Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers
Abstract
1. Introduction
2. Material and Methods
2.1. Study and Control Groups
2.2. Disease Activity
2.3. Nutritional Status
2.4. Blood Sample Collection and Serum Markers of Nutritional Status
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Subjects
3.2. Concentrations of Albumin, Transferrin and Transthyretin in CD and UC Patients
3.3. Concentrations of Albumin, Transferrin and Transthyretin vs. Disease Activity
3.4. Concentrations of Albumin, Transferrin and Transthyretin and the Nutritional Status of Patients
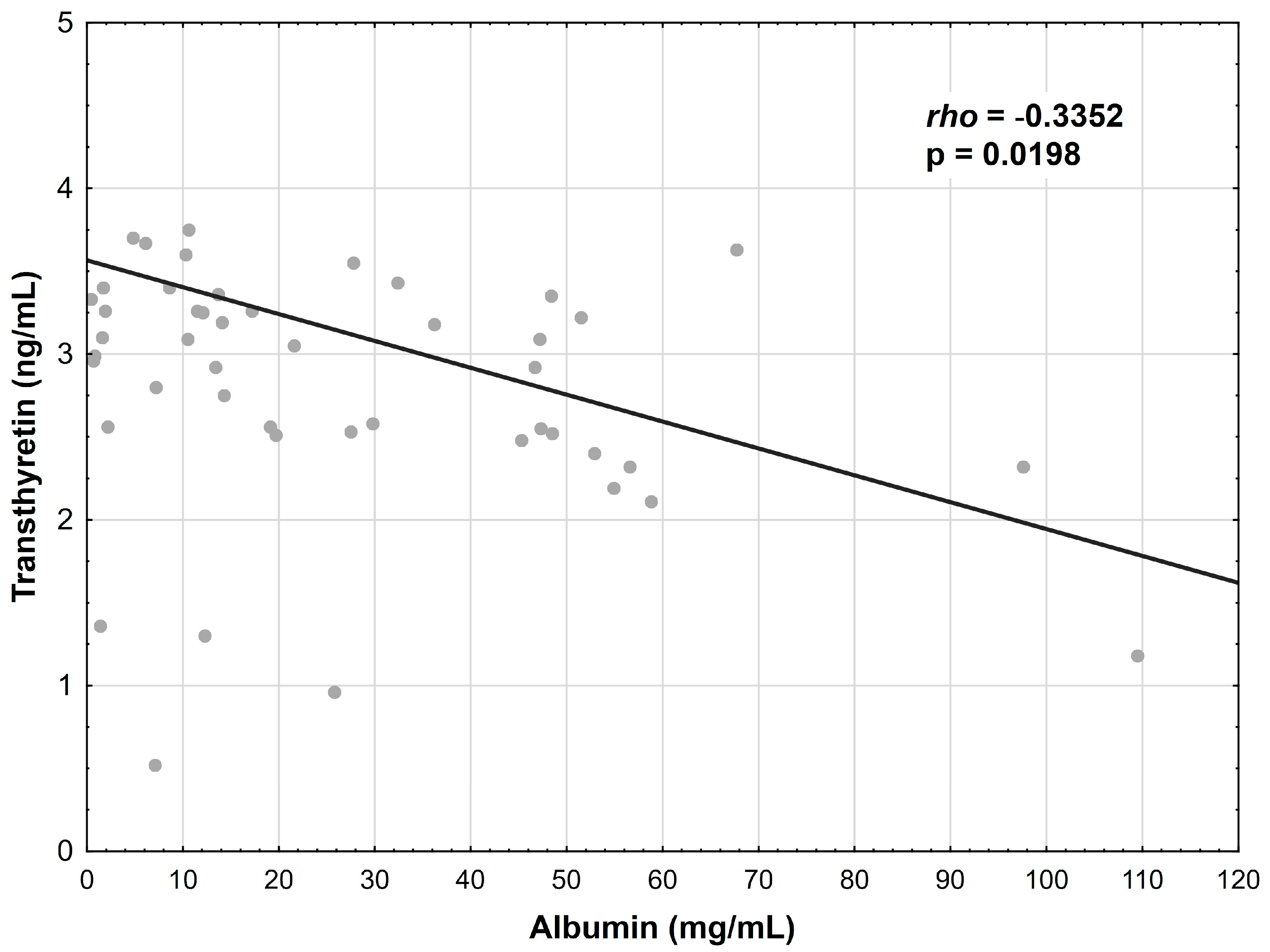
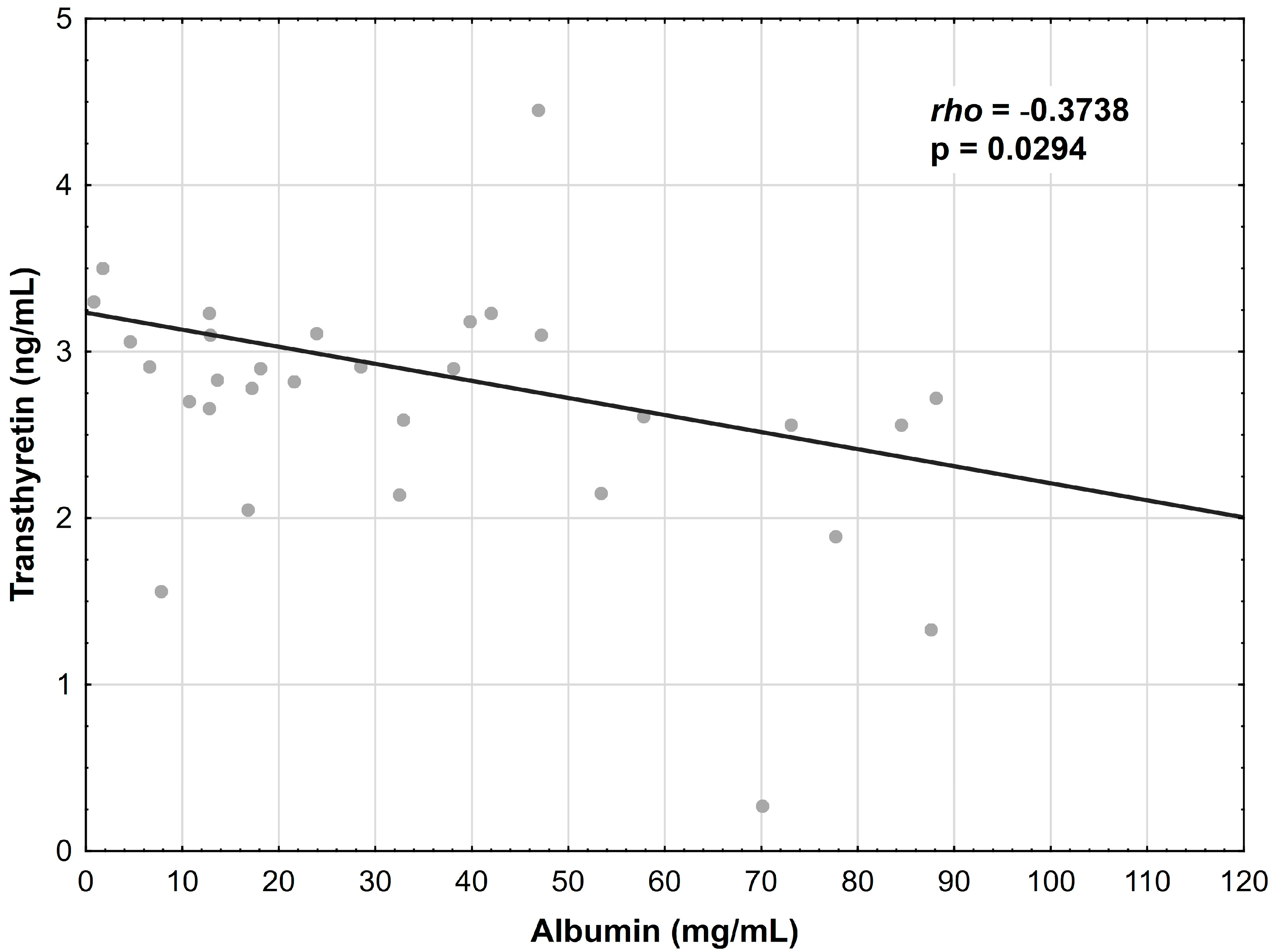
3.5. Correlations between Albumin, Transferrin and Transthyretin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Łodyga, M.; Eder, P.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of patients with Crohn’s disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Prz. Gastroenterol. 2021, 16, 257–296. [Google Scholar] [CrossRef]
- Marcil, V.; Levy, E.; Amre, D.; Bitton, A.; Guilhon de Araujo, A.M.; Szilagy, A.; Sinnett, D.; Seidman, E.D. Erratum: A Cross-Sectional Study on Malnutrition in Inflammatory Bowel Disease: Is There a Difference Based on Pediatric or Adult Age Grouping? Inflamm. Bowel. Dis. 2020, 26, 160. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Mrowiec, S. Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases. Nutrients 2023, 15, 1991. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef]
- Green, N.; Miller, T.; Suskind, D.; Lee, D. A review of dietary therapy for IBD and a vision for the future. Nutrients 2019, 11, 947. [Google Scholar] [CrossRef]
- Maconi, G.; Ardizzone, S.; Cucino, C.; Bezzio, C.; Russo, A.G.; Bianchi Porro, G. Pre-illness changes in dietary habits and diet as a risk factor for inflammatory bowel disease: A case-control study. World. J. Gastroenterol. 2010, 16, 4297–4304. [Google Scholar] [CrossRef]
- Opstelten, J.L.; de Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Vidarsdottir, J.B.; Johannsdottir, S.E.; Thorsdottir, I.; Bjornsson, E.; Ramel, A. A cross-sectional study on nutrient intake and -status in inflammatory bowel disease patients. Nutr. J. 2016, 15, 61. [Google Scholar] [CrossRef]
- Valentini, L.; Schaper, L.; Buning, C.; Hengstermann, S.; Koernicke, T.; Tillinger, W.; Guglielmi, F.W.; Norman, K.; Buhner, S.; Ockenga, J.; et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 2008, 24, 694–702. [Google Scholar] [CrossRef]
- Valentini, L.; Schulzke, J.D. Mundane, yet challenging: The assessment of malnutrition in inflammatory bowel disease. Eur. J. Int. Med. 2011, 22, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Morton, H.; Pedley, K.C.; Stewart, R.J.C.; Coad, J. Inflammatory Bowel Disease: Are Symptoms and Diet Linked? Nutrients 2020, 12, 2975. [Google Scholar] [CrossRef]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharm. 2018, 57, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef] [PubMed]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Gu, P.; Luo, J.; Kim, J.; Paul, P.; Limketkai, B.; Sauk, J.S.; Park, S.; Parekh, N.; Zheng, K.; Rudrapatna, V.; et al. Effect of Obesity on Risk of Hospitalization, Surgery, and Serious Infection in Biologic-Treated Patients With Inflammatory Bowel Diseases: A CA-IBD Cohort Study. Am. J. Gastroenterol. 2022, 117, 1639–1647. [Google Scholar] [CrossRef]
- Michalak, A.; Kasztelan-Szczerbińska, B.; Cichoż-Lach, H. Impact of Obesity on the Course of Management of Inflammatory Bowel Disease–A Review. Nutrients 2022, 14, 3983. [Google Scholar] [CrossRef]
- Caraceni, P.; Domenicali, M.; Tovoli, A.; Napoli, L.; Ricci, C.S.; Tufoni, M.; Bernardi, M. Clinical indications for the albumin use: Still a controversial issue. Eur. J. Intern. Med. 2013, 24, 721–728. [Google Scholar] [CrossRef]
- Rozga, J.; Piątek, T.; Małkowski, P. Human albumin: Old, new, and emerging applications. Ann. Transpl. 2013, 18, 205–217. [Google Scholar] [CrossRef]
- Schoenefuss, F.; Hoffmann, P. Serum γ-globulin and albumin concentrations predict secondary loss of response to anti-TNFα in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1563–1568. [Google Scholar] [CrossRef]
- Shenkin, A. Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin. Chem. 2006, 52, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. Plasma transthyretin is a nutritional biomarker in human morbidities. Front. Med. 2022, 16, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Cynober, L. Is transthyretin a good marker of nutritional status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Marco, E.; Ronquillo-Moreno, N.; Miralles, R.; Vazquez-Ibar, O.; Escalada, F.; Muniesa, J.M. Prevalence of malnutrition and sarcopenia in a post-acute care geriatric unit: Applying the new espen definition and ewgsop criteria. Clin. Nutr. 2016, 36, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M.; Izawa, S.; Enoki, H.; Okada, K.; Iguchi, A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? Clin. Nutr. 2007, 26, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; You, J.; Yeh, C.; Chen, J.; Tang, R.; Wang, J.; Chin, C. Low preoperative serum albumin in colon cancer: A risk factor for poor outcome. Int. J. Colorectal. Dis. 2011, 26, 473–481. [Google Scholar] [CrossRef]
- Asher, V.; Lee, J.; Bali, A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med. Oncol. 2012, 29, 2005–2009. [Google Scholar] [CrossRef]
- Bozzetti, F.; Braga, M.; Gianotti, L.; Gavazzi, C.; Mariani, L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: A randomized multicentre trial. Lancet 2001, 358, 1487–1492. [Google Scholar] [CrossRef]
- Loh, K.; Vriens, M.; Gerritsen, A.; Borel Rinkes, I.; van Hillegersberg, R.; Schippers, C.; Steenhagen, E.; Ong, T.; Moy, F.; Molenaar, I. Unintentional weight loss is the most important indicator of malnutrition among surgical cancer patients. Neth. J. Med. 2012, 70, 365–369. [Google Scholar]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Glinkowski, S.; Marcinkowska, D. Ulcerative colitis: Assessment of disease activity based on contemporary scales. Nowa Med. 2018, 25, 123–137. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, W.; Wang, J.; Li, J.; Qian, S.; Cai, C.; Liu, Y. Serum Albumin to Globulin Ratio is Associated with the Presence and Severity of Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Mijac, D.D.; Janković, G.L.; Jorga, J.; Krstić, M.N. Nutritional status in patients with active inflammatory bowel disease: Prevalence of malnutrition and methods for routine nutritional assessment. Eur. J. Int. Med. 2010, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Geerling, B.J.; Badart-Smook, A.; Stockbrugger, R.W.; Brummer, R.J. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur. J. Clin. Nutr. 2000, 54, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Capristo, E.; Addolorato, G.; Mingrone, G.; Greco, A.V.; Gasbarrini, G. Effect of disease localization on the anthropometric and metabolic features of Crohn’s disease. Am. J. Gastroenterol. 1998, 93, 2411–2419. [Google Scholar] [CrossRef]
- Lanfranchi, G.A.; Brignola, C.; Campieri, M.; Bazzocchi, G.; Pasquali, R.; Bassein, L.; Labò, G. Assessment of nutritional status in Crohn’s disease in remission or low activity. Hepatogastroenterology 1984, 31, 129–132. [Google Scholar]
- Benjamin, J.; Makharia, G.K.; Kalaivani, M.; Joshi, Y.K. Nutritional status of patients with Crohn’s disease. Ind. J. Gastroenterol. 2008, 27, 195–200. [Google Scholar]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition assessment of patients with inflammatory bowel disease. J. Parent. Ent. Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef]
- Loly, C.; Belaiche, J.; Louis, E. Predictors of severe Crohn’s disease. Scand. J. Gastroenterol. 2008, 43, 948–954. [Google Scholar] [CrossRef]
- Beaugerie, L.; Seksik, P.; Nion-Larmurier, I.; Gendre, J.P.; Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 2006, 130, 650–656. [Google Scholar] [CrossRef]
- Seksik, P.; Loftus, E.V.; Beaugerie, L.; Harmsen, W.S. Validation of predictors of 5-year disabling CD in a population-based cohort from Olmsted County, Minnesota 1983–1996. Gastroenterology 2007, 132, A17. [Google Scholar]
- Matusiewicz, M.; Neubauer, K.; Lewandowska, P.; Gamian, A.; Krzystek-Korpacka, M. Reduced Transferrin Levels in Active Inflammatory Bowel Disease. Biomed. Res. Int. 2017, 2017, 9541370. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Miszputen, S.J.; Figueiredo, M.S. Anemia in inflammatory bowel disease: Prevalence, differential diagnosis and association with clinical and laboratory variables. Sao Paulo Med. J. 2014, 132, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Mahadea, D.; Adamczewska, E.; Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Iron Deficiency Anemia in Inflammatory Bowel Diseases-A Narrative Review. Nutrients 2021, 13, 4008. [Google Scholar] [CrossRef]
- Gasche, C.; Befrits, A.; Berstad, R.; Beglinger, C.; Dignass, A.; Erichsen, K.; Gomollon, F. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm. Bow. Dis. 2007, 13, 1545–1553. [Google Scholar] [CrossRef]
- Cavallaro, F.L.; Duca, L.F.; Pisani, L.F.; Rigolini, R.; Spina, L.; Tontini, G.E.; Munizio, N.; Costa, E.; Cappellini, M.D.; Vecchi, M.; et al. Anti-TNF-Mediated Modulation of Prohepcidin Improves Iron Availability in Inflammatory Bowel Disease, in an IL-6-Mediated Fashion. Can. J. Gastroenterol. Hepatol. 2017, 2017, 6843976. [Google Scholar] [CrossRef]
- Repnik, K.; Koder, S.; Skok, P.; Ferkolj, I.; Potočnik, U. Transferrin Level Before Treatment and Genetic Polymorphism in HFE Gene as Predictive Markers for Response to Adalimumabin Crohn’s Disease Patients. Biochem. Genet. 2016, 54, 476–486. [Google Scholar] [CrossRef]
- El Koofy, N.M.; Moawad, E.M.I.; Yassin, N.A.; Almohammady, M.N.; Ibrahim, G.S.; El Mougy, F.A.; El Ayadi, A.A.; Tarek, S. Basic anthropometry, micronutrients status and growth velocity of patients with early-onset inflammatory bowel disease: A prospective cohort study. Arab. J. Gastroenterol. 2022, 23, 270–276. [Google Scholar] [CrossRef]
- Roma, E.; Krini, M.; Hantzi, E.; Sakka, S.; Panayiotou, I.; Margeli, A.; Papassotiriou, I.; Kanaka-Gantenbein, C. Retinol Binding Protein 4 in children with Inflammatory Bowel Disease: A negative correlation with the disease activity. Hippokratia 2012, 16, 360–365. [Google Scholar]
- Piras, C.; Soggiu, A.; Greco, V.; Cassinotti, A.; Maconi, G.; Ardizzone, S.; Amoresano, A.; Bianchi Porro, G.; Bonizzi, L.; Roncada, P. Serum protein profiling of early and advanced stage Crohn’s disease. EuPA Open. Proteom. 2014, 3, 48–59. [Google Scholar] [CrossRef]
- Weeke, B.; Jarnum, S. Serum concentration of 19 serumproteins in Crohn’s disease and ulcerative colitis. Gut 1971, 12, 297–302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doherty, N.S.; Littman, B.H.; Reilly, K.; Swindell, A.C.; Buss, J.M.; Anderson, N.L. Analysis of changes in acute-phase plasmaproteins in an acute inflammatory response and inrheumatoid arthritis using two-dimensional gelelectrophoresis. Electrophoresis 1998, 19, 355–363. [Google Scholar] [CrossRef]
- Kupčová, V.; Turecký, L.; Detkova, Z.; Prikazska, M.; Keleova, A. Changes in acute phase proteins after anti-tumor necrosisfactor antibody (infliximab) treatment in patients withCrohns disease. Physiol. Res. 2003, 52, 89–93. [Google Scholar] [CrossRef]
- Myron Johnson, A.; Merlini, G.; Sheldon, J.; Ichihara, K. Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: Transthyretin (prealbumin) in inflammation and malnutrition. Clin. Chem. Lab. Med. 2007, 45, 419–426. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Snipelisky, D.; Jentzer, J.; Batal, O.; Dardari, Z.; Mathier, M. Serum albumin concentration as an independent prognostic indicator in patients with pulmonary arterial hypertension. Clin. Cardiol. 2018, 41, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.Y.; Wu, T.H.; Liu, C.S.; Lin, C.H.; Lin, C.C.; Lai, M.M.; Lin, W.Y. Body mass index and albumin levels are prognostic factors for long-term survival in elders with limited performance status. Aging 2020, 12, 1104–1113. [Google Scholar] [CrossRef]
- Kimura, Y.; Yamada, M.; Kakehi, T.; Itagaki, A.; Tanaka, N.; Muroh, Y. Combination of Low Body Mass Index and Low Serum Albumin Level Leads to Poor Functional Recovery in Stroke Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 448–453. [Google Scholar] [CrossRef]
- Phung, D.T.; Wang, Z.; Rutherford, S.; Huang, C.; Chu, C. Body mass index and risk of pneumonia: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Viasus, D.; Garcia-Vidal, C.; Simonetti, A.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J. Infect. 2013, 66, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Farage, N.E.; Azevedo, D.L.; Viana, G.G.; Mattos, J.P.; Velarde, L.G.; Fouque, D. Impact of serum albumin and body-mass index on survival in hemodialysis patients. Int. Urol. Nephrol. 2007, 39, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Courtney, D.; Moloney, B.; Lowery, A.; Kerin, M. Patients Nutritional Status: BMI and serum albumin as a predictive indicator of survival in patients with metastatic breast cancer. Eur. J. Surg. Oncol. 2017, 43, S29–S30. [Google Scholar] [CrossRef]
- Hong, X.; Yan, J.; Xu, L.; Shen, S.; Zeng, X.; Chen, L. Relationship between nutritional status and frailty in hospitalized older patients. Clin. Interv. Aging. 2019, 14, 105–111. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Solmi, M.; Fowler, S.A.; Manzato, E.; Maggi, S.; Manu, P.; Abe, E.; Hayashi, K.; Allard, J.P.; et al. Inverse relationship between body mass index and mortality in older nursing home residents: A meta-analysis of 19,538 elderly subjects. Obes. Rev. 2015, 16, 1001–1015. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Accardi, G.; Caruso, C. Immune-inflammatory responses in the elderly: An update. Immun. Ageing 2018, 15, 11. [Google Scholar] [CrossRef]
- Filliatre-Clement, L.; Broseus, J.; Muller, M.; Hosseini, K.; Rotonda, C.; Schirmer, L.; Roth-Guepin, G.; Bonmati, C.; Feugier, P.; Béné, M.C.; et al. Serum albumin or body mass index: Which prognostic factor for survival in patients with acute myeloblastic leukaemia? Hematol. Oncol. 2019, 37, 80–84. [Google Scholar] [CrossRef]
- Ling, S.C.; Griffiths, A.M. Nutrition in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care. 2000, 3, 339–344. [Google Scholar] [CrossRef]
- Gassull, M.A. Nutrition and inflammatory bowel disease: Its relation to pathophysiology, outcome and therapy. Dig. Dis. 2003, 21, 220–227. [Google Scholar] [CrossRef]
- Mao, M.J.; Wei, X.L.; Sheng, H.; Wang, X.P.; Li, X.H.; Liu, Y.J.; Xing, S.; Huang, Q.; Dai, S.Q.; Liu, W.L. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed. Res. Int. 2017, 2017, 3083267. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. J. Parenter. Enteral. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; ASPEN Malnutrition Committee. The use of visceral proteins as nutrition markers: An ASPEN position paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Devakonda, A.; George, L.; Raoof, S.; Esan, A.; Saleh, A.; Bernstein, L.H. Transthyretin as a marker to predict outcome in critically ill patients. Clin. Biochem. 2008, 41, 1126–1130. [Google Scholar] [CrossRef]
- Han, W.X.; Chen, Z.M.; Wei, Z.J.; Xu, A.M. Preoperative pre-albumin predicts prognosis of patients after gastrectomy for adenocarcinoma of esophagogastric junction. World. J. Surg. Oncol. 2016, 14, 279–285. [Google Scholar] [CrossRef]
- Li, J.D.; Xu, X.F.; Han, J.; Wu, H.; Xing, H.; Li, C.; Yu, J.J.; Zhou, Y.H.; Gu, W.M.; Wang, H.; et al. Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: A multi-institutional study. HPB 2019, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Guo, H.R.; Chen, H.H.; Peng, C.J. Nutritional predictors of survival in terminally ill cancer patients. J. Formos. Med. Assoc. 2003, 102, 544–550. [Google Scholar]
- Isono, N.; Imamura, Y.; Ohmura, K.; Ueda, N.; Kawabata, S.; Furuse, M.; Kuroiwa, T. Transthyretin concentrations in acute stroke patients predict convalescent rehabilitation. J. Stroke. Cerebrovasc. Dis. 2017, 26, 1375–1382. [Google Scholar] [CrossRef]
- Cui, N.; Tong, H.; Li, Y.; Ge, Y.; Shi, Y.; Lv, P.; Zhao, X.; Zhang, J.; Fu, G.; Zhou, Y.; et al. Role of prealbumin in predicting the prognosis of severely and critically ill COVID-19 patients. Am. J. Trop. Med. Hyg. 2021, 105, 718–726. [Google Scholar] [CrossRef]

| IBD N(%)/Mean ± SD | Controls N(%)/Mean ± SD | |
|---|---|---|
| CD | 48 (58.5) | - |
| UC | 34 (41.5) | - |
| Age (years) | 38.1 ± 11.6 | 38.6 ± 9.1 |
| Female | 42 (51.2) | 15 (60) |
| Level of education | ||
| Secondary | 42 (51.2) | 4 (16) * |
| High | 40 (48.8) | 21 (84) * |
| Current smoking | 14 (17.1) | 3 (12) |
| Disease duration | 8.4 ± 5.7 | - |
| Surgery history | 22 (26.8) | - |
| Weight lost during last 6 months (%) | 50 (60.9)/16.5 ± 8.2 | - |
| Anthropometry | ||
| BMI (kg/m2) | 24.23 ± 4.76 | 24.64 ± 3.97 |
| Waist circumference (cm) | 88.88 ± 14.54 | 85.28 ± 9.06 |
| Fatty tissue (%) | 27.1 ± 9.6 | 30.1 ± 8.6 * |
| Medications | ||
| Biological therapy | 66 (80.5) | - |
| Immunosuppression | 33 (40.2) | - |
| Steroids | 25 (30.5) | - |
| 5-ASA | 64 (78.0) | - |
| CD n (%) | UC n(%) | |
|---|---|---|
| Self-reported clinical stage of disease | ||
| Remission | 23 (47.9) | 17 (50) |
| Moderate | 16 (33.3) | 11 (32.4) |
| Active | 15 (31.2) | 6 (17.6) |
| CDAI | ||
| 0 | 17 (35.4) | - |
| 1 | 10 (20.9) | - |
| 2 | 17 (35.4) | - |
| 3 | 4 (8.3) | - |
| Montreal classification | ||
| Age at diagnosis (A1/A2/A3) | 8 (16.7)/36 (75)/4 (8.3) | - |
| Disease location (L1/L2/L3) | 17 (35.4)/7 (14.6)/24 (50) | - |
| Disease behavior (B1/B2/B3) | 21 (43.8)/18 (37.5) /15 (3.2) | - |
| Partial Mayo Score | ||
| 0 | - | 17 (50.0) |
| 1 | - | 0 (0) |
| 2 | - | 11 (32.4) |
| 3 | - | 6 (17.6) |
| Montreal classification | ||
| Disease location (E1/E2/E3) | - | 4 (11.8)/16 (47.0)/14 (41.2) |
| Severity of relapse (S0/S1/S2/S3) | - | 12 (35.3)/10 (29.4)/11 (32.4)/3 (8.8) |
| Analyzed Marker | Study Group | Statistical Parameters | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Q1–Q3 | Min.–Max. | |||
| Albumins (mg/mL) | CD | 26.42 | 25.25 | 15.75 | 7.90–46.95 | 0.44–109.50 | <0.0001 ** |
| UC | 38.96 | 34.07 | 32.70 | 12.90–53.40 | 0.80–163.30 | ||
| Control | 78.40 | 87.74 | 43.20 | 12.10–101.90 | 8.90–266.70 | ||
| Transferrin (mg/mL) | CD | 3.21 | 3.75 | 1.32 | 8.85–5.00 | 0.15–14.01 | =0.3548 |
| UC | 2.70 | 2.91 | 1.55 | 0.94–3.34 | 0.18–10.85 | ||
| Control | 4.22 | 3.57 | 1.90 | 1.60–8.34 | 0.46–0.85 | ||
| Transthyretin (ng/mL) | CD | 3.14 | 2.48 | 3.02 | 2.51–3.34 | 0.52–19.20 | =0.0001 ** |
| UC | 2.83 | 1.22 | 2.82 | 2.56–3.11 | 0.27–8.20 | ||
| Control | 1.32 | 0.56 | 1.40 | 0.94–1.66 | 0.37–2.24 | ||
| Albumins (mg/mL) Me [Q1–Q3] | Transferrin (mg/mL) Me [Q1–Q3] | Transthyretin (ng/mL) Me [Q1–Q3] | |
|---|---|---|---|
| Disease duration | |||
| <5 | 19.7 [13.4–42.0] | 1.3 [0.9–2.9] | 2.8 [2.3–3.1] |
| 5–10 | 12.6 [7.2–47.3] | 1.9 [1.0–5.2] | 3.0 [2.5–4.3] |
| >10 | 27.8 [12.8–51.5] | 1.3 [0.7–3.3] | 3.0 [2.6–3.3] |
| p-value | 0.4217 | 0.4213 | 0.2840 |
| Biology therapy | |||
| YES | 19.4 [10.3–46.7] | 1.3 [0.9–4.2] | 3.1 [2.6–3.3] |
| NO | 37.2 [13.8–63.9] | 1.5 [0.2–2.9] | 2.1 [1.9–3.1] |
| p-value | 0.0753 | 0.3086 | 0.01224 * |
| Surgery history | |||
| YES | 31.1 [12.1–47.2] | 2.1 [1.2–6.7] | 3.0 [2.5–3.3] |
| NO | 17.6 [9.6–47.6] | 1.3 [0.8–3.1] | 2.9 [2.4–3.2] |
| p-value | 0.5300 | 0.1164 | 0.6717 |
| Albumins (mg/mL) Me [Q1–Q3] | Transferrin (mg/mL) Me [Q1–Q3] | Transthyretin (ng/mL) Me [Q1–Q3] | |
|---|---|---|---|
| Partial Mayo Score | |||
| 0 | 42.5 [32.9–70.1] | 1.4 [1.0–3.3] | 3.1 [2.6–37.4] |
| 2 | 28.5 [12.8–47.2] | 2.0 [0.5–3.4] | 2.7 [2.1–2.9] |
| 3 | 17.6 [12.8–53.4] | 2.2 [0.9–3.6] | 2.7 [1.9–3.1] |
| p-value | 0.0151 * | 0.8247 | 0.2583 |
| Montreal Classification | |||
| E1 | 43.5 [26.3–67.6] | 2.7 [1.5–7.0] | 3.2 [2.9–5.7] |
| E2 | 23.9 [12.8–42.0] | 1.4 [0.2–3.4] | 2.8 [2.6–3.1] |
| E3 | 38.1 [16.8–70.1] | 1.5 [1.1–2.9] | 2.6 [1.9–3.1] |
| p-value | 0.5090 | 0.6870 | 0.1897 |
| S0 | 23.9 [7.8–47.2] | 1.4 [1.1–3.3] | 3.1 [2.0–3.2] |
| S1 | 39.4 [17.2–42.0] | 1.8 [1.0–2.9] | 2.8 [2.6–3.2] |
| S2 | 28.1 [12.8–73.1] | 2.3 [0.9–3.6] | 2.7 [2.1–2.9] |
| S3 | 58.5 [46.9–70.1] | 1.2 [0.9–1.5] | 2.4 [0.3–4.4] |
| p-value | 0.6145 | 0.8998 | 0.4560 |
| Albumins (mg/mL) Me [Q1–Q3] | Transferrin (mg/mL) Me [Q1–Q3] | Transthyretin (ng/mL) Me [Q1–Q3] | |
|---|---|---|---|
| Age at onset (years) | |||
| A1 <16 | 9.4 [4.0–36.2] | 1.3 [1.0–5.3] | 3.2 [2.0–3.5] |
| A2 17–40 | 17.2 [10.5–47.3] | 1.3 [0.8–5.2] | 3.0 [2.5–3.3] |
| A3 >40 | 23.6 [16.9–38.0] | 2.8 [1.4–5.1] | 2.5 [2.5–2.9] |
| p-value | 0.0488 * | 0.7563 | 0.4829 |
| Localization | |||
| L1 Ileum | 13.4 [7.2–25.8] | 1.2 [0.8–2.4] | 2.9 [2.5–3.3] |
| L2 Colon | 46.8 [11.1–52.9] | 3.2 [0.3–6.2] | 2.4 [2.3–3.3] |
| L3 Ileum + colon | 21.6 [8.6–47.2] | 1.7 [0.9–8.3] | 3.1 [2.6–3.4] |
| p-value | 0.2663 | 0.3985 | 0.4127 |
| Course of the disease | |||
| B1 No stenoses or fistulas | 19.1 [11.1–32.4] | 1.3 [0.8–6.2] | 2.8 [2.3–3.4] |
| B2 Stenoses | 15.3 [6.1–46.7] | 1.5 [1.2–6.4] | 3.1 [2.9–3.6] |
| B3 Fistulas | 46.7 [6.1–52.9] | 1.3 [0.9–5.2] | 2.9 [2.4–3.1] |
| Perianal lesions | 25.1 [1.3–50.6] | 0.5 [0.3–2.1] | 3.1 [2.7–3.3] |
| p-value | 0.7083 | 0.7711 | 0.7006 |
| CDAI | |||
| <150 | 47.2 [14.3–58.8] | 1.2 [0.5–1.9] | 3.2 [2.6–3.4] |
| 150–220 | 19.7 [11.1–46.7] | 2.0 [0.9–6.4] | 2.9 [2.5–3.3] |
| 221–450 | 17.2 [6.1–52.9] | 2.5 [1.3–5.2] | 2.8 [2.4–3.3] |
| >450 | 13.7 [2.2–27.8] | 0.9 [0.2–1.3] | 2.7 [2.1–3.1] |
| p-value | 0.0334 * | 0.1008 | 0.4962 |
| Albumins (mg/mL) Me [Q1–Q3] | Transferrin (mg/mL) Me [Q1–Q3] | Transthyretin (ng/mL) Me [Q1–Q3] | |
|---|---|---|---|
| BMI (kg/m2) | |||
| <18.5 | 28.1 [6.1–5.6] | 1.3 [0.9–2.0] | 2.8 [2.1–3.0] |
| 18.5–24.9 | 34.3 [12.3–51.5] | 1.8 [0.9–4.8] | 2.9 [2.5–3.2] |
| >25 | 18.4 [8.6–39.4] | 1.2 [0.8–3.6] | 3.1 [2.6–3.5] |
| >30 | 14.8 [9.6–29.1] | 2.4 [1.2–5.2] | 2.6 [1.8–3.1] |
| p-value | 0.0251 * | 0.6566 | 0.2153 |
| Waist circumference (cm) | |||
| Normal | 27.8 [10.5–51.5] | 1.3 [0.9–3.4] | 3.0 [2.5–3.3] |
| High | 16.8 [10.7–29.8] | 1.4 [0.9–4.2] | 2.7 [2.5–3.2] |
| p-value | 0.0213 * | 0.6057 | 0.2923 |
| Weight reduction in 6 months (%) | |||
| 5–10 | 27.1 [6.6–51.5] | 1.4 [0.8–3.7] | 2.8 [2.5–3.2] |
| >10 | 20.6 [12.2–43.6] | 1.5 [0.9–4.8] | 3.0 [2.6–3.3] |
| p-value | 0.0463 * | 0.4265 | 0.4540 |
| Adipose tissue (%) | |||
| Low | 22.6 [11.1–46.7] | 1.3 [0.2–2.4] | 2.6 [2.1–3.0] |
| Normal | 34.1 [6.1–58.8] | 1.4 [0.7–5.2] | 3.1 [2.6–3.3] |
| High | 13.7 [10.5–45.3] | 1.6 [1.0–4.2] | 2.9 [2.8–3.3] |
| p-value | 0.04866 * | 0.4542 | 0.1560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godala, M.; Gaszyńska, E.; Walczak, K.; Małecka-Wojciesko, E. Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers. Nutrients 2023, 15, 3479. https://doi.org/10.3390/nu15153479
Godala M, Gaszyńska E, Walczak K, Małecka-Wojciesko E. Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers. Nutrients. 2023; 15(15):3479. https://doi.org/10.3390/nu15153479
Chicago/Turabian StyleGodala, Małgorzata, Ewelina Gaszyńska, Konrad Walczak, and Ewa Małecka-Wojciesko. 2023. "Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers" Nutrients 15, no. 15: 3479. https://doi.org/10.3390/nu15153479
APA StyleGodala, M., Gaszyńska, E., Walczak, K., & Małecka-Wojciesko, E. (2023). Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers. Nutrients, 15(15), 3479. https://doi.org/10.3390/nu15153479








