Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Construction of Expression Vectors
2.3. Cell Lines and Culture Conditions
2.4. Preparation of Caco-2 Cells Conditionally Expressing EGFP-Tagged MCT13
2.5. Localization Analysis
2.6. Uptake Study
2.7. Directional Transport Study
2.8. Accumulation Study
2.9. Statistics
3. Results
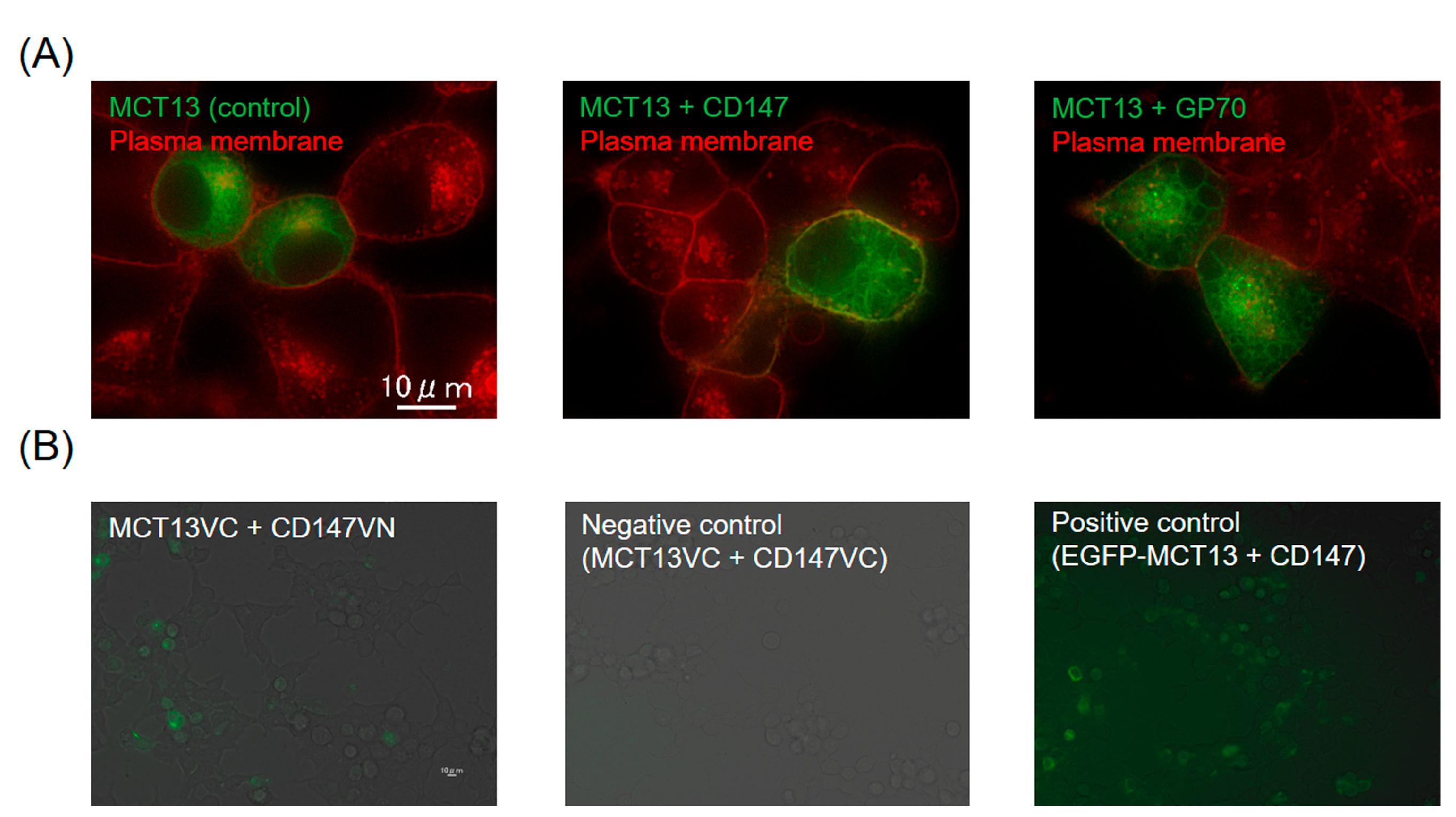
3.1. Enhanced Expression of MCT13 at the Plasma Membrane by CD147
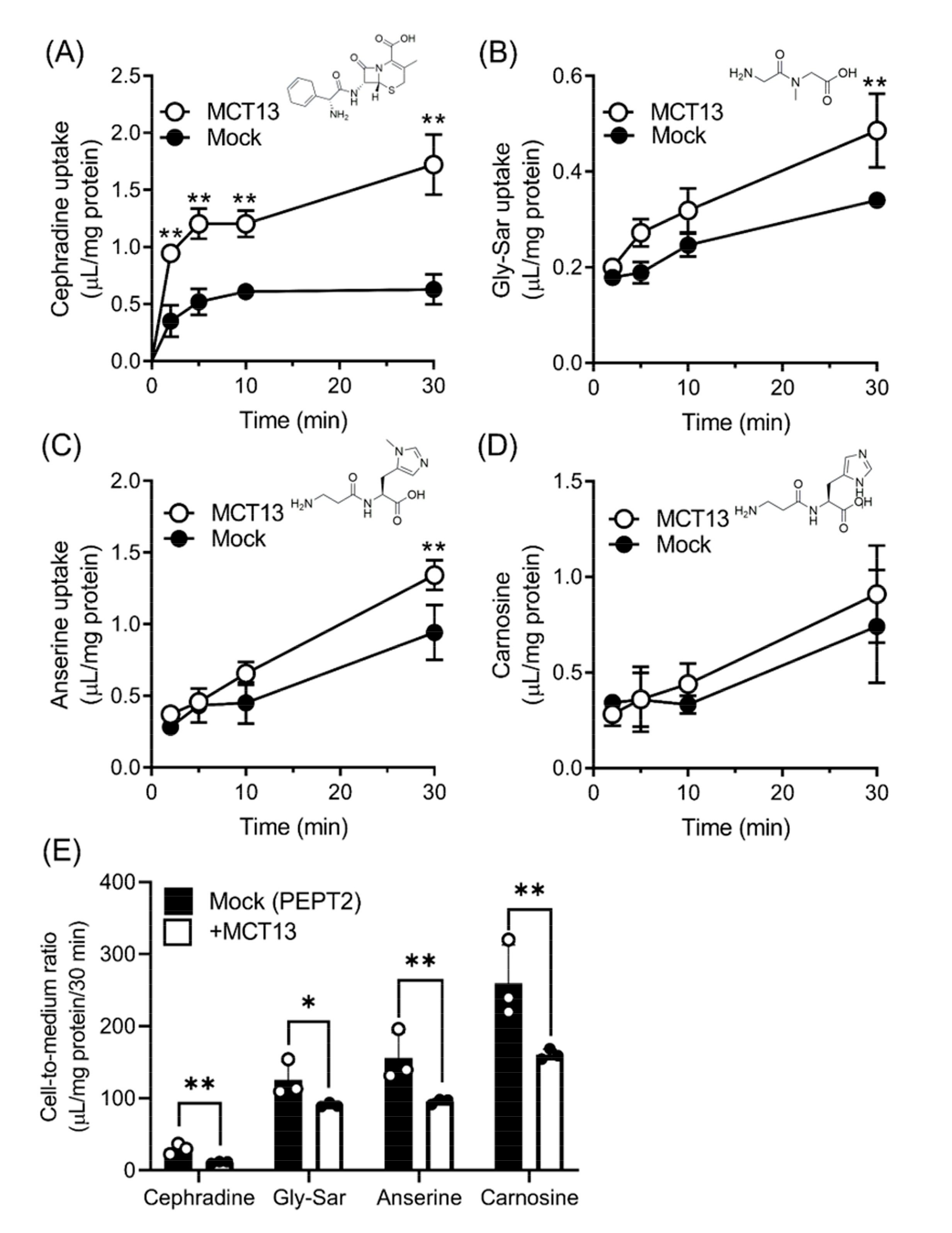
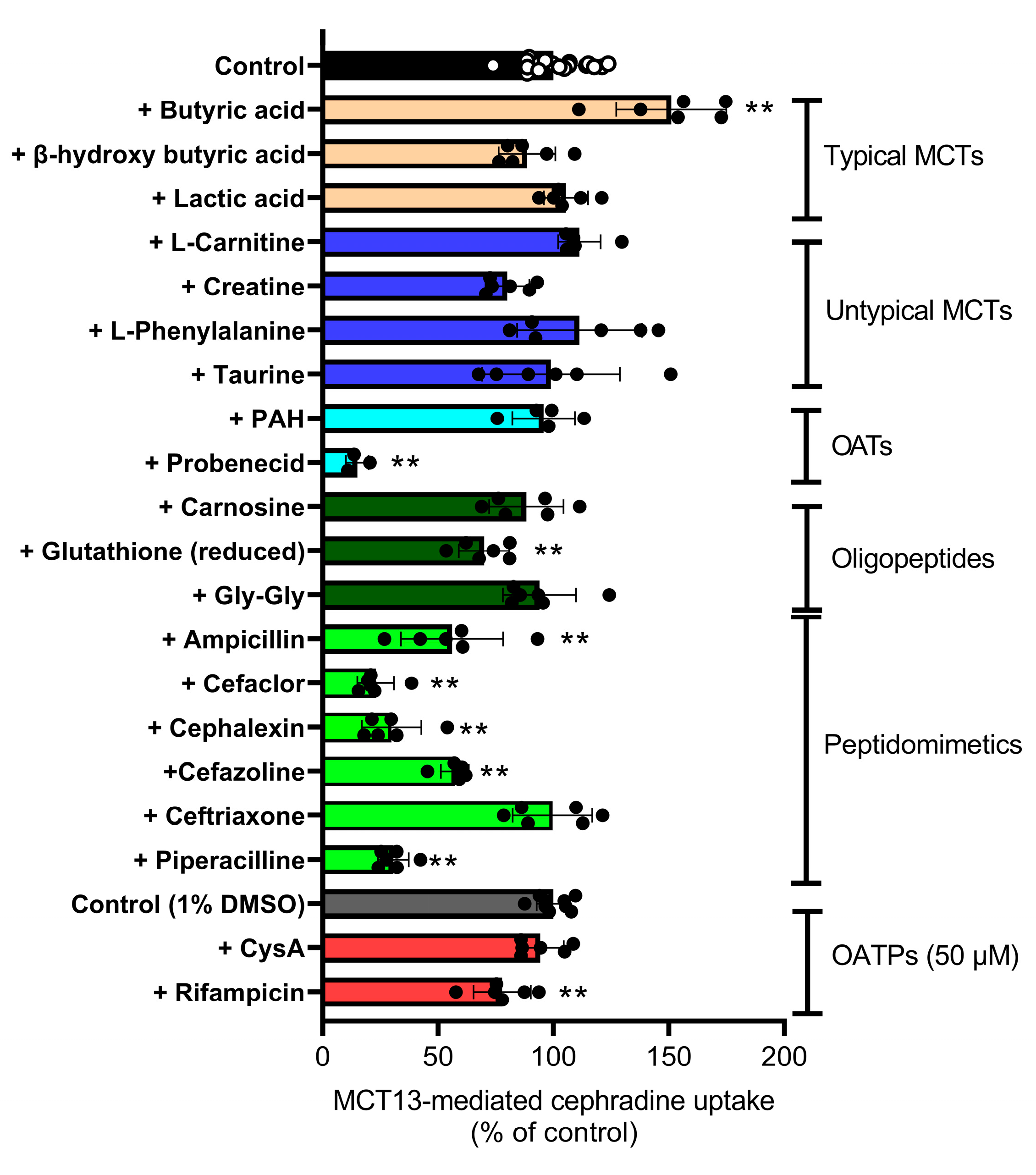
3.2. MCT13-Mediated Transport of Oligopeptides and Peptidomimetics
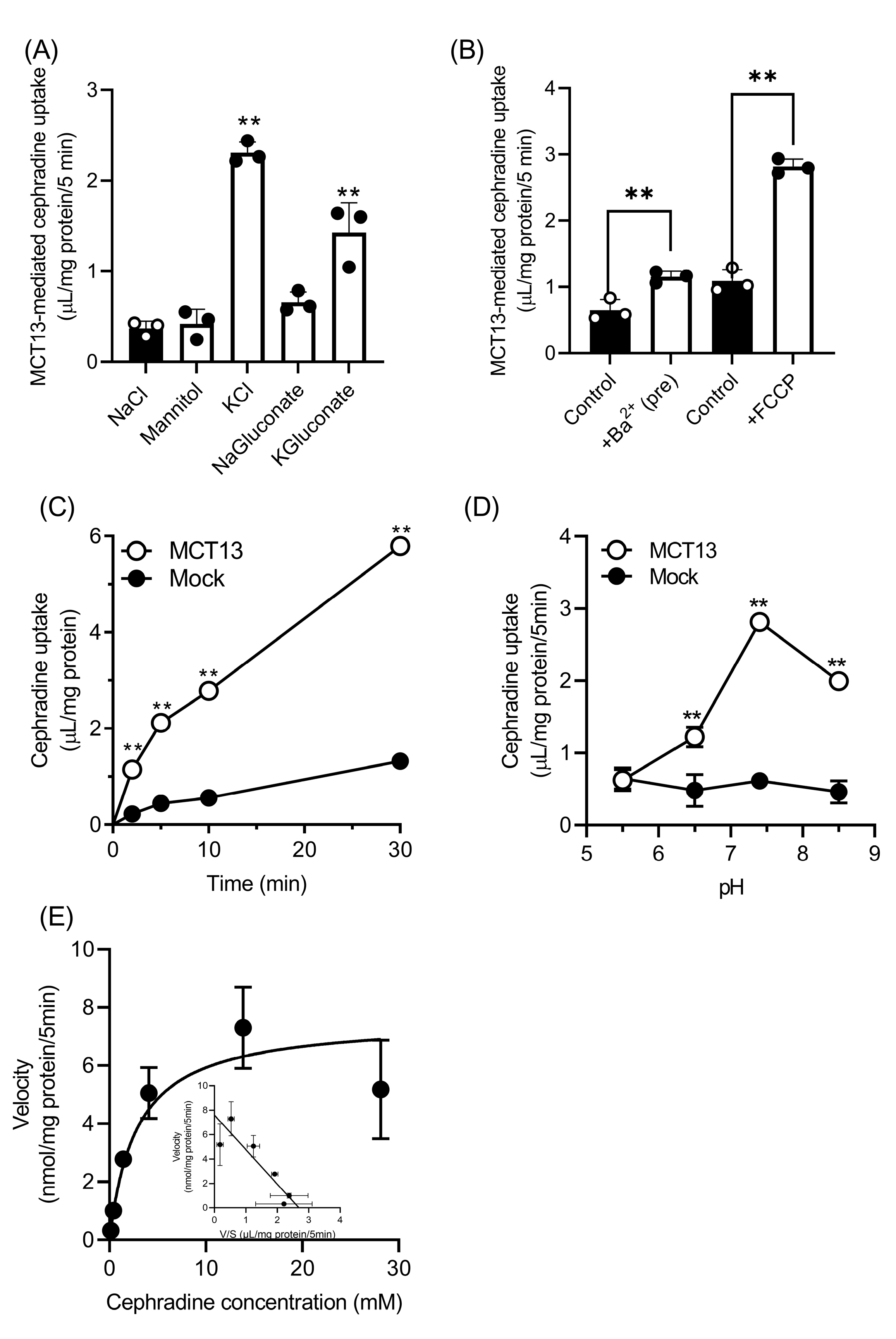
3.3. Characteristics of Cephradine Transport by MCT13
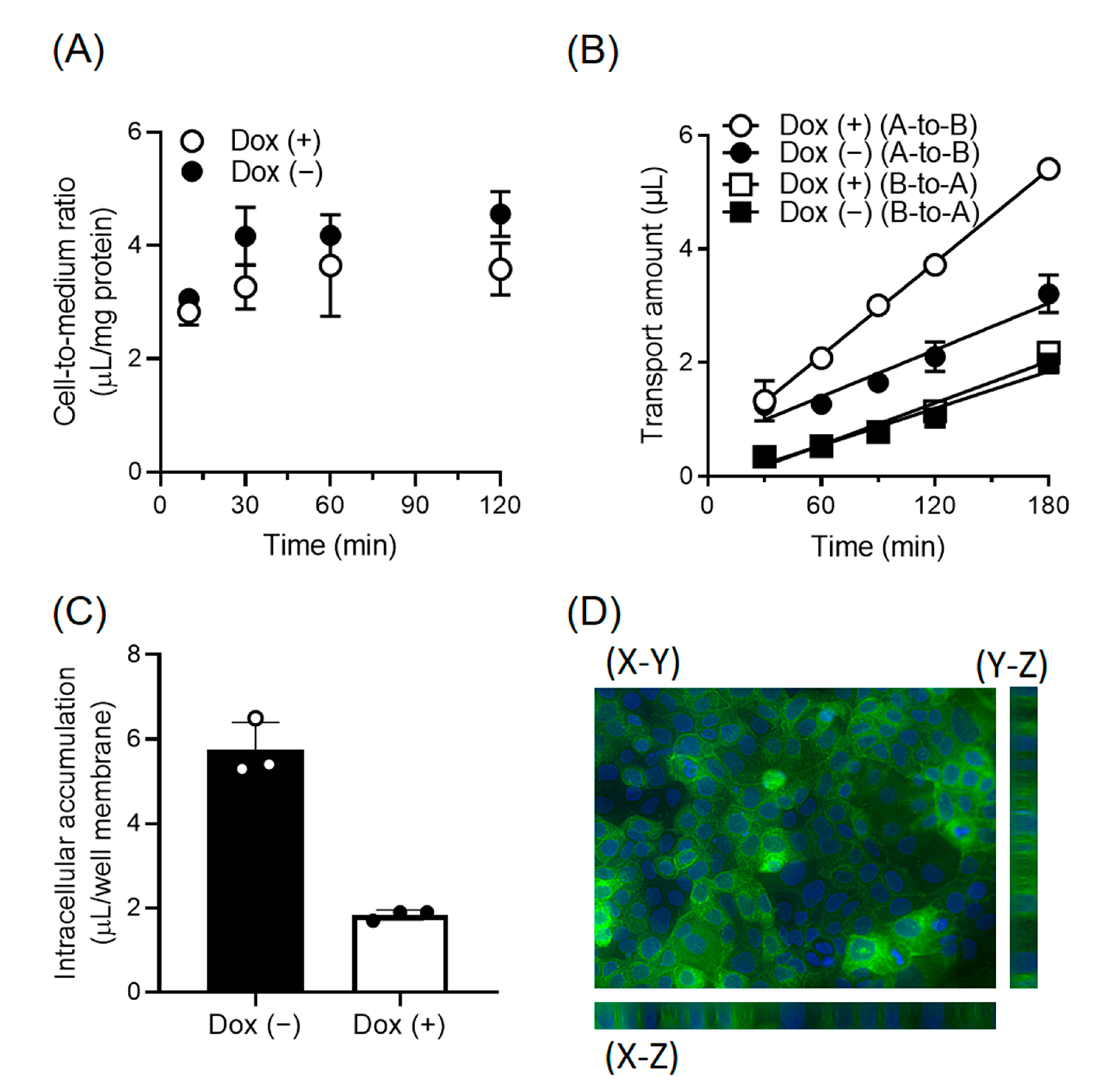
3.4. Enhancement of the Directional Transport of Cephradine across Caco-2 Cells by MCT13
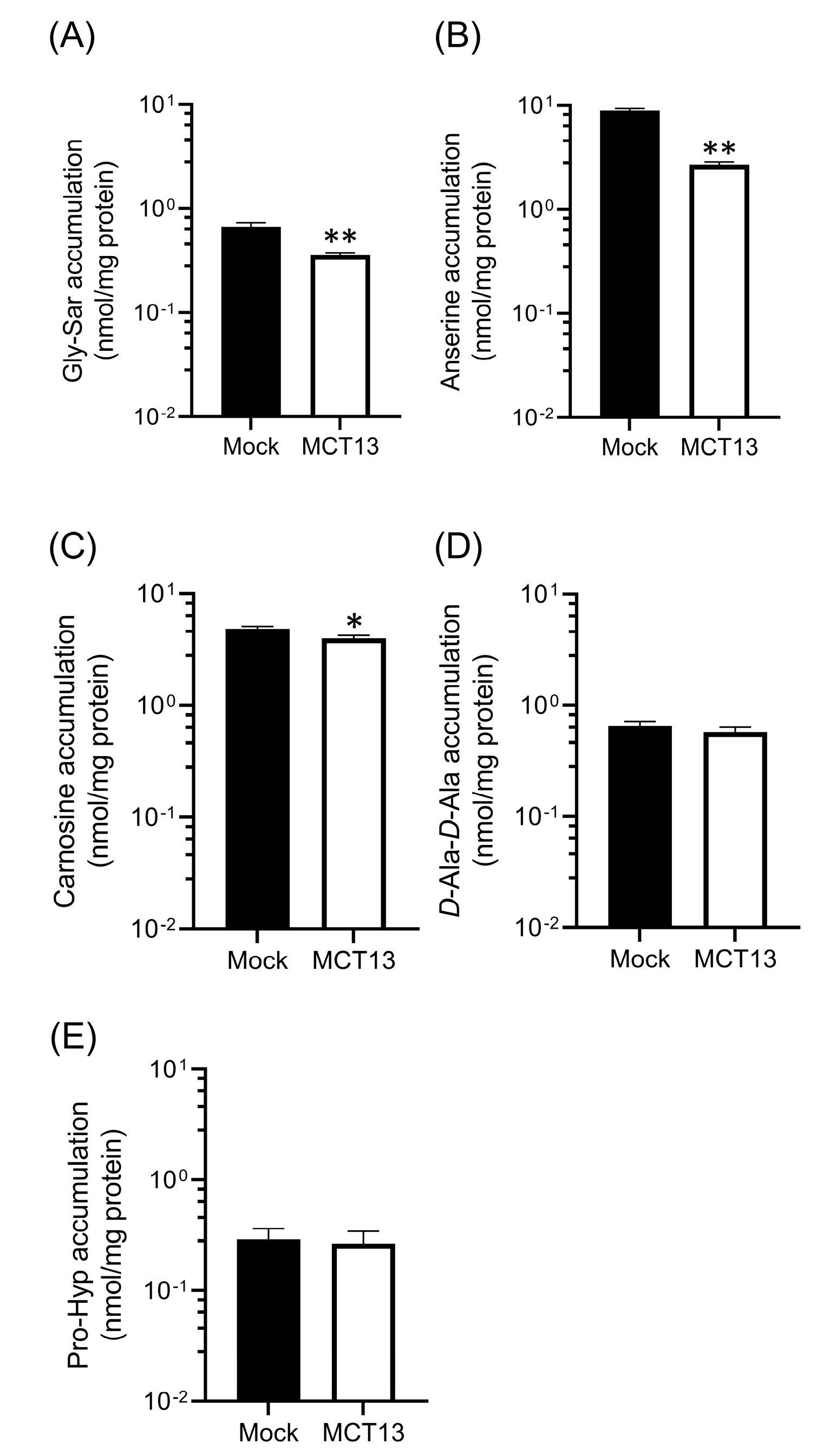
3.5. Regulation of Intracellular Oligopeptide Levels by MCT13
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganapathy, M.E.; Brandsch, M.; Prasad, P.D.; Ganapathy, V.; Leibach, F.H. Differential Recognition of Beta -Lactam Antibiotics by Intestinal and Renal Peptide Transporters, PEPT 1 and PEPT 2. J. Biol. Chem. 1995, 270, 25672–25677. [Google Scholar] [CrossRef]
- Liang, R.; Fei, Y.J.; Prasad, P.D.; Ramamoorthy, S.; Han, H.; Yang-Feng, T.L.; Hediger, M.A.; Ganapathy, V.; Leibach, F.H. Human Intestinal H+/Peptide Cotransporter. Cloning, Functional Expression, and Chromosomal Localization. J. Biol. Chem. 1995, 270, 6456–6463. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Inui, K. Dipeptide Transporters in Apical and Basolateral Membranes of the Human Intestinal Cell Line Caco-2. Am. J. Physiol. 1993, 265, G289–G294. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Brown, C.D.; Hirst, B.H.; Simmons, N.L. Transepithelial Glycylsarcosine Transport in Intestinal Caco-2 Cells Mediated by Expression of H(+)-Coupled Carriers at Both Apical and Basal Membranes. J. Biol. Chem. 1993, 268, 7640–7642. [Google Scholar] [CrossRef]
- Terada, T.; Sawada, K.; Saito, H.; Hashimoto, Y.; Inui, K. Functional Characteristics of Basolateral Peptide Transporter in the Human Intestinal Cell Line Caco-2. Am. J. Physiol. 1999, 276, G1435–G1441. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Terada, T.; Sawada, K.; Saito, H.; Inui, K. Recognition and Transport Characteristics of Nonpeptidic Compounds by Basolateral Peptide Transporter in Caco-2 Cells. J. Pharmacol. Exp. Ther. 2001, 298, 711–717. [Google Scholar] [PubMed]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Ondrej, C.; Jitka, V.; Ales, V.; Petra, B.; Vladimir, S., Jr. Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment. Nutrients 2023, 15, 1770. [Google Scholar]
- Xu, M.; Sun, B.; Li, D.; Mao, R.; Li, H.; Li, Y.; Wang, J. Beneficial Effects of Small Molecule Oligopeptides Isolated from Panax Ginseng Meyer on Pancreatic Beta-Cell Dysfunction and Death in Diabetic Rats. Nutrients 2017, 9, 1061. [Google Scholar] [CrossRef]
- Seki, E.; Yamamoto, A.; Fujiwara, Y.; Yamane, T.; Satsu, H.; Ohkubo, I. Dipeptidyl Peptidase-IV Inhibitory Activity of Katsuobushi-Derived Peptides in Caco-2 Cell Assay and Oral Glucose Tolerance Test in ICR Mice. J. Agric. Food Chem. 2020, 68, 6355–6367. [Google Scholar] [CrossRef]
- Rohm, F.; Daniel, H.; Spanier, B. Transport Versus Hydrolysis: Reassessing Intestinal Assimilation of Di- and Tripeptides by LC-MS/MS Analysis. Mol. Nutr. Food Res. 2019, 63, e1900263. [Google Scholar] [CrossRef]
- Geissler, S.; Zwarg, M.; Knütter, I.; Markwardt, F.; Brandsch, M. The Bioactive Dipeptide Anserine Is Transported by Human Proton-Coupled Peptide Transporters. FEBS J. 2010, 277, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 Gene Family-Structure, Role and Regulation in Health and Disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Contreras-Baeza, Y.; Sandoval, P.Y.; Alarcón, R.; Galaz, A.; Cortés-Molina, F.; Alegría, K.; Baeza-Lehnert, F.; Arce-Molina, R.; Guequén, A.; Flores, C.A.; et al. Monocarboxylate Transporter 4 (MCT4) Is a High Affinity Transporter Capable of Exporting Lactate in High-Lactate Microenvironments. J. Biol. Chem. 2019, 294, 20135–20147. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Y.; Vera, J.C.; Chaganti, R.S.; Golde, D.W. Human Monocarboxylate Transporter 2 (MCT2) Is a High Affinity Pyruvate Transporter. J. Biol. Chem. 1998, 273, 28959–28965. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kishimoto, H.; Shirasaka, Y.; Inoue, K. Functional Characterization of Monocarboxylate Transporter 12 (SLC16A12/MCT12) as a Facilitative Creatine Transporter. Drug Metab. Pharmacokinet. 2020, 35, 281–287. [Google Scholar] [CrossRef]
- Suhre, K.; Shin, S.-Y.; Petersen, A.-K.; Mohney, R.P.; Meredith, D.; Wägele, B.; Altmaier, E.; Deloukas, P.; Erdmann, J.; Grundberg, E.; et al. Human Metabolic Individuality in Biomedical and Pharmaceutical Research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Higuchi, K.; Sugiyama, K.; Tomabechi, R.; Kishimoto, H.; Inoue, K. Mammalian Monocarboxylate Transporter 7 (MCT7/Slc16a6) Is a Novel Facilitative Taurine Transporter. J. Biol. Chem. 2022, 298, 101800. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Wilson, M.C.; Meredith, D.; Fox, J.E.M.; Manoharan, C.; Davies, A.J.; Halestrap, A.P. Basigin (CD147) Is the Target for Organomercurial Inhibition of Monocarboxylate Transporter Isoforms 1 and 4: The Ancillary Protein for the Insensitive MCT2 Is EMBIGIN (Gp70). J. Biol. Chem. 2005, 280, 27213–27221. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Wilson, M.C.; Heddle, C.; Brown, M.H.; Barclay, A.N.; Halestrap, A.P. CD147 Is Tightly Associated with Lactate Transporters MCT1 and MCT4 and Facilitates Their Cell Surface Expression. EMBO J. 2000, 19, 3896–3904. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Saksena, S.; Alrefai, W.A.; Sarwar, Z.; Goldstein, J.L.; Carroll, R.E.; Ramaswamy, K.; Dudeja, P.K. Expression and Membrane Localization of MCT Isoforms along the Length of the Human Intestine. Am. J. Physiol. Cell Physiol. 2005, 289, C846–C852. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Iwanaga, T.; Kishimoto, A. Cellular Distributions of Monocarboxylate Transporters: A Review. Biomed. Res. Tokyo Jpn. 2015, 36, 279–301. [Google Scholar] [CrossRef]
- Hara, K.; Fujita, H.; Johnson, T.A.; Yamauchi, T.; Yasuda, K.; Horikoshi, M.; Peng, C.; Hu, C.; Ma, R.C.W.; Imamura, M.; et al. Genome-Wide Association Study Identifies Three Novel Loci for Type 2 Diabetes. Hum. Mol. Genet. 2014, 23, 239–246. [Google Scholar] [CrossRef]
- Zheng, H.; Pu, S.; Zhang, Y.; Fan, Y.; Yang, J. The Association between the Rs312457 Genotype of the SLC16a13 Gene and Diabetes Mellitus in a Chinese Population. Comput. Math. Methods Med. 2021, 2021, 9918055. [Google Scholar] [CrossRef]
- Schumann, T.; König, J.; von Loeffelholz, C.; Vatner, D.F.; Zhang, D.; Perry, R.J.; Bernier, M.; Chami, J.; Henke, C.; Kurzbach, A.; et al. Deletion of the Diabetes Candidate Gene Slc16a13 in Mice Attenuates Diet-Induced Ectopic Lipid Accumulation and Insulin Resistance. Commun. Biol. 2021, 4, 826. [Google Scholar] [CrossRef]
- Natsume, T.; Kiyomitsu, T.; Saga, Y.; Kanemaki, M.T. Rapid Protein Depletion in Human Cells by Auxin-Inducible Degron Tagging with Short Homology Donors. Cell Rep. 2016, 15, 210–218. [Google Scholar] [CrossRef]
- Kodama, Y.; Hu, C.-D. An Improved Bimolecular Fluorescence Complementation Assay with a High Signal-to-Noise Ratio. BioTechniques 2010, 49, 793–805. [Google Scholar] [CrossRef]
- Shyu, Y.J.; Liu, H.; Deng, X.; Hu, C.-D. Identification of New Fluorescent Protein Fragments for Bimolecular Fluorescence Complementation Analysis under Physiological Conditions. BioTechniques 2006, 40, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Komae, S.; Kasamatsu, S.; Uchida, K.; Ihara, H. Quantitative Determination of 2-Oxo-Imidazole-Containing Dipeptides by High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Antioxidants 2022, 11, 2401. [Google Scholar] [CrossRef] [PubMed]
- Hille, B.; Schwarz, W. Potassium Channels as Multi-Ion Single-File Pores. J. Gen. Physiol. 1978, 72, 409–442. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Jo, I.; Pak, K.; Bae, S.-W.; Rhim, H.; Suh, S.-H.; Park, J.; Zhu, H.; So, I.; Kim, K.W. FCCP Depolarizes Plasma Membrane Potential by Activating Proton and Na+ Currents in Bovine Aortic Endothelial Cells. Pflug. Arch. 2002, 443, 344–352. [Google Scholar] [CrossRef]
- Inui, K.; Yamamoto, M.; Saito, H. Transepithelial Transport of Oral Cephalosporins by Monolayers of Intestinal Epithelial Cell Line Caco-2: Specific Transport Systems in Apical and Basolateral Membranes. J. Pharmacol. Exp. Ther. 1992, 261, 195–201. [Google Scholar]
- Matsumoto, S.; Saito, H.; Inui, K. Transcellular Transport of Oral Cephalosporins in Human Intestinal Epithelial Cells, Caco-2: Interaction with Dipeptide Transport Systems in Apical and Basolateral Membranes. J. Pharmacol. Exp. Ther. 1994, 270, 498–504. [Google Scholar]
- Baye, E.; Ukropec, J.; de Courten, M.P.; Vallova, S.; Krumpolec, P.; Kurdiova, T.; Aldini, G.; Ukropcova, B.; de Courten, B. Effect of Carnosine Supplementation on the Plasma Lipidome in Overweight and Obese Adults: A Pilot Randomised Controlled Trial. Sci. Rep. 2017, 7, 17458. [Google Scholar] [CrossRef]
- Yuasa, H.; Amidon, G.L.; Fleisher, D. Peptide Carrier-Mediated Transport in Intestinal Brush Border Membrane Vesicles of Rats and Rabbits: Cephradine Uptake and Inhibition. Pharm. Res. 1993, 10, 400–404. [Google Scholar] [CrossRef]
- Luckner, P.; Brandsch, M. Interaction of 31 Beta-Lactam Antibiotics with the H+/Peptide Symporter PEPT2: Analysis of Affinity Constants and Comparison with PEPT1. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2005, 59, 17–24. [Google Scholar] [CrossRef]
- Mackenzie, B.; Loo, D.D.; Fei, Y.; Liu, W.J.; Ganapathy, V.; Leibach, F.H.; Wright, E.M. Mechanisms of the Human Intestinal H+-Coupled Oligopeptide Transporter HPEPT1. J. Biol. Chem. 1996, 271, 5430–5437. [Google Scholar] [CrossRef]
- Brandsch, M.; Knütter, I.; Bosse-Doenecke, E. Pharmaceutical and Pharmacological Importance of Peptide Transporters. J. Pharm. Pharmacol. 2008, 60, 543–585. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Luck, K.; Kim, D.-K.; Lambourne, L.; Spirohn, K.; Begg, B.E.; Bian, W.; Brignall, R.; Cafarelli, T.; Campos-Laborie, F.J.; Charloteaux, B.; et al. A Reference Map of the Human Binary Protein Interactome. Nature 2020, 580, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Hubbert, M.; Haywood, J.; Craddock, A.L.; Zerangue, N.; Christian, W.V.; Ballatori, N. The Heteromeric Organic Solute Transporter Alpha-Beta, Ostalpha-Ostbeta, Is an Ileal Basolateral Bile Acid Transporter. J. Biol. Chem. 2005, 280, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, K.; Kunieda, M.; Sugiyama, K.; Tomabechi, R.; Kishimoto, H.; Inoue, K. Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter. Nutrients 2023, 15, 3527. https://doi.org/10.3390/nu15163527
Higuchi K, Kunieda M, Sugiyama K, Tomabechi R, Kishimoto H, Inoue K. Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter. Nutrients. 2023; 15(16):3527. https://doi.org/10.3390/nu15163527
Chicago/Turabian StyleHiguchi, Kei, Misato Kunieda, Koki Sugiyama, Ryuto Tomabechi, Hisanao Kishimoto, and Katsuhisa Inoue. 2023. "Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter" Nutrients 15, no. 16: 3527. https://doi.org/10.3390/nu15163527
APA StyleHiguchi, K., Kunieda, M., Sugiyama, K., Tomabechi, R., Kishimoto, H., & Inoue, K. (2023). Monocarboxylate Transporter 13 (MCT13/SLC16A13) Functions as a Novel Plasma Membrane Oligopeptide Transporter. Nutrients, 15(16), 3527. https://doi.org/10.3390/nu15163527





