.; Moretto-Zita, M.; Chang, C.; Horii, M.; Pizzo, D.; Nelson, K.K.; Laurent, L.C.; Parast, M.M. Hypoxia directs human extravillous trophoblast differentiation in a hypoxia-inducible factor–dependent manner. Am. J. Pathol. 2017, 187, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Cowden Dahl, K.D.; Fryer, B.H.; Mack, F.A.; Compernolle, V.; Maltepe, E.; Adelman, D.M.; Carmeliet, P.; Simon, M.C. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol. Cell. Biol. 2005, 25, 10479–10491. [Google Scholar] [CrossRef]
- Caniggia, I.; Mostachfi, H.; Winter, J.; Gassmann, M.; Lye, S.J.; Kuliszewski, M.; Post, M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ 3. J. Clin. Investig. 2000, 105, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Rumi, M.K.; Soares, M. NK cells, hypoxia and trophoblast cell differentiation. Cell Cycle 2012, 11, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tanaka, S.; Oda, M.; Makino, T.; Ohgane, J.; Shiota, K. Retinoic acid promotes differentiation of trophoblast stem cells to a giant cell fate. Dev. Biol. 2001, 235, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Schoonjans, K.; Guibourdenche, J.; Bidart, J.M.; Vidaud, M.; Auwerx, J.; Rochette-Egly, C.; Evain-Brion, D. PPARγ/RXRα heterodimers are involved in human CGβ synthesis and human trophoblast differentiation. Endocrinology 2001, 142, 4504–4514. [Google Scholar] [CrossRef]
- Wendling, O.; Chambon, P.; Mark, M. Retinoid X receptors are essential for early mouse development and placentogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Sarlis, N.J.; Richards, R.; Handwerger, S. Expression of retinoic acid receptor subtypes and cellular retinoic acid binding protein-II mRNAs during differentiation of human trophoblast cells. Biochem. Biophys. Res. Commun. 1994, 202, 772–780. [Google Scholar] [CrossRef]
- Norton, J.D.; Deed, R.W.; Craggs, G.; Sablitzky, F. Id helix—Loop—Helix proteins in cell growth and differentiation. Trends Cell Biol. 1998, 8, 58–65. [Google Scholar]
- Lu, X.; Deb, S.; Soares, M.J. Spontaneous differentiation of trophoblast cells along the spongiotrophoblast cell pathway: Expression of members of the placental prolactin gene family and modulation by retinoic acid. Dev. Biol. 1994, 163, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, T.; Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu. Rev. Cell Dev. Biol. 1996, 12, 335–363. [Google Scholar] [CrossRef]
- Schaiff, W.T.; Carlson, M.G.; Smith, S.D.; Levy, R.; Nelson, D.M.; Sadovsky, Y. Peroxisome proliferator-activated receptor-γ modulates differentiation of human trophoblast in a ligand-specific manner. J. Clin. Endocrinol. Metab. 2000, 85, 3874–3881. [Google Scholar] [CrossRef][Green Version]
- Tarrade, A.; Schoonjans, K.; Pavan, L.; Auwerx, J.; Rochette-Egly, C.; Evain-Brion, D.; Fournier, T. PPARγ/RXRα heterodimers control human trophoblast invasion. J. Clin. Endocrinol. Metab. 2001, 86, 5017–5024. [Google Scholar] [CrossRef][Green Version]
- Kohan-Ghadr, H.; Kadam, L.; Jain, C.; Armant, D.R.; Drewlo, S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016, 10, 126–135. [Google Scholar] [CrossRef]
- Ng, R.K.; Dean, W.; Dawson, C.; Lucifero, D.; Madeja, Z.; Reik, W.; Hemberger, M. Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat. Cell Biol. 2008, 10, 1280–1290. [Google Scholar] [CrossRef]
- Hattori, N.; Imao, Y.; Nishino, K.; Hattori, N.; Ohgane, J.; Yagi, S.; Tanaka, S.; Shiota, K. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells 2007, 12, 387–396. [Google Scholar] [CrossRef]
- Hattori, N.; Nishino, K.; Ko, Y.; Hattori, N.; Ohgane, J.; Tanaka, S.; Shiota, K. Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J. Biol. Chem. 2004, 279, 17063–17069. [Google Scholar] [CrossRef]
- Murray, A.; Sienerth, A.R.; Hemberger, M. Plet1 is an epigenetically regulated cell surface protein that provides essential cues to direct trophoblast stem cell differentiation. Sci. Rep. 2016, 6, 25112. [Google Scholar] [CrossRef]
- Blond, J.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Ruebner, M.; Strissel, P.L.; Ekici, A.B.; Stiegler, E.; Dammer, U.; Goecke, T.W.; Faschingbauer, F.; Fahlbusch, F.B.; Beckmann, M.W.; Strick, R. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promoter region. PLoS ONE 2013, 8, e56145. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Muralimanoharan, S.; Gogate, A.A.; Mendelson, C.R. Human trophoblast differentiation is associated with profound gene regulatory and epigenetic changes. Endocrinology 2019, 160, 2189–2203. [Google Scholar] [CrossRef]
- Rahnama, F.; Shafiei, F.; Gluckman, P.D.; Mitchell, M.D.; Lobie, P.E. Epigenetic regulation of human trophoblastic cell migration and invasion. Endocrinology 2006, 147, 5275–5283. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, K.; Leach, R. 5-Aza-dC treatment induces mesenchymal-to-epithelial transition in 1st trimester trophoblast cell line HTR8/SVneo. Biochem. Biophys. Res. Commun. 2013, 432, 116–122. [Google Scholar] [CrossRef][Green Version]
- Chiu, R.W.; Chim, S.S.; Wong, I.H.; Wong, C.S.; Lee, W.; To, K.F.; Tong, J.H.; Yuen, R.K.; Shum, A.S.; Chan, J.K. Hypermethylation of RASSF1A in human and rhesus placentas. Am. J. Pathol. 2007, 170, 941–950. [Google Scholar] [CrossRef]
- Novakovic, B.; Rakyan, V.; Ng, H.K.; Manuelpillai, U.; Dewi, C.; Wong, N.C.; Morley, R.; Down, T.; Beck, S.; Craig, J.M.; et al. Specific tumour-associated methylation in normal human term placenta and first-trimester cytotrophoblasts. Mol. Hum. Reprod. 2008, 14, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Guilleret, I.; Osterheld, M.; Braunschweig, R.; Gastineau, V.; Taillens, S.; Benhattar, J. Imprinting of tumor-suppressor genes in human placenta. Epigenetics 2009, 4, 62–68. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Wang, K.; Qian, C.; Leach, R. DNA methylation is associated with transcription of Snail and Slug genes. Biochem. Biophys. Res. Commun. 2013, 430, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Gerlitz, G.; Bustin, M. Efficient cell migration requires global chromatin condensation. J. Cell Sci. 2010, 123, 2207–2217. [Google Scholar] [CrossRef]
- Fu, Y.; Chin, L.K.; Bourouina, T.; Liu, A.Q.; VanDongen, A.M. Nuclear deformation during breast cancer cell transmigration. Lab Chip 2012, 12, 3774–3778. [Google Scholar] [CrossRef]
- Song, S.J.; Poliseno, L.; Song, M.S.; Ala, U.; Webster, K.; Ng, C.; Beringer, G.; Brikbak, N.J.; Yuan, X.; Cantley, L.C. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 2013, 154, 311–324. [Google Scholar] [CrossRef]
- Shan, Y.; Chen, Y.; Brkić, J.; Fournier, L.; Ma, H.; Peng, C. miR-218-5p induces interleukin-1β and endovascular trophoblast differentiation by targeting the transforming growth factor β-SMAD2 pathway. Front. Endocrinol. 2022, 13, 842587. [Google Scholar] [CrossRef]
- Nadeem, U.; Ye, G.; Salem, M.; Peng, C. MicroRNA-378a-5p targets cyclin G2 to inhibit fusion and differentiation in BeWo cells. Biol. Reprod. 2014, 91, 76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Moreau, P.; Contu, L.; Alba, F.; Lai, S.; Simoes, R.; Orrù, S.; Carcassi, C.; Roger, M.; Rabreau, M.; Carosella, E.D. HLA-G gene polymorphism in human placentas: Possible association of G* 0106 allele with preeclampsia and miscarriage. Biol. Reprod. 2008, 79, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Ho, J.F.; Chong, Y.S.; Loganath, A.; Chan, Y.H.; Ravichandran, J.; Lee, C.G.; Chong, S.S. Paternal contribution of HLA-G* 0106 significantly increases risk for pre-eclampsia in multigravid pregnancies. Mol. Hum. Reprod. 2008, 14, 317–324. [Google Scholar] [CrossRef]
- Hemberger, M. Genetic-epigenetic intersection in trophoblast differentiation: Implications for extraembryonic tissue function. Epigenetics 2010, 5, 24–29. [Google Scholar] [CrossRef]
- Wen, J.; Zeng, Y.; Fang, Z.; Gu, J.; Ge, L.; Tang, F.; Qu, Z.; Hu, J.; Cui, Y.; Zhang, K. Single-cell analysis reveals lineage segregation in early post-implantation mouse embryos. J. Biol. Chem. 2017, 292, 9840–9854. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.G.; Natale, D.R.; Begay, V.; Hughes, M.; Leutz, A.; Cross, J.C. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development 2008, 135, 2083–2091. [Google Scholar] [CrossRef]
- Pearton, D.J.; Smith, C.S.; Redgate, E.; van Leeuwen, J.; Donnison, M.; Pfeffer, P.L. Elf5 counteracts precocious trophoblast differentiation by maintaining Sox2 and 3 and inhibiting Hand1 expression. Dev. Biol. 2014, 392, 344–357. [Google Scholar] [CrossRef]
- Hesse, M.; Franz, T.; Tamai, Y.; Taketo, M.M.; Magin, T.M. Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J. 2000, 19, 5060–5070. [Google Scholar] [CrossRef]
- Jaquemar, D.; Kupriyanov, S.; Wankell, M.; Avis, J.; Benirschke, K.; Baribault, H.; Oshima, R.G. Keratin 8 protection of placental barrier function. J. Cell Biol. 2003, 161, 749–756. [Google Scholar] [CrossRef]
- Mould, A.; Morgan, M.A.; Li, L.; Bikoff, E.K.; Robertson, E.J. Blimp1/Prdm1 governs terminal differentiation of endovascular trophoblast giant cells and defines multipotent progenitors in the developing placenta. Genes Dev. 2012, 26, 2063–2074. [Google Scholar] [CrossRef]
- Simmons, D.G.; Rawn, S.; Davies, A.; Hughes, M.; Cross, J.C. Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genom. 2008, 9, 352. [Google Scholar] [CrossRef]
- Sharma, A.; Lacko, L.A.; Argueta, L.B.; Glendinning, M.D.; Stuhlmann, H. miR-126 regulates glycogen trophoblast proliferation and DNA methylation in the murine placenta. Dev. Biol. 2019, 449, 21–34. [Google Scholar] [CrossRef]
- El-Hashash, A.H.; Warburton, D.; Kimber, S.J. Genes and signals regulating murine trophoblast cell development. Mech. Dev. 2010, 127, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.T.; Linzer, D.I. GATA-2 restricts prolactin-like protein A expression to secondary trophoblast giant cells in the mouse. Biol. Reprod. 2000, 63, 570–574. [Google Scholar] [CrossRef][Green Version]
- Gasperowicz, M.; Surmann-Schmitt, C.; Hamada, Y.; Otto, F.; Cross, J.C. The transcriptional co-repressor TLE3 regulates development of trophoblast giant cells lining maternal blood spaces in the mouse placenta. Dev. Biol. 2013, 382, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dupressoir, A.; Vernochet, C.; Bawa, O.; Harper, F.; Pierron, G.; Opolon, P.; Heidmann, T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA 2009, 106, 12127–12132. [Google Scholar] [CrossRef] [PubMed]


| Cell Types | Specifics for scRNAseq ID | Additional Markers | |
|---|---|---|---|
| Positive | Negative | ||
| Cytotrophoblasts | Krt8/18 Spint1 Hai-1 | HLA-G | |
| Syncytiotrophoblasts | Krt8/18 hCG hPL Syncytin-1 | HLA-G | Cyp19a1 |
| Extravillous Trophoblasts | Krt8/18 HLA-G VE-Cadherin | Notch2, Erbb2, Tcf-4, Tead2 | |
| Interstitial Extravillous Trophoblasts | Krt8/18 Pappa2 Dao Serpine1 | ||
| Endovascular Extravillous Trophoblasts | Krt8/18 Itga1/5 VE-Cadherin Pecam1 | ||
| Cell Types | Specifics for scRNAseq ID | Additional Markers | |
|---|---|---|---|
| Positive | Negative | ||
| Spongiotrophoblasts | Krt8/18 Tpbpα | Hand1 Cdkn1c Pcdh12 | Prl5a1 (E8.5-9.5); Prl3c1, 3a1, 8a8, 8a6 (E14.5-term) |
| Glycogen Trophoblasts | Krt8/18 Tpbpα Cdkn1c Pcdh12 | Hand1 | Prl6a1, 7b1, 7c1 (after E12.5) |
| Trophoblast Giant Cells | Krt8/18 Hand1 Tpbpα (+/−) | ||
| Parietal Trophoblast Giant Cells | Krt8/18 Hand1 Tpbpα (+/−) Prl3d1 | Prl6a1 Prl7b1 Prl7c1 | Prl4a1 (before E12.5); Prl2b1, 7a1, 3b1 |
| Spiral Artery Trophoblast Giant Cells | Krt8/18 Hand1 Tpbpα Prl6a1 Prl7b1 | Prl3d1 Prl3b1 | Prl7d1, 2c2 |
| Canal Trophoblast Giant Cells | Krt8/18 Hand1 Tpbpα (+/−) Prl7b1 Prl3b1 | Prl6a1 | Prl2c2, 7d1 |
| Sinusoidal Trophoblast Giant Cells | Krt8/18 Hand1 Ctsq | Tpbpα | Prl2b1, 3b1, 7d1 |
| Syncytiotrophoblast-I | Krt8/18 Syna | Hand1 Tpbpα Gcm1 Synb Cebpa | |
| Syncytiotrophoblast-II | Krt8/18 Gcm1 Synb Cebpa | Hand1 Tpbpα Syna | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawless, L.; Qin, Y.; Xie, L.; Zhang, K. Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications. Nutrients 2023, 15, 3564. https://doi.org/10.3390/nu15163564
Lawless L, Qin Y, Xie L, Zhang K. Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications. Nutrients. 2023; 15(16):3564. https://doi.org/10.3390/nu15163564
Chicago/Turabian StyleLawless, Lauren, Yushu Qin, Linglin Xie, and Ke Zhang. 2023. "Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications" Nutrients 15, no. 16: 3564. https://doi.org/10.3390/nu15163564
APA StyleLawless, L., Qin, Y., Xie, L., & Zhang, K. (2023). Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications. Nutrients, 15(16), 3564. https://doi.org/10.3390/nu15163564





