The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly
Abstract
1. Introduction
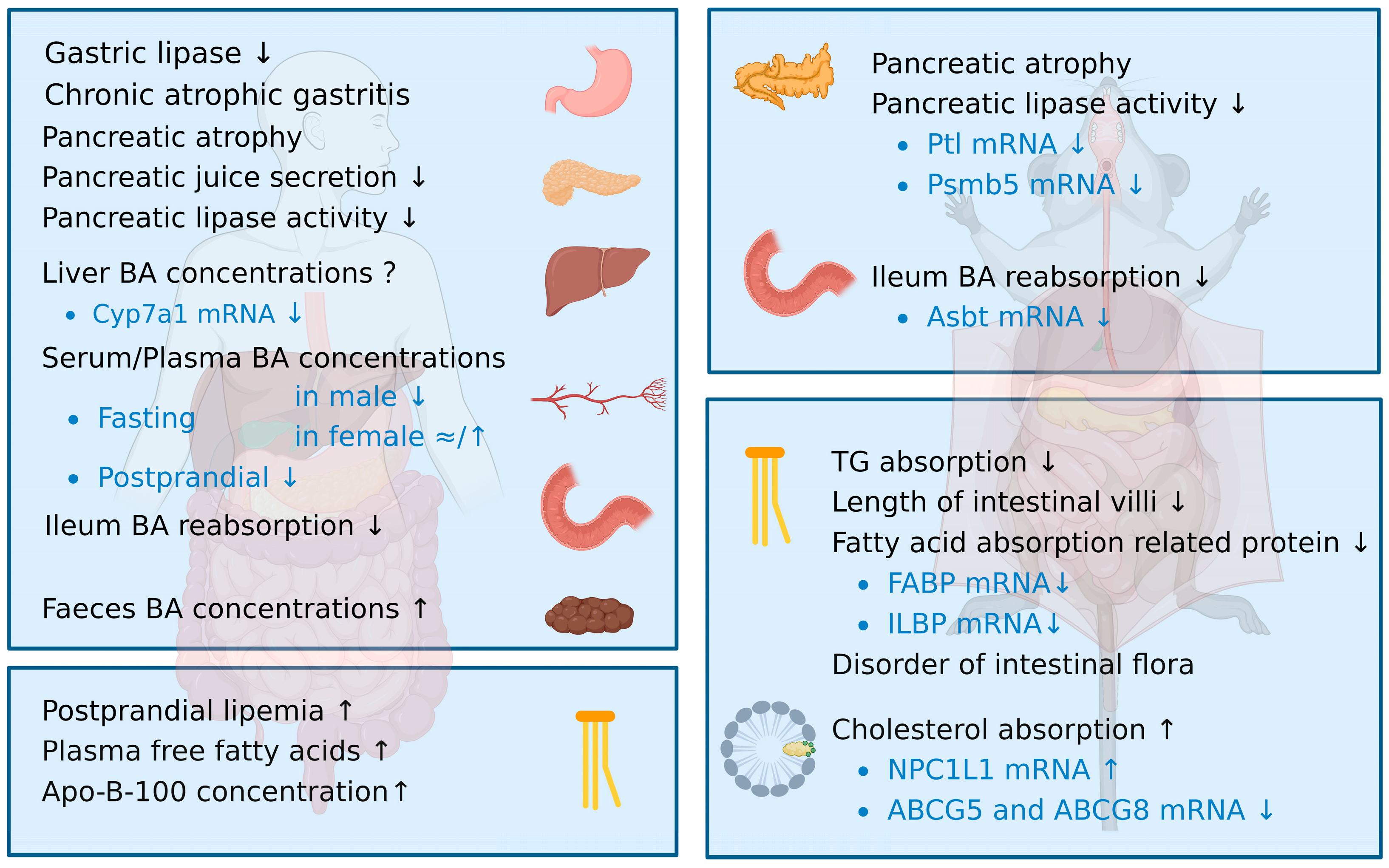
2. Disorder of Lipid Digestion and Absorption in the Elderly
2.1. Age-Related Changes in Lipid Digestion
2.1.1. Gastric Lipase Activity Decreases with Age
2.1.2. Pancreatic Function Declines with Age Due to Decreased Pancreatic Lipase Expression
2.1.3. Aging Is Mainly Associated with a Decline in Bile Acid Levels Due to the Lower Ileum Reabsorption
2.2. Age-Related Changes in Lipid Absorption
2.2.1. Triglyceride Absorption Decreases with Age
2.2.2. Cholesterol Absorption Increases with Age
3. Disorder of Lipid Anabolism and Catabolism in the Elderly
3.1. Disorder of Plasma Lipoprotein Metabolism during Aging
3.2. Age-Related Changes in Triglyceride Metabolism
3.2.1. Fatty Acid Uptake and Triglyceride Synthesis Increase in Liver with Age
3.2.2. Mitochondrial Dysfunction Leads to Impairment of the β-Oxidation
3.3. Age-Related Changes in Cholesterol Metabolism
3.3.1. Endogenous Cholesterol Synthesis Increases during Aging
3.3.2. Bile Acid Enterohepatic Circulation and Lipoprotein Dynamics Changes with Age
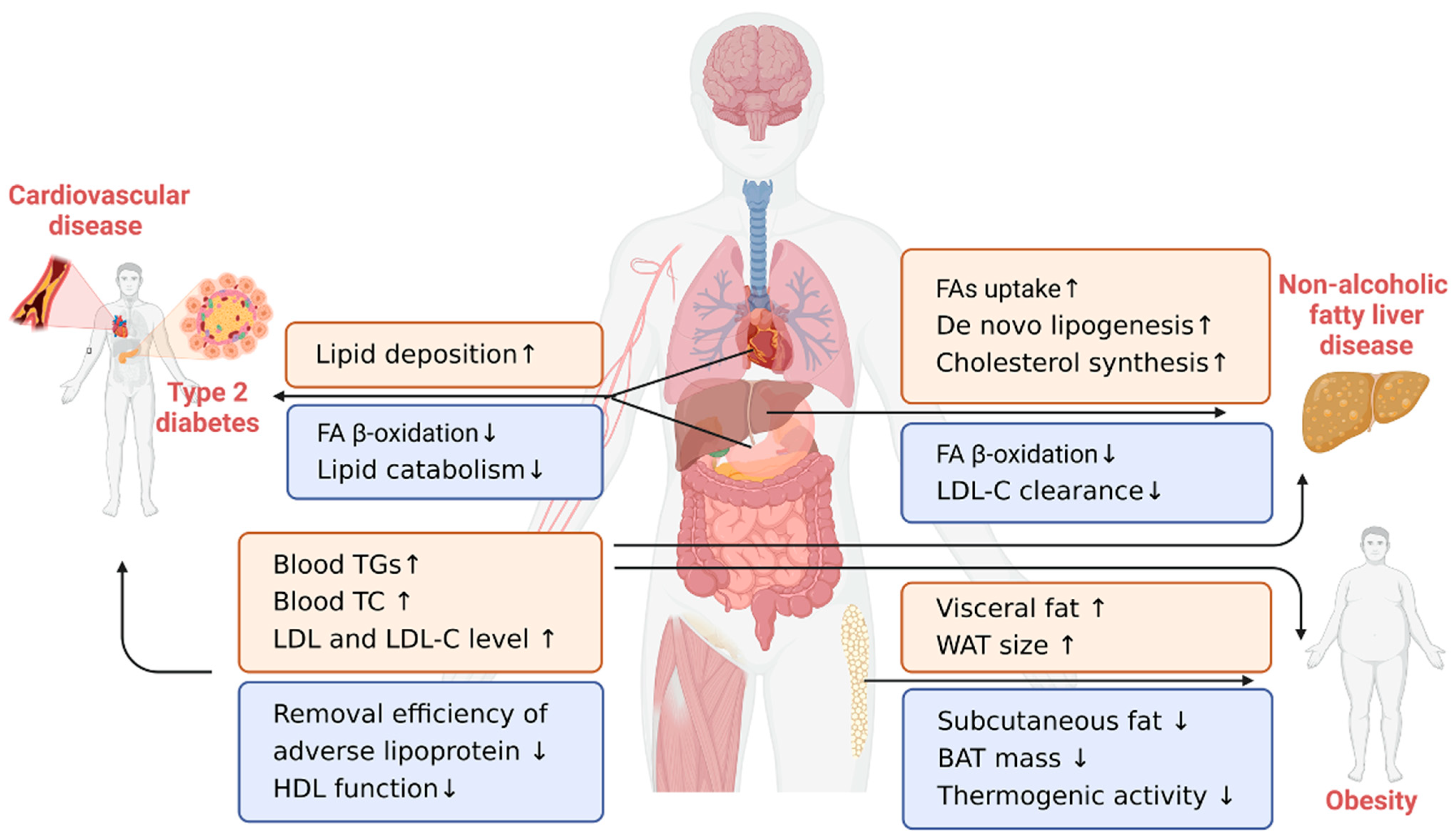
4. Lipid-Related Chronic Diseases in the Elderly
4.1. Cardiovascular Disease
4.2. Type 2 Diabetes
4.3. Obesity
4.4. Nonalcoholic Fatty Liver Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights. 2019. ST/ESA/SER.A/423. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 15 June 2022).
- Katsiki, N.; Kolovou, G.; Perez-Martinez, P.; Mikhailidis, D.P. Dyslipidaemia in the Elderly: To Treat or Not to Treat? Expert Rev. Clin. Pharmacol. 2018, 11, 259–278. [Google Scholar] [CrossRef]
- Zemedikun, D.T.; Gray, L.J.; Khunti, K.; Davies, M.J.; Dhalwani, N.N. Patterns of Multimorbidity in Middle-Aged and Older Adults: An Analysis of the UK Biobank Data. Mayo Clin. Proc. 2018, 93, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, T.; Zhang, M.; Li, C.; Liu, Q.; Li, F. Prevalence, Awareness and Control of Type 2 Diabetes Mellitus and Risk Factors in Chinese Elderly Population. BMC Public Health 2022, 22, 1382. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Prolonging Healthy Aging: Longevity Vitamins and Proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Bergman, P.; Brighenti, S. Targeted Nutrition in Chronic Disease. Nutrients 2020, 12, 1682. [Google Scholar] [CrossRef]
- Milan, A.M.; Cameron-Smith, D. Digestion and Postprandial Metabolism in the Elderly. Adv. Food Nutr. Res. 2015, 76, 79–124. [Google Scholar] [CrossRef]
- Van der Wulp, M.Y.M.; Verkade, H.J.; Groen, A.K. Regulation of Cholesterol Homeostasis. Mol. Cell Endocrinol. 2013, 368, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Fani, A.; Singh, H. Biophysical Insights into Modulating Lipid Digestion in Food Emulsions. Prog. Lipid Res. 2022, 85, 101129. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Moreau, H.; Laugier, R.; Gargouri, Y.; Ferrato, F.; Verger, R. Human Preduodenal Lipase Is Entirely of Gastric Fundic Origin. Gastroenterology 1988, 95, 1221–1226. [Google Scholar] [CrossRef]
- Sams, L.; Paume, J.; Giallo, J.; Carrière, F. Relevant PH and Lipase for in Vitro Models of Gastric Digestion. Food Funct. 2016, 7, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; McArthur, K.; Huet, B.; Lee, E. Effects of Aging and Gastritis on Gastric Acid and Pepsin Secretion in Humans: A Prospective Study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, H.; Lin, G.; Zheng, S. Magnetic Resonance Imaging Investigation of Age-Related Morphological Changes in the Pancreases of 226 Chinese. Aging Med. 2021, 4, 297–303. [Google Scholar] [CrossRef]
- Jiang, Z.-E.; Jiang, C.; Chen, B.; Koh, C.S.; Yong, J.-H.; Park, D.-H.; Won, M.-H.; Lee, Y.-L. Age-Associated Changes in Pancreatic Exocrine Secretion of the Isolated Perfused Rat Pancreas. Lab. Anim. Res. 2013, 29, 19–26. [Google Scholar] [CrossRef]
- Torigoe, T.; Ito, K.; Yamamoto, A.; Kanki, A.; Yasokawa, K.; Tamada, T.; Yoshida, K. Age-Related Change of the Secretory Flow of Pancreatic Juice in the Main Pancreatic Duct: Evaluation with Cine-Dynamic MRCP Using Spatially Selective Inversion Recovery Pulse. AJR Am. J. Roentgenol. 2014, 202, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Laugier, R.; Bernard, J.P.; Berthezene, P.; Dupuy, P. Changes in Pancreatic Exocrine Secretion with Age: Pancreatic Exocrine Secretion Does Decrease in the Elderly. Digestion 1991, 50, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Carriere, F.; Barrowman, J.A.; Verger, R.; Laugier, R. Secretion and Contribution to Lipolysis of Gastric and Pancreatic Lipases during a Test Meal in Humans. Gastroenterology 1993, 105, 876–888. [Google Scholar] [CrossRef]
- Vellas, B.; Balas, D.; Moreau, J.; Bouisson, M.; Senegas-Balas, F.; Guidet, M.; Ribet, A. Exocrine Pancreatic Secretion in the Elderly. Int. J. Pancreatol. 1988, 3, 497–502. [Google Scholar] [CrossRef]
- Yamamoto, K.; E, S.; Hatakeyama, Y.; Sakamoto, Y.; Tsuduki, T. High-Fat Diet Intake from Senescence Inhibits the Attenuation of Cell Functions and the Degeneration of Villi with Aging in the Small Intestine, and Inhibits the Attenuation of Lipid Absorption Ability in SAMP8 Mice. J. Clin. Biochem. Nutr. 2015, 57, 204–211. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kitano, Y.; Shuang, E.; Hatakeyama, Y.; Sakamoto, Y.; Honma, T.; Tsuduki, T. Decreased Lipid Absorption Due to Reduced Pancreatic Lipase Activity in Aging Male Mice. Biogerontology 2014, 15, 463–473. [Google Scholar] [CrossRef]
- Kovacs, D.; Szabo, B.; Pancsa, R.; Tompa, P. Intrinsically Disordered Proteins Undergo and Assist Folding Transitions in the Proteome. Arch. Biochem. Biophys. 2013, 531, 80–89. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Rainteau, D.; Tuvignon, N.; Wolf, C.; Seksik, P.; Laugier, R.; Carrière, F. Postprandial Bile Acid Levels in Intestine and Plasma Reveal Altered Biliary Circulation in Chronic Pancreatitis Patients. J. Lipid Res. 2018, 59, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, F.M.; van der Werf, S.D.; Lamers, H.L.; Hectors, M.P.; Buys, W.C.; van Tongeren, J.M. Influence of Age, Intestinal Transit Time, and Dietary Composition on Fecal Bile Acid Profiles in Healthy Subjects. Dig. Dis. Sci. 1988, 33, 673–678. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Paumgartner, G.; Berr, F. Long-Term Effects of Cholecystectomy on Bile Acid Metabolism. Hepatology 1995, 21, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Feridooni, H.A.; Howlett, S.E.; Pelis, R.M. Influence of Age on Intestinal Bile Acid Transport in C57BL/6 Mice. Pharmacol. Res. Perspect. 2017, 5, e00287. [Google Scholar] [CrossRef]
- Vergès, B. Intestinal Lipid Absorption and Transport in Type 2 Diabetes. Diabetologia 2022, 65, 1587–1600. [Google Scholar] [CrossRef]
- Hussain, M.M. Intestinal Lipid Absorption and Lipoprotein Formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef]
- Cohn, J.S.; McNamara, J.R.; Cohn, S.D.; Ordovas, J.M.; Schaefer, E.J. Postprandial Plasma Lipoprotein Changes in Human Subjects of Different Ages. J. Lipid Res. 1988, 29, 469–479. [Google Scholar] [CrossRef]
- Issa, J.S.; Diament, J.; Forti, N. Postprandial Lipemia: Influence of Aging. Arq. Bras. Cardiol. 2005, 85, 15–19. [Google Scholar] [CrossRef][Green Version]
- Puga, G.M.; Meyer, C.; Everman, S.; Mandarino, L.J.; Katsanos, C.S. Postprandial Lipemia in the Elderly Involves Increased Incorporation of Ingested Fat in Plasma Free Fatty Acids and Small (Sf 20-400) Triglyceride-Rich Lipoproteins. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E356–E361. [Google Scholar] [CrossRef]
- Puga, G.M.; Meyer, C.; Mandarino, L.J.; Katsanos, C.S. Postprandial Spillover of Dietary Lipid into Plasma Is Increased with Moderate Amounts of Ingested Fat and Is Inversely Related to Adiposity in Healthy Older Men. J. Nutr. 2012, 142, 1806–1811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gebert, N.; Cheng, C.-W.; Kirkpatrick, J.M.; Di Fraia, D.; Yun, J.; Schädel, P.; Pace, S.; Garside, G.B.; Werz, O.; Rudolph, K.L.; et al. Region-Specific Proteome Changes of the Intestinal Epithelium during Aging and Dietary Restriction. Cell Rep. 2020, 31, 107565. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, T.D.; Drozdowski, L.A.; Wild, G.E.; Clandinin, M.T.; Agellon, L.B.; Thomson, A.B.R. The Age-Related Decline in Intestinal Lipid Uptake Is Associated with a Reduced Abundance of Fatty Acid-Binding Protein. Lipids 2004, 39, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.-H. Aging Per Se Is an Independent Risk Factor for Cholesterol Gallstone Formation in Gallstone Susceptible Mice. J. Lipid Res. 2002, 43, 1950–1959. [Google Scholar] [CrossRef]
- Duan, L.-P.; Wang, H.H.; Ohashi, A.; Wang, D.Q.-H. Role of Intestinal Sterol Transporters Abcg5, Abcg8, and Npc1l1 in Cholesterol Absorption in Mice: Gender and Age Effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G269–G276. [Google Scholar] [CrossRef]
- Wang, D.Q.-H. Regulation of Intestinal Cholesterol Absorption. Annu. Rev. Physiol. 2007, 69, 221–248. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Chung, K.W. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Jalbert, S.M.; Lamon-Fava, S.; Dolnikowski, G.G.; Welty, F.K.; Barrett, H.R.; Schaefer, E.J.; Lichtenstein, A.H. TRL, IDL, and LDL Apolipoprotein B-100 and HDL Apolipoprotein A-I Kinetics as a Function of Age and Menopausal Status. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1691–1696. [Google Scholar] [CrossRef]
- Feng, L.; Nian, S.; Tong, Z.; Zhu, Y.; Li, Y.; Zhang, C.; Bai, X.; Luo, X.; Wu, M.; Yan, Z. Age-Related Trends in Lipid Levels: A Large-Scale Cross-Sectional Study of the General Chinese Population. BMJ Open 2020, 10, e034226. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chen, W.; McDermott, J.; Han, J.-D.J. Molecular and Phenotypic Biomarkers of Aging. F1000Res 2017, 6, 860. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, X.; Lu, Y.; Wang, E.; Zhang, Z.; Yang, J.; Zhang, H.; Li, X. Hepatic Steatosis Exacerbated by Endoplasmic Reticulum Stress-Mediated Downregulation of FXR in Aging Mice. J. Hepatol. 2014, 60, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Papsdorf, K.; Brunet, A. Linking Lipid Metabolism to Chromatin Regulation in Aging. Trends Cell Biol. 2019, 29, 97–116. [Google Scholar] [CrossRef]
- Greenfield, M.S.; Kraemer, F.; Tobey, T.; Reaven, G. Effect of Age on Plasma Triglyceride Concentrations in Man. Metabolism 1980, 29, 1095–1099. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The Metabolic Footprint of Aging in Mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef]
- Parini, P.; Angelin, B.; Rudling, M. Cholesterol and Lipoprotein Metabolism in Aging: Reversal of Hypercholesterolemia by Growth Hormone Treatment in Old Rats. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 832–839. [Google Scholar] [CrossRef]
- Ericsson, S.; Eriksson, M.; Vitols, S.; Einarsson, K.; Berglund, L.; Angelin, B. Influence of Age on the Metabolism of Plasma Low Density Lipoproteins in Healthy Males. J. Clin. Investig. 1991, 87, 591–596. [Google Scholar] [CrossRef]
- Maranhão, R.C.; Pala, D.; Freitas, F.R. Lipoprotein Removal Mechanisms and Aging: Implications for the Cardiovascular Health of the Elderly. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 104–109. [Google Scholar] [CrossRef]
- Mutharasan, R.K.; Thaxton, C.S.; Berry, J.; Daviglus, M.L.; Yuan, C.; Sun, J.; Ayers, C.; Lloyd-Jones, D.M.; Wilkins, J.T. HDL Efflux Capacity, HDL Particle Size, and High-Risk Carotid Atherosclerosis in a Cohort of Asymptomatic Older Adults: The Chicago Healthy Aging Study. J. Lipid Res. 2017, 58, 600–606. [Google Scholar] [CrossRef]
- Berrougui, H.; Khalil, A. Age-Associated Decrease of High-Density Lipoprotein-Mediated Reverse Cholesterol Transport Activity. Rejuvenation Res. 2009, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.K.; Oredipe, O.A.; Stiles, M.R.; Shipp, J.C. Increased Fatty Acid Uptake, a Factor in Increased Hepatic Triacylglycerol Synthesis in Aging Rats. Mech. Ageing Dev. 1986, 37, 49–54. [Google Scholar] [CrossRef]
- Sheedfar, F.; Sung, M.M.; Aparicio-Vergara, M.; Kloosterhuis, N.J.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Febbraio, M.; Jacobs, R.L.; de Bruin, A.; Vinciguerra, M.; et al. Increased Hepatic CD36 Expression with Age Is Associated with Enhanced Susceptibility to Nonalcoholic Fatty Liver Disease. Aging 2014, 6, 281–295. [Google Scholar] [CrossRef]
- Xu, S.-F.; Hu, A.-L.; Xie, L.; Liu, J.-J.; Wu, Q.; Liu, J. Age-Associated Changes of Cytochrome P450 and Related Phase-2 Gene/Proteins in Livers of Rats. PeerJ 2019, 7, e7429. [Google Scholar] [CrossRef]
- Zhou, J.; Febbraio, M.; Wada, T.; Zhai, Y.; Kuruba, R.; He, J.; Lee, J.H.; Khadem, S.; Ren, S.; Li, S.; et al. Hepatic Fatty Acid Transporter Cd36 Is a Common Target of LXR, PXR, and PPARgamma in Promoting Steatosis. Gastroenterology 2008, 134, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile Acids Lower Triglyceride Levels via a Pathway Involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef]
- Ishizuka, K.; Kon, K.; Lee-Okada, H.-C.; Arai, K.; Uchiyama, A.; Yamashina, S.; Yokomizo, T.; Ikejima, K. Aging Exacerbates High-Fat Diet-Induced Steatohepatitis through Alteration in Hepatic Lipid Metabolism in Mice. J. Gastroenterol. Hepatol. 2020, 35, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Teofilović, A.; Vratarić, M.; Veličković, N.; Vojnović Milutinović, D.; Mladenovic, A.; Prvulovic, M.; Djordjevic, A. Late-Onset Calorie Restriction Improves Lipid Metabolism and Aggravates Inflammation in the Liver of Old Wistar Rats. Front. Nutr. 2022, 9, 899255. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J.A. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, Z.R.; Smith, M.R.; Hu, X.; Orr, M.; Liu, K.H.; Quyyumi, A.A.; Jones, D.P.; Go, Y.-M. Plasma Acylcarnitine Levels Increase with Healthy Aging. Aging 2020, 12, 13555–13570. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wu, X.; Chen, H.; Xia, X.; Song, X.; Chen, S.; Lu, X.; Jin, J.; Su, Q.; Cai, D.; et al. Aging-Induced Aberrant RAGE/PPARα Axis Promotes Hepatic Steatosis via Dysfunctional Mitochondrial β Oxidation. Aging Cell 2020, 19, e13238. [Google Scholar] [CrossRef]
- Chung, K.W.; Lee, E.K.; Lee, M.K.; Oh, G.T.; Yu, B.P.; Chung, H.Y. Impairment of PPARα and the Fatty Acid Oxidation Pathway Aggravates Renal Fibrosis during Aging. J. Am. Soc. Nephrol. 2018, 29, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Petrashen, A.P.; Sanders, J.A.; Peterson, A.L.; Sedivy, J.M. SLC1A5 Glutamine Transporter Is a Target of MYC and Mediates Reduced MTORC1 Signaling and Increased Fatty Acid Oxidation in Long-Lived Myc Hypomorphic Mice. Aging Cell 2019, 18, e12947. [Google Scholar] [CrossRef]
- Gimeno-Mallench, L.; Mas-Bargues, C.; Inglés, M.; Olaso, G.; Borras, C.; Gambini, J.; Vina, J. Resveratrol Shifts Energy Metabolism to Increase Lipid Oxidation in Healthy Old Mice. Biomed. Pharmacother. 2019, 118, 109130. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xue, M.; Xia, F.; Zhu, L.; Li, Y.; Jia, D.; Chen, S.; Xu, G.; Lei, Y. Astragaloside IV Ameliorates Fat Metabolism in the Liver of Ageing Mice through Targeting Mitochondrial Activity. J. Cell Mol. Med. 2021, 25, 8863–8876. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol Metabolism: A Review of How Ageing Disrupts the Biological Mechanisms Responsible for Its Regulation. Ageing Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Kuhla, A.; Blei, T.; Jaster, R.; Vollmar, B. Aging Is Associated with a Shift of Fatty Metabolism toward Lipogenesis. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H.-S. Reactive Oxygen Species-Induced Changes in Glucose and Lipid Metabolism Contribute to the Accumulation of Cholesterol in the Liver during Aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [PubMed]
- Mc Auley, M.T.; Mooney, K.M. LDL-C Levels in Older People: Cholesterol Homeostasis and the Free Radical Theory of Ageing Converge. Med. Hypotheses 2017, 104, 15–19. [Google Scholar] [CrossRef]
- Pallottini, V.; Martini, C.; Cavallini, G.; Bergamini, E.; Mustard, K.J.; Hardie, D.G.; Trentalance, A. Age-Related HMG-CoA Reductase Deregulation Depends on ROS-Induced P38 Activation. Mech. Ageing Dev. 2007, 128, 688–695. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. History of Discovery: The LDL Receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.S.; Lichtenstein, A.H.; Cuchel, M.; Dolnikowski, G.G.; Hachey, D.L.; Cohn, J.S.; Schaefer, E.J. Impact of Age on the Metabolism of VLDL, IDL, and LDL Apolipoprotein B-100 in Men. J. Lipid Res. 1995, 36, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.; Bhuvaneswaran, C.; Udupa, K.B. Age-Related Alteration in Hepatic Acyl-CoA: Cholesterol Acyltransferase and Its Relation to LDL Receptor and MAPK. Mech. Ageing Dev. 2005, 126, 740–751. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Pan, T.; Dong, L.; Ding, L.; Wang, Z.; Song, R.; Wang, X.; Wang, N.; Zhang, Y.; et al. Kanglexin, a New Anthraquinone Compound, Attenuates Lipid Accumulation by Activating the AMPK/SREBP-2/PCSK9/LDLR Signalling Pathway. Biomed. Pharmacother. 2021, 133, 110802. [Google Scholar] [CrossRef]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Molecular Biology of PCSK9: Its Role in LDL Metabolism. Trends Biochem. Sci. 2007, 32, 71–77. [Google Scholar] [CrossRef]
- Chae, H.-S.; You, B.H.; Kim, D.-Y.; Lee, H.; Ko, H.W.; Ko, H.-J.; Choi, Y.H.; Choi, S.S.; Chin, Y.-W. Sauchinone Controls Hepatic Cholesterol Homeostasis by the Negative Regulation of PCSK9 Transcriptional Network. Sci. Rep. 2018, 8, 6737. [Google Scholar] [CrossRef]
- Dubuc, G.; Tremblay, M.; Paré, G.; Jacques, H.; Hamelin, J.; Benjannet, S.; Boulet, L.; Genest, J.; Bernier, L.; Seidah, N.G.; et al. A New Method for Measurement of Total Plasma PCSK9: Clinical Applications. J. Lipid Res. 2010, 51, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Ju, X.; Yang, T.; Zhang, M.; Tang, W.; Chen, Q.; Hu, Y.; Haas, J.V.; Troutt, J.S.; Pickard, R.T.; et al. Serum PCSK9 Is Associated with Multiple Metabolic Factors in a Large Han Chinese Population. Atherosclerosis 2010, 213, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wong, C.; Song, Y.; Shen, H.; Mori, D.; Rotllan, N.; Price, N.; Dobrian, A.D.; Meng, H.; Kleinstein, S.H.; et al. Age-Associated Vascular Inflammation Promotes Monocytosis during Atherogenesis. Aging Cell 2016, 15, 766–777. [Google Scholar] [CrossRef]
- Einarsson, K.; Nilsell, K.; Leijd, B.; Angelin, B. Influence of Age on Secretion of Cholesterol and Synthesis of Bile Acids by the Liver. N. Engl. J. Med. 1985, 313, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Abate, N.; Bertolotti, S.; Loria, P.; Concari, M.; Messora, R.; Carubbi, F.; Pinetti, A.; Carulli, N. Effect of Aging on Cholesterol 7 Alpha-Hydroxylation in Humans. J. Lipid Res. 1993, 34, 1001–1007. [Google Scholar] [CrossRef]
- Bertolotti, M.; Gabbi, C.; Anzivino, C.; Crestani, M.; Mitro, N.; Del Puppo, M.; Godio, C.; De Fabiani, E.; Macchioni, D.; Carulli, L.; et al. Age-Related Changes in Bile Acid Synthesis and Hepatic Nuclear Receptor Expression. Eur. J. Clin. Investig. 2007, 37, 501–508. [Google Scholar] [CrossRef]
- Uchida, K.; Satoh, T.; Chikai, T.; Takase, H.; Nomura, Y.; Nakao, H.; Takeuchi, N. Influence of Cholesterol Feeding on Bile Acid Metabolism in Young and Aged Germ-Free Rats. Jpn. J. Pharmacol. 1996, 71, 113–118. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.-K.J.; Saupe, K.W.; Klaassen, C.D. Energy Restriction Does Not Compensate for the Reduced Expression of Hepatic Drug-Processing Genes in Mice with Aging. Drug Metab. Dispos. 2010, 38, 1122–1131. [Google Scholar] [CrossRef]
- Fu, Z.D.; Csanaky, I.L.; Klaassen, C.D. Gender-Divergent Profile of Bile Acid Homeostasis during Aging of Mice. PLoS ONE 2012, 7, e32551. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Jiang, S.; Hu, W.; Li, T.; Di, S.; Wang, D.; Yang, Y. A Global Perspective on FOXO1 in Lipid Metabolism and Lipid-Related Diseases. Prog. Lipid Res. 2017, 66, 42–49. [Google Scholar] [CrossRef]
- Castro, A.; Signini, É.F.; De Oliveira, J.M.; Di Medeiros Leal, M.C.B.; Rehder-Santos, P.; Millan-Mattos, J.C.; Minatel, V.; Pantoni, C.B.F.; Oliveira, R.V.; Catai, A.M.; et al. The Aging Process: A Metabolomics Perspective. Molecules 2022, 27, 8656. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Eum, J.Y.; Lee, J.C.; Yi, S.S.; Kim, I.Y.; Seong, J.K.; Moon, M.H. Aging-Related Lipidomic Changes in Mouse Serum, Kidney, and Heart by Nanoflow Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1618, 460849. [Google Scholar] [CrossRef]
- Pereira, T.M.; Nogueira, B.V.; Lima, L.C.; Porto, M.L.; Arruda, J.A.; Vasquez, E.C.; Meyrelles, S.S. Cardiac and Vascular Changes in Elderly Atherosclerotic Mice: The Influence of Gender. Lipids Health Dis. 2010, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.R.; Bouïs, D.; Mann, K.M.; Rashid, I.M.; McCubbrey, A.L.; Hyman, M.C.; Goldstein, D.R.; Mei, A.; Pinsky, D.J. CD73 Promotes Age-Dependent Accretion of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wilkins, J.T.; Zheng, Y.; Joyce, B.T.; Jacobs, D.R.; Schreiner, P.J.; Horvath, S.; Greenland, P.; Lloyd-Jones, D.; Hou, L. Plasma Lipid Profiles in Early Adulthood Are Associated with Epigenetic Aging in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin. Epigenet. 2022, 14, 16. [Google Scholar] [CrossRef]
- Cao, X.; Tang, Z.; Zhang, J.; Li, H.; Singh, M.; Sun, F.; Li, X.; Li, C.; Wang, Y.; Guo, X.; et al. Association between High-Density Lipoprotein Cholesterol and Type 2 Diabetes Mellitus among Chinese: The Beijing Longitudinal Study of Aging. Lipids Health Dis. 2021, 20, 71. [Google Scholar] [CrossRef]
- Zheng, D.; Li, H.; Ai, F.; Sun, F.; Singh, M.; Cao, X.; Jiang, J.; He, Y.; Tang, Z.; Guo, X. Association between the Triglyceride to High-Density Lipoprotein Cholesterol Ratio and the Risk of Type 2 Diabetes Mellitus among Chinese Elderly: The Beijing Longitudinal Study of Aging. BMJ Open Diabetes Res. Care 2020, 8, e000811. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Pérez-Sousa, M.Á.; González-Ruíz, K.; Cano-Gutierrez, C.A.; Schmidt-RioValle, J.; Correa-Rodríguez, M.; Izquierdo, M.; Romero-García, J.A.; Campos-Rodríguez, A.Y.; Triana-Reina, H.R.; et al. Obesity- and Lipid-Related Parameters in the Identification of Older Adults with a High Risk of Prediabetes According to the American Diabetes Association: An Analysis of the 2015 Health, Well-Being, and Aging Study. Nutrients 2019, 11, 2654. [Google Scholar] [CrossRef]
- Lambert, J.E.; Parks, E.J. Postprandial Metabolism of Meal Triglyceride in Humans. Biochim. Biophys. Acta 2012, 1821, 721–726. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, L.; Yang, J.-K.; Wang, B.; Wu, K.K.L.; Hallenborg, P.; Xu, A.; Cheng, K.K.Y. The Dysfunctional MDM2-P53 Axis in Adipocytes Contributes to Aging-Related Metabolic Complications by Induction of Lipodystrophy. Diabetes 2018, 67, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, E.; Johansen, R.F.; Jensen, M.D.; Nielsen, S. Postprandial VLDL-TG Metabolism in Type 2 Diabetes. Metabolism 2017, 75, 25–35. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and Ageing: Two Sides of the Same Coin. Obes. Rev. 2020, 21, e12991. [Google Scholar] [CrossRef]
- Da Silva Alexandre, T.; Aubertin-Leheudre, M.; Carvalho, L.P.; de Oliveira Máximo, R.; Corona, L.P.; de Brito, T.R.P.; Nunes, D.P.; Santos, J.L.F.; de Oliveira Duarte, Y.A.; Lebrão, M.L. Dynapenic Obesity as an Associated Factor to Lipid and Glucose Metabolism Disorders and Metabolic Syndrome in Older Adults—Findings from SABE Study. Clin. Nutr. 2018, 37, 1360–1366. [Google Scholar] [CrossRef]
- Arner, P.; Bernard, S.; Appelsved, L.; Fu, K.-Y.; Andersson, D.P.; Salehpour, M.; Thorell, A.; Rydén, M.; Spalding, K.L. Adipose Lipid Turnover and Long-Term Changes in Body Weight. Nat. Med. 2019, 25, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Guillermier, C.; Fazeli, P.K.; Kim, S.; Lun, M.; Zuflacht, J.P.; Milian, J.; Lee, H.; Francois-Saint-Cyr, H.; Horreard, F.; Larson, D.; et al. Imaging Mass Spectrometry Demonstrates Age-Related Decline in Human Adipose Plasticity. JCI Insight 2017, 2, e90349. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White Adipose Tissue Dysfunction in Obesity and Aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef]
- Bonzón-Kulichenko, E.; Moltó, E.; Pintado, C.; Fernández, A.; Arribas, C.; Schwudke, D.; Gallardo, N.; Shevchenko, A.; Andrés, A. Changes in Visceral Adipose Tissue Plasma Membrane Lipid Composition in Old Rats Are Associated With Adipocyte Hypertrophy With Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1139–1146. [Google Scholar] [CrossRef]
- Feng, J.; Xu, H.; Pan, F.; Hu, J.; Wu, Y.; Lin, N.; Zhang, X.; Ji, C.; Hu, Y.; Zhong, H.; et al. An Integrated Analysis of MRNA and LncRNA Expression Profiles Indicates Their Potential Contribution to Brown Fat Dysfunction with Aging. Front. Endocrinol. 2020, 11, 46. [Google Scholar] [CrossRef]
- Silva, K.R.; Baptista, L.S. Adipose-Derived Stromal/Stem Cells from Different Adipose Depots in Obesity Development. World J. Stem Cells 2019, 11, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi-Hotta, M.; Hasegawa, S.; Igarashi, T.; Yamada, T.; Takahashi, M.; Numata, S.; Kobayashi, T.; Iwata, Y.; Arima, M.; Yamamoto, N.; et al. Enhancement of Individual Differences in Proliferation and Differentiation Potentials of Aged Human Adipose-Derived Stem Cells. Regen. Ther. 2017, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, S.; Zhu, Y.; Pu, S.; Zhou, Z. Antiobesity Effects of Adipose-Derived Stromal/Stem Cells in a Naturally Aged Mouse Model. Obesity 2021, 29, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M.; Ahima, R.S. Pathophysiology of Lipid Droplet Proteins in Liver Diseases. Exp. Cell Res. 2016, 340, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-P.; Lai, M.; Lin, W.-Y.; Huang, K.-C.; Yang, K.-C. Metabolic Profiles and Fibrosis of Nonalcoholic Fatty Liver Disease in the Elderly: A Community-Based Study. J. Gastroenterol. Hepatol. 2020, 35, 1636–1643. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular Senescence Drives Age-Dependent Hepatic Steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, Z.; Huang, C.; Lin, C.; Tsai, T. Cisd2 Slows down Liver Aging and Attenuates Age-related Metabolic Dysfunction in Male Mice. Aging Cell 2021, 20, e13523. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, B.; Yang, G.; Hu, W.; Zhang, L.; Liu, R.; Li, M.; Wang, K.; Gu, H.F.; Guan, Y.; et al. JAZF1 Ameliorates Age and Diet-Associated Hepatic Steatosis through SREBP-1c -Dependent Mechanism. Cell Death Dis. 2018, 9, 859. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Hu, M.; Qin, X.; Qiu, L.; Wang, P.; Zhang, X.; Liu, R.; Wang, X. The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly. Nutrients 2023, 15, 3433. https://doi.org/10.3390/nu15153433
Song R, Hu M, Qin X, Qiu L, Wang P, Zhang X, Liu R, Wang X. The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly. Nutrients. 2023; 15(15):3433. https://doi.org/10.3390/nu15153433
Chicago/Turabian StyleSong, Rui, Mengxiao Hu, Xiyu Qin, Lili Qiu, Pengjie Wang, Xiaoxu Zhang, Rong Liu, and Xiaoyu Wang. 2023. "The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly" Nutrients 15, no. 15: 3433. https://doi.org/10.3390/nu15153433
APA StyleSong, R., Hu, M., Qin, X., Qiu, L., Wang, P., Zhang, X., Liu, R., & Wang, X. (2023). The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly. Nutrients, 15(15), 3433. https://doi.org/10.3390/nu15153433






