Hypoglycemia in Children: Major Endocrine-Metabolic Causes and Novel Therapeutic Perspectives
Abstract
1. Introduction
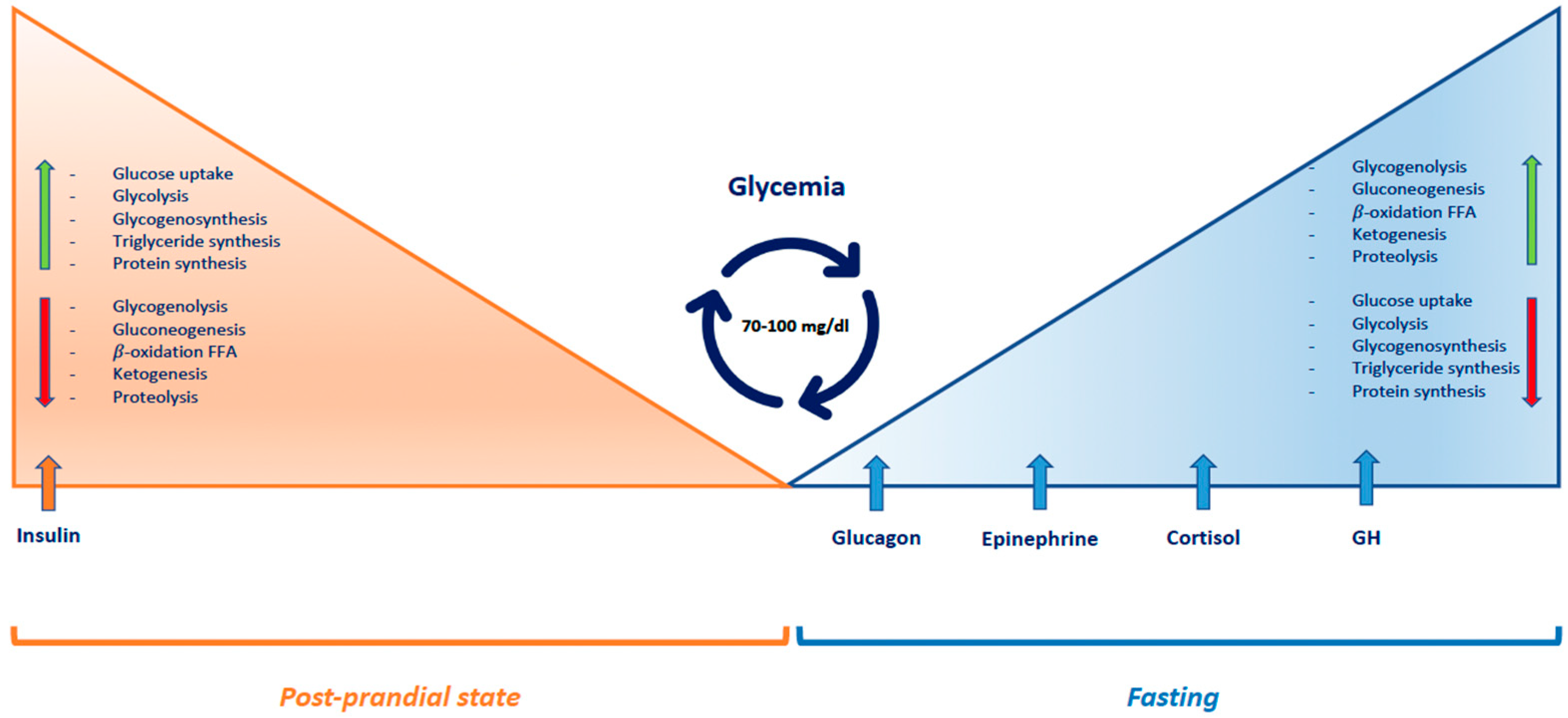
1.1. Pathophysiology
1.2. Adaptation of Glucose Homeostasis from Intrauterine to Neonatal Life
2. Etiology
2.1. Hormonal Causes of Hypoglycemia (Table 1)
2.1.1. Hyperinsulinism
2.1.2. Counter-Regulatory Hormone Defects
2.1.3. Other Endocrine Causes: Insulin-Like Growth Factor-II (IGF-II) Production
| Type | Causes | Clinical Features | Biochemical Features | Treatment | Potential New Therapies | |
|---|---|---|---|---|---|---|
| Hyperinsulinism |  Insulin Insulin | Hypoglycemic symptoms | Hypoglycemia, increased insulin and C-peptide, normal ketones and fatty acids, absence of M.A., positive glycemic response to glucagon | Diazoxide, somatostatin analogs (octreotide), long-acting release somatostatin analogs (lanreotide) | Glucagon-like peptide-1 receptor antagonists, pharmacological chaperones | |
| GH deficiency |  GH GH | Hypoglycemic symptoms, prolonged and recurrent jaundice, growth deceleration | Hypoglycemia, increased ketones, fasting M.A. | Hormone replacement therapy | ||
| Endocrine disorders | Adrenal insufficiency |  Cortisol Cortisol | Hypoglycemic symptoms, cholestasis | Fasting hypoglycemia, increased ketones, fasting M.A. | Hormone replacement therapy | |
| Pediatric neoplastic formations (Wilms’ tumor, nephroblastoma, lymphomas/leukemias) |  IGF-II IGF-II | Hypoglycemic symptoms | Fasting hypoglycemia and postprandial hyperglycemia, suppressed insulin secretion, low ketones and fatty acids, suppressed glucagon and GH release | Surgical treatment, glucocorticoids and GH administration | Anti-IGF-II monoclonal antibodies |
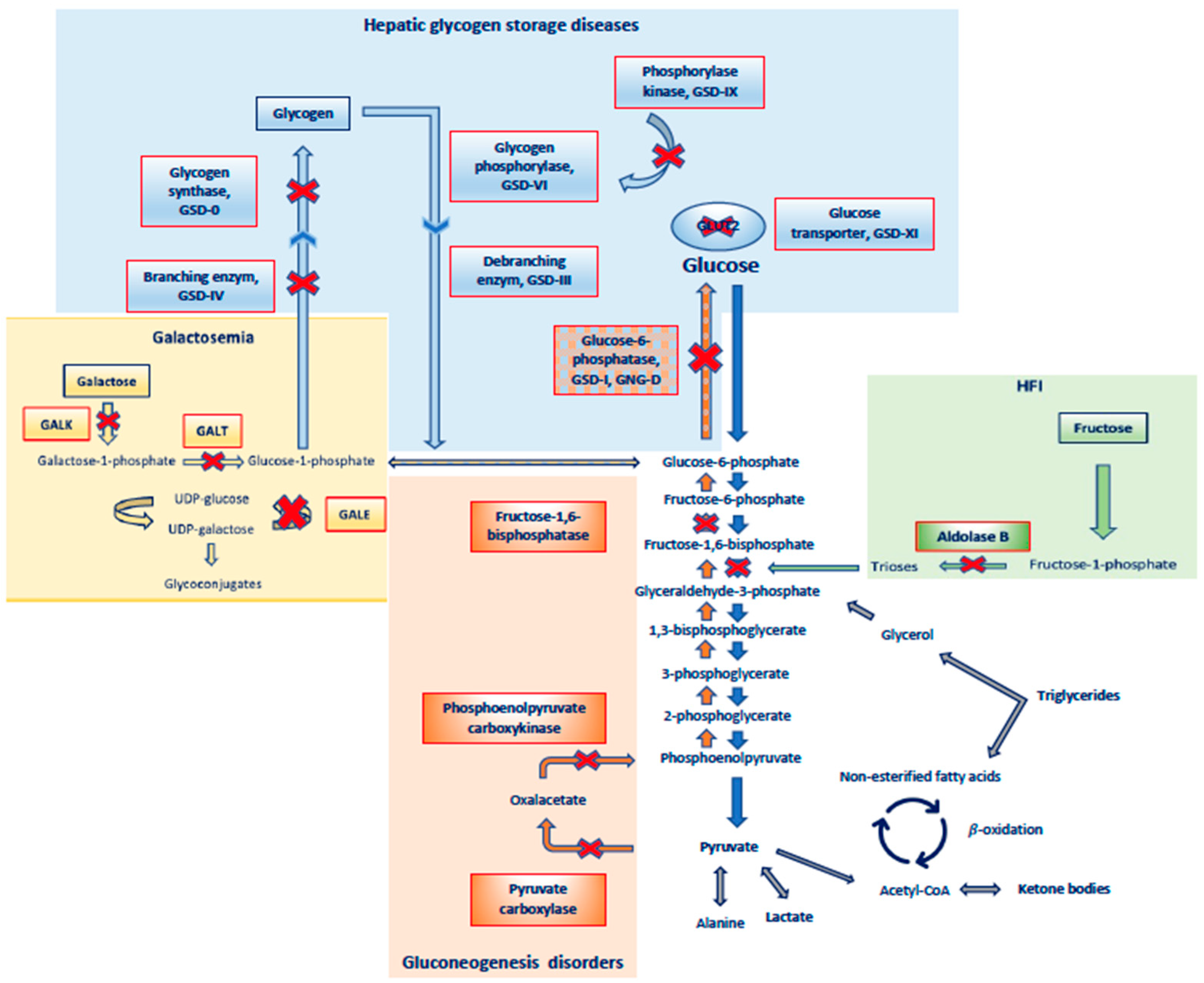
2.2. Metabolic Causes of Hypoglycemia, (Table 2 and Figure 2)
2.2.1. Glycogen Storage Disorders
| Type | Causes | Clinical Features | Biochemical Features | Treatment | Potential New Therapies | |
|---|---|---|---|---|---|---|
| GSD 0 |  Glycogen synthase Glycogen synthase | Hypoglycemic symptoms, normal liver size | Postprandial hyperglycemia and hyperlactatemia, fasting ketotic hypoglycemia | Dietary approach | ||
| Metabolic disorders | GSD Ia |  Glucose-6- phosphatase Glucose-6- phosphatase | Hypoglycemic symptoms, hepatomegaly, nephromegaly, failure to thrive | Fasting hypoglycemia, mild ketosis, hyperlactatemia, hyperuricemia, hypertriglyceridemia | Dietary approach | Gene therapy (initial phase of clinical trials) |
| GSD Ib |  Glucose-6-phosphatase transporter Glucose-6-phosphatase transporter | Hypoglycemic symptoms, hepatomegaly, nephromegaly, failure to thrive, neutropenia, inflammatory bowel disease | Fasting hypoglycemia, mild ketosis, hyperlactatemia, hyperuricemia, hypertriglyceridemia | Dietary approach | ||
| GSD III |  Glycogen debranching enzyme Glycogen debranching enzyme | Hypoglycemic symptoms, hepatomegaly, chronic myopathy, delayed growth | Fasting ketotic hypoglycemia, hyperlipidemia, increased CPK and transaminases | Dietary approach | ||
| GSD VI, IX |  Glycogen phosphorylase, Phosphorylase kinase Glycogen phosphorylase, Phosphorylase kinase | Hypoglycemic symptoms, hepatomegaly, failure to thrive, hypotonia | Fasting ketotic hypoglycemia, mild hyperlipidemia | Dietary approach | ||
| GSD XI |  GLUT2 transporter GLUT2 transporter | Hypoglycemic symptoms, hepatomegaly, failure to thrive, Fanconi tubulopaty | Fasting hypoglycemia, hyperaminoaciduria, acidosis, hyperphosphaturia, glycosuria | Dietary approach | ||
| HFI |  Aldolase B Aldolase B | Acute signs and symptoms (nausea, vomiting, abdominal pain, lethargy, seizures) and chronic signs (failure to thrive, hepatic and renal insufficiency) | Postprandial hypoglycemia, lactic acidemia, hypophosphatemia, hyperuricemia, hypermagnesemia, hyperalaninemia | Dietary restriction of fructose, sucrose, sucralose, and sorbitol | ||
| Galactosemia |  GALT, GALK, GALE GALT, GALK, GALE | Hypoglicemic symptoms, poor feeding, vomiting, jaundice, hepatomegaly, hypotonia, lethargy, cataracts, ovarian failure, failure to thrive | Postprandial hypoglycemia, hyperchloremic metabolic acidosis, hypophosphatemia, increased transaminases and direct/indirect bilirubin, aminoaciduria | Galactose restricted diet | Gene therapy, pharmacological chaperones, enzyme inhibitors, endoplasmic reticulum stress-reducing agents | |
| Gluconeogenesis disorders |  Fructose-1,6-bisphosphatase Fructose-1,6-bisphosphatase | Hypoglycemic symptoms, hepatomegaly, delayed growth | Fasting ketotic hypoglycemia, lactic acidosis, hyperuricemia, hypertriglyceridemia | Dietary approach | ||
 Pyruvate carboxylase Pyruvate carboxylase | Hypoglycemic symptoms, severe encephalopathy, developmental delay, seizures, growth retardation | Fasting ketotic hypoglycemia, metabolic acidosis | Dietary approach | |||
 Phosphoenolpyruvate carboxykinase Phosphoenolpyruvate carboxykinase | Hypoglycemic symptoms | Fasting ketotic hypoglycemia, metabolic acidosis | Dietary approach | |||
| Fatty-acid-oxidation disorders | Defects of enzymes involved in transport and beta oxidation of F.A. in the mitochondria | Hypoglycemic symptoms, cardiomyopathy, myopathy, hepatomegaly, Reye-like syndrome | Hypoketotic hypoglycemia, elevated free fatty acids, increased transaminases and CPK, hyperammonemia | Dietary approach | Triheptanoin for long-chain FAO disorders |
2.2.2. Hereditary Fructose Intolerance
2.2.3. Galactosemia
2.2.4. Gluconeogenesis Disorders
2.2.5. Fatty-Acid-Oxidation Disorders
3. Idiopathic Ketotic Hypoglycemia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casertano, A.; Rossi, A.; Fecarotta, S.; Rosanio, F.M.; Moracas, C.; Di Candia, F.; Parenti, G.; Franzese, A.; Mozzillo, E. An Overview of Hypoglycemia in Children Including a Comprehensive Practical Diagnostic Flowchart for Clinical Use. Front. Endocrinol. 2021, 12, 684011. [Google Scholar] [CrossRef]
- Thornton, P.S.; Stanley, C.A.; De Leon, D.D.; Harris, D.; Haymond, M.W.; Hussain, K.; Levitsky, L.L.; Murad, M.H.; Rozance, P.J.; Simmons, R.A.; et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J. Pediatr. 2015, 167, 238–245. [Google Scholar]
- Ghosh, A.; Banerjee, I.; Morris, A.A.M. Recognition, assessment and management of hypoglycaemia in childhood. Arch. Dis. Child. 2016, 101, 575–580. [Google Scholar] [CrossRef]
- Sharma, A.; Davis, A.; Shekhawat, P.S. Hypoglycemia in the preterm neonate: Etiopathogenesis, diagnosis, management and long-term outcomes. Transl. Pediatr. 2017, 6, 335–348. [Google Scholar]
- Thompson-Branch, A.; Havranek, T. Neonatal Hypoglycemia. Pediatr. Rev. 2017, 38, 147–157. [Google Scholar] [CrossRef]
- Cryer, P.E. Hypoglycemia, functional brain failure, and brain death. J. Clin. Investig. 2007, 117, 868–870. [Google Scholar] [CrossRef]
- Stanley, C.A.; Rozance, P.J.; Thornton, P.S.; De Leon, D.D.; Harris, D.; Haymond, M.W.; Hussain, K.; Levitsky, L.L.; Murad, M.H.; Simmons, R.A.; et al. Re-Evaluating “Transitional Neonatal Hypoglycemia”: Mechanism and Implications for Management. J. Pediatr. 2015, 166, 1520–1525.e1. [Google Scholar]
- Harris, D.L.; Weston, P.J.; Gamble, G.D.; Harding, J.E. Glucose Profiles in Healthy Term Infants in the First 5 Days: The Glucose in Well Babies (GLOW) Study. J. Pediatr. 2020, 223, 34–41.e4. [Google Scholar]
- Cornblath, M.; Hawdon, J.M.; Williams, A.F.; Aynsley-Green, A.; Ward-Platt, M.P.; Schwartz, R.; Kalhan, S.C. Controversies Regarding Definition of Neonatal Hypoglycemia: Suggested Operational Thresholds. Pediatrics 2000, 105, 1141–1145. [Google Scholar]
- Yager, J.Y. Hypoglycemic injury to the immature brain. Clin. Perinatol. 2002, 29, 651–674. [Google Scholar] [CrossRef]
- Saudubray, J.M.; De Lonlay, P.; Touati, G.; Martin, D.; Nassogne, M.C.; Castelnau, P.; Sevin, C.; Laborde, C.; Baussan, C.; Brivet, M.; et al. Genetic hypoglycaemia in infancy and childhood: Pathophysiology and diagnosis. J. Inherit Metab. Dis. 2000, 23, 197–214. [Google Scholar]
- Sprague, J.E.; Arbeláez, A.M. Glucose counterregulatory responses to hypoglycemia. Pediatr. Endocrinol. Rev. 2011, 9, 463–473. [Google Scholar]
- Blanco, C.L.; Kim, J. Neonatal Glucose Homeostasis. Clin. Perinatol. 2022, 49, 393–404. [Google Scholar] [CrossRef]
- Hay, W.W.; Raju, T.N.K.; Higgins, R.D.; Kalhan, S.C.; Devaskar, S.U. Knowledge Gaps and Research Needs for Understanding and Treating Neonatal Hypoglycemia: Workshop Report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. J. Pediatr. 2009, 155, 612–617. [Google Scholar]
- Adamkin, D.H. Postnatal Glucose Homeostasis in Late-Preterm and Term Infants. Pediatrics 2011, 127, 575–579. [Google Scholar] [CrossRef]
- Kaiser, J.R.; Bai, S.; Rozance, P.J. Newborn Plasma Glucose Concentration Nadirs by Gestational-Age Group. Neonatology 2018, 113, 353–359. [Google Scholar] [CrossRef]
- Lang, T.F.; Hussain, K. Pediatric Hypoglycemia. Pediatrics 2014, 63, 211–245. [Google Scholar]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef]
- Adamkin, D.H. Neonatal hypoglycemia. Semin. Fetal Neonatal Med. 2017, 22, 36–41. [Google Scholar] [CrossRef]
- Hussain, K. Diagnosis and management of hyperinsulinaemic hypoglycaemia of infancy. Horm. Res. 2008, 69, 2–13. [Google Scholar]
- Lang, T.F.; Cardy, D.; Carson, D.; Loughrey, C.M.; Hanna, E. Audit of acute hypoglycaemia in children: Re-audit of procedures for diagnosis. Ann. Clin. Biochem. Int. J. Lab. Med. 2008, 45, 486–488. [Google Scholar]
- Bolmasova, A.V.; Melikyan, M.A.; Krylova, N.A.; Ionov, O.V.; Ryumina, I.; Bockeria, E.L.; Pekareva, N.A.; Degtyareva, A.V. Transient hyperinsulinism in neonates. Probl. Endocrinol. 2020, 66, 61–67. [Google Scholar] [CrossRef]
- Palladino, A.A.; Bennett, M.J.; Stanley, C.A. Hyperinsulinism in Infancy and Childhood: When an Insulin Level Is Not Always Enough. Clin. Chem. 2008, 54, 256–263. [Google Scholar]
- Stanley, C.A. Hyperinsulinism in Infants and Children. Pediatr. Clin. N. Am. 1997, 44, 363–374. [Google Scholar] [CrossRef]
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar]
- Lord, K.; De León, D.D. Hyperinsulinism in the Neonate. Clin. Perinatol. 2018, 45, 61–74. [Google Scholar] [CrossRef]
- Rahman, S.A.; Nessa, A.; Hussain, K. Molecular mechanisms of congenital hyperinsulinism. J. Mol. Endocrinol. 2015, 54, R119–R129. [Google Scholar] [CrossRef]
- Dunne, M.J.; Cosgrove, K.E.; Shepherd, R.M.; Aynsley-Green, A.; Lindley, K.J. Hyperinsulinism in infancy: From basic science to clinical disease. Physiol. Rev. 2004, 84, 239–275. [Google Scholar]
- Arnoux, J.-B.; Verkarre, V.; Saint-Martin, C.; Montravers, F.; Brassier, A.; Valayannopoulos, V.; Brunelle, F.; Fournet, J.-C.; Robert, J.-J.; Aigrain, Y.; et al. Congenital hyperinsulinism: Current trends in diagnosis and therapy. Orphanet J. Rare Dis. 2011, 6, 63. [Google Scholar]
- Galcheva, S.; Demirbilek, H.; Al-Khawaga, S.; Hussain, K. The Genetic and Molecular Mechanisms of Congenital Hyperinsulinism. Front. Endocrinol. 2019, 10, 111. [Google Scholar] [CrossRef]
- Valayannopoulos, V.; Romano, S.; Mention, K.; Vassault, A.; Rabier, D.; Polak, M.; Robert, J.-J.; de Keyzer, Y.; de Lonlay, P. What’s new in metabolic and genetic hypoglycaemias: Diagnosis and management. Eur. J. Pediatr. 2008, 167, 257–265. [Google Scholar]
- Danowitz, M.; De Leon, D.D. The Role of GLP-1 Signaling in Hypoglycemia due to Hyperinsulinism. Front. Endocrinol. 2022, 13, 863184. [Google Scholar] [CrossRef]
- Kapoor, R.R.; Flanagan, S.E.; Arya, V.B.; Shield, J.P.; Ellard, S.; Hussain, K. Clinical and molecular characterisation of 300 patients with congenital hyperinsulinism. Eur. J. Endocrinol. 2013, 168, 557–564. [Google Scholar] [CrossRef]
- Taylor-Miller, T.; Houghton, J.; Munyard, P.; Kumar, Y.; Puvirajasinghe, C.; Giri, D. Congenital hyperinsulinism due to compound heterozygous mutations in ABCC8 responsive to diazoxide therapy. J. Pediatr. Endocrinol. Metab. 2020, 33, 671–674. [Google Scholar]
- De Franco, E.; Saint-Martin, C.; Brusgaard, K.; Knight Johnson, A.E.; Aguilar-Bryan, L.; Bowman, P.; Arnoux, J.-B.; Larsen, A.R.; Sanyoura, M.; Greeley, S.A.W. Update of variants identified in the pancreatic β-cell KATP channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum. Mutat. 2020, 41, 884–905. [Google Scholar] [CrossRef]
- Sims, K. Congenital Hyperinsulinism. Neoreviews 2021, 22, e230–e240. [Google Scholar] [CrossRef]
- Demirbilek, H.; Hussain, K. Congenital Hyperinsulinism: Diagnosis and Treatment Update. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 69–87. [Google Scholar]
- Ismail, D.; Hussain, K. Role of 18F-DOPA PET/CT imaging in congenital hyperinsulinism. Rev. Endocr. Metab. Disord. 2010, 11, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Salomon-Estebanez, M.; Shah, P.; Nicholson, J.; Cosgrove, K.E.; Dunne, M.J. Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia. Diabetes Med. 2019, 36, 9–21. [Google Scholar] [CrossRef]
- Hussain, K.; Aynsley-Green, A. Management of hyperinsulinism in infancy and childhood. Ann. Med. 2000, 32, 544–551. [Google Scholar] [CrossRef]
- Brar, P.C.; Heksch, R.; Cossen, K.; De Leon, D.D.; Kamboj, M.K.; Marks, S.D.; Marshall, B.A.; Miller, R.; Page, L.; Stanley, T.; et al. Management and Appropriate Use of Diazoxide in Infants and Children with Hyperinsulinism. J. Clin. Endocrinol. Metab. 2020, 105, 3750–3761. [Google Scholar] [CrossRef]
- Novokreshhennyx, E.E.; Gubaeva, D.N.; Melikyan, M.A. The use of long-acting somatostatin analogs in congenital hyperinsulinism. Probl. Endokrinol. 2020, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Tang, F.; Seeholzer, S.H.; Zou, Y.; de León, D.D. Population pharmacokinetics of exendin-(9-39) and clinical dose selection in patients with congenital hyperinsulinism. Br. J. Clin. Pharmacol. 2018, 84, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Stefanovski, D.; Vajravelu, M.E.; Givler, S.; de León, D.D. Exendin-(9-39) Effects on Glucose and Insulin in Children With Congenital Hyperinsulinism During Fasting and During a Meal and a Protein Challenge. Diabetes Care 2022, 45, 1381–1390. [Google Scholar] [CrossRef]
- Martin, G.M.; Sung, M.W.; Shyng, S.-L. Pharmacological chaperones of ATP-sensitive potassium channels: Mechanistic insight from cryoEM structures. Mol. Cell Endocrinol. 2020, 502, 110667. [Google Scholar]
- Chen, P.-C.; Olson, E.M.; Zhou, Q.; Kryukova, Y.; Sampson, H.M.; Thomas, D.Y.; Shyng, S.-L. Carbamazepine as a Novel Small Molecule Corrector of Trafficking-impaired ATP-sensitive Potassium Channels Identified in Congenital Hyperinsulinism. J. Biol. Chem. 2013, 288, 20942–20954. [Google Scholar] [PubMed]
- Baujat, G.; Rio, M.; Rossignol, S.; Sanlaville, D.; Lyonnet, S.; Le Merrer, M.; Munnich, A.; Gicquel, C.; Cormier-Daire, V.; Colleaux, L. Paradoxical NSD1 Mutations in Beckwith-Wiedemann Syndrome and 11p15 Anomalies in Sotos Syndrome. Am. J. Hum. Genet. 2004, 74, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.M.; Boodhansingh, K.E.; Bhatti, T.R.; Ganguly, A.; Conlin, L.K.; Becker, S.A.; Givler, S.; Mighion, L.; Palladino, A.A.; Adzick, N.S.; et al. Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J. Med. Genet. 2016, 53, 53–61. [Google Scholar] [CrossRef]
- Zenker, M.; Mohnike, K.; Palm, K. Syndromic forms of congenital hyperinsulinism. Front. Endocrinol. 2023, 14, 1013874. [Google Scholar] [CrossRef]
- Barthlen, W.; Mohnike, W.; Mohnike, K. Techniques in pediatric surgery: Congenital hyperinsulinism. Horm. Res. Paediatr. 2011, 75, 304–310. [Google Scholar]
- Di Iorgi, N.; Morana, G.; Allegri, A.E.M.; Napoli, F.; Gastaldi, R.; Calcagno, A.; Patti, G.; Loche, S.; Maghnie, M. Classical and non-classical causes of GH deficiency in the paediatric age. Best. Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 705–736. [Google Scholar]
- Mullis, P.E. Genetics of growth hormone deficiency. Endocrinol. Metab. Clin. N. Am. 2007, 36, 17–36. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J. Endocrinology Update: Hypopituitarism. FP Essent. 2016, 451, 25–30. [Google Scholar]
- Donato, J.; Wasinski, F.; Furigo, I.C.; Metzger, M.; Frazão, R. Central Regulation of Metabolism by Growth Hormone. Cells 2021, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.O.; Krag, M.; Jessen, N.; Nørrelund, H.; Vestergaard, E.T.; Møller, N.; Christiansen, J.S. Growth Hormone and Glucose Homeostasis. Horm. Res. Paediatr. 2004, 62, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Profka, E.; Rodari, G.; Giacchetti, F.; Giavoli, C.G.H. Deficiency and Replacement Therapy in Hypopituitarism: Insight Into the Relationships With Other Hypothalamic-Pituitary Axes. Front. Endocrinol. 2021, 12, 678778. [Google Scholar]
- Ara, L.B.I.; Katugampola, H.; Dattani, M.T. Congenital Hypopituitarism During the Neonatal Period: Epidemiology, Pathogenesis, Therapeutic Options, and Outcome. Front. Pediatr. 2021, 8, 600962. [Google Scholar]
- Shulman, D.I.; Palmert, M.R.; Kemp, S.F. Adrenal Insufficiency: Still a Cause of Morbidity and Death in Childhood. Pediatrics 2007, 119, e484–e494. [Google Scholar] [PubMed]
- Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jørgensen, J.O.L.; Møller, N. Effects of Cortisol on Carbohydrate, Lipid, and Protein Metabolism: Studies of Acute Cortisol Withdrawal in Adrenocortical Failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar]
- Ioakim, K.J.; Sydney, G.I.; Paschou, S.A. Glucose metabolism disorders in patients with adrenal gland disorders: Pathophysiology and management. Hormones 2020, 19, 135–143. [Google Scholar]
- Djurhuus, C.B.; Gravholt, C.H.; Nielsen, S.; Mengel, A.; Christiansen, J.S.; Schmitz, O.E.; Møller, N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E172–E177. [Google Scholar] [CrossRef] [PubMed]
- Gujral, J.; Yau, M.; Yang, A.C.; Kastury, R.; Romero, C.J.; Wallach, E.; Costin, G.; Rapaport, R. Primary Cortisol Deficiency and Growth Hormone Deficiency in a Neonate With Hypoglycemia: Coincidence or Consequence? J. Endocr. Soc. 2019, 3, 838–846. [Google Scholar] [PubMed]
- Keenan, D.M.; Veldhuis, J.D.; Basu, A.; Basu, R. A novel measure of glucose homeostasis (or loss thereof) comprising the joint dynamics of glucose, insulin, glucagon, and cortisol. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E998–E1011. [Google Scholar] [PubMed]
- Prete, A.; Auchus, R.J.; Ross, R.J. Clinical advances in the pharmacotherapy of congenital adrenal hyperplasia. Eur. J. Endocrinol. 2022, 186, R1–R14. [Google Scholar] [CrossRef]
- Blyth, A.J.; Kirk, N.S.; Forbes, B.E. Understanding IGF-II Action through Insights into Receptor Binding and Activation. Cells 2020, 9, 2276. [Google Scholar] [CrossRef]
- Dynkevich, Y.; Rother, K.I.; Whitford, I.; Qureshi, S.; Galiveeti, S.; Szulc, A.L.; Danoff, A.; Breen, T.L.; Kaviani, N.; Shanik, M.H.; et al. Tumors, IGF-2, and hypoglycemia: Insights from the clinic, the laboratory, and the historical archive. Endocr. Rev. 2013, 34, 798–826. [Google Scholar]
- O’Dell, S.D.; Day, I.N. Insulin-like growth factor II (IGF-II). Int. J. Biochem. Cell Biol. 1998, 30, 767–771. [Google Scholar]
- Alvino, C.L.; Ong, S.C.; McNeil, K.A.; Delaine, C.; Booker, G.W.; Wallace, J.C.; Forbes, B.E. Understanding the mechanism of insulin and insulin-like growth factor (IGF) receptor activation by IGF-II. PLoS ONE 2011, 6, e27488. [Google Scholar]
- Feng, Y.; Zhu, Z.; Xiao, X.; Choudhry, V.; Barrett, J.C.; Dimitrov, D.S. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol. Cancer Ther. 2006, 5, 114–120. [Google Scholar]
- Wright, T.L.F.; Umaña, L.A.; Ramirez, C.M. Update on glycogen storage disease: Primary hepatic involvement. Curr. Opin. Pediatr. 2022, 34, 496–502. [Google Scholar]
- Molares-Vila, A.; Corbalán-Rivas, A.; Carnero-Gregorio, M.; González-Cespón, J.L.; Rodríguez-Cerdeira, C. Biomarkers in Glycogen Storage Diseases: An Update. Int. J. Mol. Sci. 2021, 22, 4381. [Google Scholar] [PubMed]
- Kishnani, P.S.; Sun, B.; Koeberl, D.D. Gene therapy for glycogen storage diseases. Hum. Mol. Genet. 2019, 28, R31–R41. [Google Scholar] [CrossRef] [PubMed]
- Rake, J.; Visser, G.; Labrune, P.; Leonard, J.; Ullrich, K.; Smit, P. Glycogen storage disease type I: Diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur. J. Pediatr. 2002, 161, S20–S34. [Google Scholar]
- Kishnani, P.S.; Austin, S.L.; Abdenur, J.E.; Arn, P.; Bali, D.S.; Boney, A.; Chung, W.K.; Dagli, A.I.; Dale, D.; Koeberl, D.; et al. Diagnosis and management of glycogen storage disease type I: A practice guideline of the American College of Medical Genetics and Genomics. Genet. Med. 2014, 16, e1–e29. [Google Scholar]
- Sim, S.W.; Weinstein, D.A.; Lee, Y.M.; Jun, H.S. Glycogen storage disease type Ib: Role of glucose-6-phosphate transporter in cell metabolism and function. FEBS Lett. 2020, 594, 3–18. [Google Scholar] [PubMed]
- Sentner, C.P.; Hoogeveen, I.J.; Weinstein, D.A.; Santer, R.; Murphy, E.; McKiernan, P.J.; Steuerwald, U.; Beauchamp, N.J.; Taybert, J.; Laforêt, P.; et al. Glycogen storage disease type III: Diagnosis, genotype, management, clinical course and outcome. J. Inherit. Metab. Dis. 2016, 39, 697–704. [Google Scholar]
- Kishnani, P.S.; Austin, S.L.; Arn, P.; Bali, D.S.; Boney, A.; Case, L.E.; Chung, W.K.; Desai, D.M.; El-Gharbawy, A.; Haller, R.; et al. Glycogen storage disease type III diagnosis and management guidelines. Genet. Med. 2010, 12, 446–463. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Goldstein, J.; Austin, S.L.; Arn, P.; Bachrach, B.; Bali, D.S.; Chung, W.K.; El-Gharbawy, A.; Brown, L.M.; Kahler, S.; et al. Diagnosis and management of glycogen storage diseases type VI and IX: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2019, 21, 772–789. [Google Scholar]
- Derks, T.G.J.; Rodriguez-Buritica, D.F.; Ahmad, A.; de Boer, F.; Couce, M.L.; Grünert, S.C.; Labrune, P.; Maldonado, N.L.; de Souza, C.F.M.; Riba-Wolman, R.; et al. Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs. Nutrients 2021, 13, 3828. [Google Scholar]
- Salabarria, S.; Nair, J.; Clement, N.; Smith, B.; Raben, N.; Fuller, D.; Byrne, B.; Corti, M. Advancements in AAV-mediated Gene Therapy for Pompe Disease. J. Neuromuscul. Dis. 2020, 7, 15–31. [Google Scholar] [CrossRef]
- Mayatepek, E.; Hoffmann, B.; Meissner, T. Inborn errors of carbohydrate metabolism. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Sarma, M.S. Hereditary fructose intolerance: A comprehensive review. World J. Clin. Pediatr. 2022, 11, 321–329. [Google Scholar] [PubMed]
- Hegde, V.S.; Sharman, T. Hereditary Fructose Intolerance; NIH: Bethesda, MD, USA, 2022. [Google Scholar]
- Saborido-Fiaño, R.; Martinón-Torres, N.; Crujeiras-Martinez, V.; Couce, M.L.; Leis, R. Letter to the editor concerning the article ‘Safety of vaccines administration in hereditary fructose intolerance’. Hum. Vaccin. Immunother. 2021, 17, 2593–2594. [Google Scholar]
- Timson, D.J. The molecular basis of galactosemia—Past, present and future. Gene 2016, 589, 133–141. [Google Scholar] [PubMed]
- Demirbas, D.; Coelho, A.I.; Rubio-Gozalbo, M.E.; Berry, G.T. Hereditary galactosemia. Metabolism 2018, 83, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S. GALT Deficiency Galactosemia. MCN Am. J. Matern. Child. Nurs. 2018, 43, 44–51. [Google Scholar] [CrossRef]
- Welling, L.; Bernstein, L.E.; Berry, G.T.; Burlina, A.B.; Eyskens, F.; Gautschi, M.; Grünewald, S.; Gubbels, C.S.; Knerr, I.; Labrune, P.; et al. International clinical guideline for the management of classical galactosemia: Diagnosis, treatment, and follow-up. J. Inherit Metab. Dis. 2017, 40, 171–176. [Google Scholar]
- Cuthbert, C.; Klapper, H.; Elsas, L. Diagnosis of Inherited Disorders of Galactose Metabolism. Curr. Protoc. Hum. Genet. 2008, 56. [Google Scholar] [CrossRef]
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry, Molecular Genetics, Newborn Screening, and Treatment. Biomolecules 2022, 12, 968. [Google Scholar]
- Yu, S.; Meng, S.; Xiang, M.; Ma, H. Phosphoenolpyruvate carboxykinase in cell metabolism: Roles and mechanisms beyond gluconeogenesis. Mol. Metab. 2021, 53, 101257. [Google Scholar]
- Marin-Valencia, I.; Roe, C.R.; Pascual, J.M. Pyruvate carboxylase deficiency: Mechanisms, mimics and anaplerosis. Mol. Genet. Metab. 2010, 101, 9–17. [Google Scholar]
- Lebigot, E.; Brassier, A.; Zater, M.; Imanci, D.; Feillet, F.; Thérond, P.; De Lonlay, P.; Boutron, A. Fructose 1,6-bisphosphatase deficiency: Clinical, biochemical and genetic features in French patients. J. Inherit Metab. Dis. 2015, 38, 881–887. [Google Scholar]
- Gorce, M.; Lebigot, E.; Arion, A.; Brassier, A.; Cano, A.; de Lonlay, P.; Feillet, F.; Gay, C.; Labarthe, F.; Nassogne, M.-C. Fructose-1,6-bisphosphatase deficiency causes fatty liver disease and requires long-term hepatic follow-up. J. Inherit Metab. Dis. 2022, 45, 215–222. [Google Scholar]
- Habarou, F.; Brassier, A.; Rio, M.; Chrétien, D.; Monnot, S.; Barbier, V.; Barouki, R.; Bonnefont, J.; Boddaert, N.; Chadefaux-Vekemans, B.; et al. Pyruvate carboxylase deficiency: An underestimated cause of lactic acidosis. Mol. Genet. Metab. Rep. 2015, 2, 25–31. [Google Scholar] [PubMed]
- Wang, D.; de Vivo, D. Pyruvate Carboxylase Deficiency; NIH: Bethesda, MD, USA, 1993. [Google Scholar]
- Grosse, S.D.; Khoury, M.J.; Greene, C.L.; Crider, K.S.; Pollitt, R.J. The epidemiology of medium chain acyl-CoA dehydrogenase deficiency: An update. Genet. Med. 2006, 8, 205–212. [Google Scholar]
- Longo, N.; Amat di San Filippo, C.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin. Med. Genet. 2006, 142C, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. part 1, classification, dietary sources and biological functions. Biomed. Pap. 2011, 155, 117–130. [Google Scholar]
- El-Gharbawy, A.; Vockley, J. Inborn Errors of Metabolism with Myopathy. Pediatr. Clin. N. Am. 2018, 65, 317–335. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; Ijlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar]
- Wilcken, B. Disorders of the carnitine cycle and detection by newborn screening. Ann. Acad Med. Singap. 2008, 37, 71–73. [Google Scholar]
- Shekhawat, P.S.; Matern, D.; Strauss, A.W. Fetal Fatty Acid Oxidation Disorders, Their Effect on Maternal Health and Neonatal Outcome: Impact of Expanded Newborn Screening on Their Diagnosis and Management. Pediatr. Res. 2005, 57, 78R–86R. [Google Scholar] [PubMed]
- Long-chain fatty acid oxidation disorders and current management strategies. Am. J. Manag. Care 2020, 26, S147–S154. [CrossRef] [PubMed]
- White, K.; Truong, L.; Aaron, K.; Mushtaq, N.; Thornton, P.S. The Incidence and Etiology of Previously Undiagnosed Hypoglycemic Disorders in the Emergency Department. Pediatr. Emerg. Care 2020, 36, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.P.; Osterhoudt, K.C.; Weinzimer, S.A. Presenting features of idiopathic ketotic hypoglycemia. J. Emerg. Med. 2003, 25, 39–43. [Google Scholar] [CrossRef]
- Mitchell, G.A.; Kassovska-Bratinova, S.; Boukaftane, Y.; Robert, M.F.; Wang, S.P.; Ashmarina, L.; Lambert, M.; Lapierre, P.; Potier, E. Medical aspects of ketone body metabolism. Clin. Investig. Med. 1995, 18, 193–216. [Google Scholar]
- Drachmann, D.; Hoffmann, E.; Carrigg, A.; Davis-Yates, B.; Weaver, V.; Thornton, P.; Weinstein, D.A.; Petersen, J.S.; Shah, P.; Christesen, H.T. Towards enhanced understanding of idiopathic ketotic hypoglycemia: A literature review and introduction of the patient organization, Ketotic Hypoglycemia International. Orphanet J. Rare Dis. 2021, 16, 173. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarta, A.; Iannucci, D.; Guarino, M.; Blasetti, A.; Chiarelli, F. Hypoglycemia in Children: Major Endocrine-Metabolic Causes and Novel Therapeutic Perspectives. Nutrients 2023, 15, 3544. https://doi.org/10.3390/nu15163544
Quarta A, Iannucci D, Guarino M, Blasetti A, Chiarelli F. Hypoglycemia in Children: Major Endocrine-Metabolic Causes and Novel Therapeutic Perspectives. Nutrients. 2023; 15(16):3544. https://doi.org/10.3390/nu15163544
Chicago/Turabian StyleQuarta, Alessia, Daniela Iannucci, Miriana Guarino, Annalisa Blasetti, and Francesco Chiarelli. 2023. "Hypoglycemia in Children: Major Endocrine-Metabolic Causes and Novel Therapeutic Perspectives" Nutrients 15, no. 16: 3544. https://doi.org/10.3390/nu15163544
APA StyleQuarta, A., Iannucci, D., Guarino, M., Blasetti, A., & Chiarelli, F. (2023). Hypoglycemia in Children: Major Endocrine-Metabolic Causes and Novel Therapeutic Perspectives. Nutrients, 15(16), 3544. https://doi.org/10.3390/nu15163544







