Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children
Abstract
1. Introduction
1.1. COVID-19 and Vitamin D
1.2. Pneumonia and Vitamin D
1.3. Bronchiolitis and Vitamin D
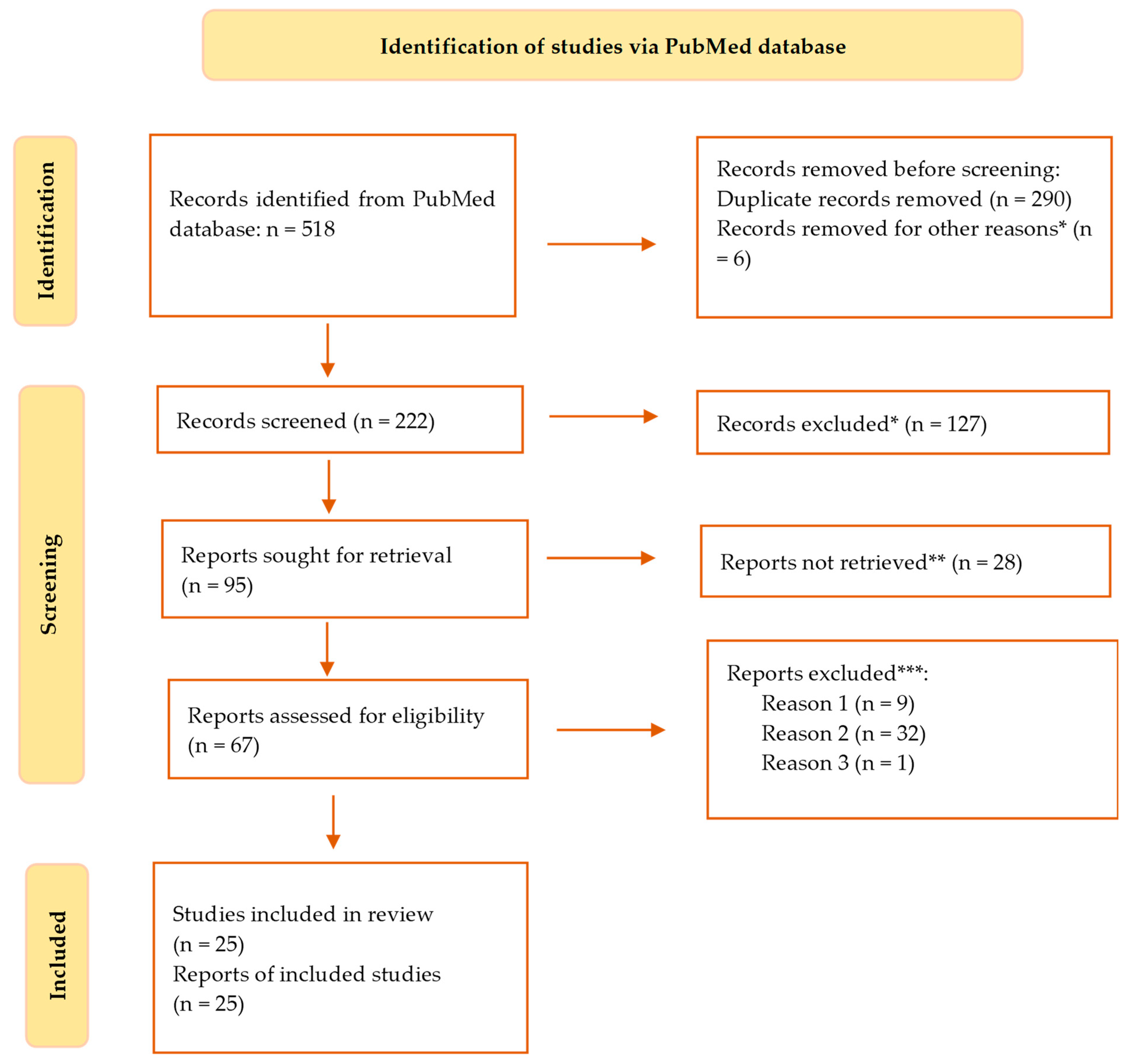
2. Materials and Methods
- Search results for “respiratory tract infections and vitamin D”—217 results;
- Search results for “vitamin D pneumonia”—134 results;
- Search results for ”vitamin D and COVID-19”—104 results;
- Search results for ”vitamin D and SARS-CoV-2”—63 results.
3. Results
Statistics of the Main Findings
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Indicator Metadata Registry Details. Children Aged <5 Years with Acute Respiratory Infection (ARI) Symptoms Taken to Facility (%). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3147 (accessed on 27 June 2023).
- Institutul National de Sanatate Publica. 2021. Available online: https://insp.gov.ro/centrul-national-de-evaluare-si-promovare-a-starii-de-sanatate-cnepss/starea-de-sanatate/rapoarte-si-studii-despre-starea-de-sanatate/sanatatea-copiilor/ (accessed on 18 June 2023).
- Simoes, E.A.F.; Cherian, T.; Chow, J.; Shahid-Salles, S.A.; Laxminarayan, R.; John, T.J. Acute Respiratory Infections in Children. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA; Oxford University Press: New York, NY, USA, 2006; Chapter 25; Available online: https://www.ncbi.nlm.nih.gov/books/NBK11786/ (accessed on 27 June 2023).
- Kesson, A.M. Respiratory virus infections. Paediatr. Respir. Rev. 2007, 8, 240–248. [Google Scholar] [CrossRef]
- Thomas, M.; Bomar, P.A. Upper Respiratory Tract Infection; Updated 27 June 2022; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK532961/ (accessed on 27 June 2023).
- Pappas, D.E.; Hendley, J.O. The Common Cold. Princ. Pract. Pediatr. Infect. Dis. 2008, 203–206, 199–202.e1. [Google Scholar] [CrossRef]
- Pneumonia in Children Statistics—UNICEF DATA. Available online: https://data.unicef.org/topic/child-health/pneumonia/ (accessed on 27 June 2023).
- Bleakley, A.S.; Licciardi, P.V.; Binks, M.J. Vitamin D Modulation of the Innate Immune Response to Paediatric Respiratory Pathogens Associated with Acute Lower Respiratory Infections. Nutrients 2021, 13, 276. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Wong, C.K.; Ho, C.Y.; Ko, F.W.S.; Chan, C.H.S.; Ho, A.S.S.; Hui, D.S.C.; Lam, C.W.K. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin. Exp. Immunol. 2001, 125, 177–183. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Mohan, M.; Cherlan, J.J.; Sharma, A. Exploring links between vitamin D deficiency and COVID-19. PloS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 27 June 2023).
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Vitamin D and COVID-19: Where are we now? Postgrad. Med. 2023, 135, 195–207. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Chen, C.-S.; Chan, Y.-J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Rice, M.; Zhang, H.; Sha, D.; Li, M.; Su, Y.; Yang, C. The Impact of Policy Measures on Human Mobility, COVID-19 Cases, and Mortality in the US: A Spatiotemporal Perspective. Int. J. Environ. Res. Public Health 2021, 18, 996. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022, 13, 836738. [Google Scholar] [CrossRef]
- Mocanu, A.; Lazureanu, V.E.; Marinescu, A.R.; Cut, T.G.; Laza, R.; Rusu, L.C.; Marza, A.M.; Nelson-Twakor, A.; Negrean, R.A.; Popescu, I.M.; et al. A Retrospective Assessment of Laboratory Findings and Cytokine Markers in Severe SARS-CoV-2 Infection among Patients of Roma Population. J. Clin. Med. 2022, 11, 6777. [Google Scholar] [CrossRef]
- Mihai, C.M.; Chisnoiu, T.; Balasa, A.L.; Frecus, C.E.; Mihai, L.; Pantazi, A.C.; Stuparu, A.Z.; Axelerad, A. Clinical Characteristics and Laboratory Findings in Children with Multisystem Inflammatory Syndrome (MIS-C)—A Retrospective Study of a Tertiary Care Center from Constanta, Romania. Healthcare 2023, 11, 544. [Google Scholar] [CrossRef]
- Khashim Alswailmi, F.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular Mechanisms of Vitamin D-Mediated Immunomodulation. Galen Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef]
- Tintut, Y.; Demer, L.L. Potential impact of the steroid hormone, vitamin D, on the vasculature. Am. Heart J. 2021, 239, 147–153. [Google Scholar] [CrossRef]
- Sirajudeen, S.; Shah, I.; Al Menhali, A. A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues. Int. J. Mol. Sci. 2019, 20, 3832. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Vaidya, A.; Brown, J.M.; Williams, J.S. The renin-angiotensin-aldosterone system and calcium-regulatory hormones. J. Hum. Hypertens. 2015, 29, 515–521. [Google Scholar] [CrossRef]
- Kota, S.K.; Kota, S.K.; Jammula, S.; Meher, L.K.; Panda, S.; Tripathy, P.R.; Modi, K.D. Renin-angiotensin system activity in vitamin D deficient, obese individuals with hypertension: An urban Indian study. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. S4), S395–S401. [Google Scholar] [CrossRef]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.L.; Hou, A.N.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Chun Li, Y. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013, 27, 2116–2125. [Google Scholar] [CrossRef]
- Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719. [CrossRef]
- Ghelani, D.; Alesi, S.; Mousa, A. Vitamin D and COVID-19: An Overview of Recent Evidence. Int. J. Mol. Sci. 2021, 22, 10559. [Google Scholar] [CrossRef]
- Pocket Book of Hospital Care for Children, 2nd ed.; WHO: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/978-92-4-154837-3 (accessed on 27 June 2023).
- Martin Gimenez, V.M.; Inserra, F.; Tajer, C.D. Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 2020, 254, 117808. [Google Scholar] [CrossRef]
- Ye, Q.; Lai, E.Y.; Luft, F.C.; Persson, P.B.; Mao, J. SARS-CoV-2 effects on the renin-angiotensin-aldosterone system, therapeutic implications. Acta Physiol. 2021, 231, e13608. [Google Scholar] [CrossRef]
- Georgakopoulou, V.E.; Mantzouranis, K.; Damaskos, C.; Karakou, E.; Melemeni, D.; Mermigkis, D.; Petsinis, G.; Sklapani, P.; Trakas, N.; Tsiafaki, X. Correlation Between Serum Levels of 25-Hydroxyvitamin D and Severity of Community-Acquired Pneumonia in Hospitalized Patients Assessed by Pneumonia Severity Index: An Observational Descriptive Study. Cureus 2020, 12, e8947. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine 2019, 98, e17252. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: Past, present and future. J. Steroid Biochem. Mol. Biol. 2010, 121, 234–238. [Google Scholar] [CrossRef]
- Yang, C.; Lu, Y.; Wan, M.; Xu, D.; Yang, X.; Yang, L.; Wang, S.; Sun, G. Efficacy of High-Dose Vitamin D Supplementation as an Adjuvant Treatment on Pneumonia: Systematic Review and a Meta-Analysis of Randomized Controlled Studies. Nutr. Clin. Pract. 2021, 36, 368–384. [Google Scholar] [CrossRef]
- Piedimonte, G.; Perez, M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 2015, 35, 519–530, Erratum in Pediatr. Rev. 2015, 36, 85. [Google Scholar] [CrossRef]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.K. Vitamin D-Cathelicidin Axis: At the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef]
- Beigelman, A.; Castro, M.; Schweiger, T.L.; Wilson, B.S.; Zheng, J.; Yin-DeClue, H.; Sajol, G.; Giri, T.; Sierra, O.L.; Isaacson-Schmid, M.; et al. Vitamin D Levels Are Unrelated to the Severity of Respiratory Syncytial Virus Bronchiolitis among Hospitalized Infants. J. Pediatric Infect. Dis. Soc. 2015, 4, 182–188. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus. 2020, 5, e10405. [Google Scholar] [CrossRef]
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive Effects of Vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018, 37, 749–754. [Google Scholar] [CrossRef]
- Chowdhury, F.; Bin Shahid, A.S.M.S.; Tabassum, M.; Parvin, I.; Ghosh, P.K.; Hossain, M.I.; Alam, N.H.; Faruque, A.S.G.; Huq, S.; Shahrin, L.; et al. Vitamin D supplementation among Bangladeshi children under-five years of age hospitalised for severe pneumonia: A randomised placebo controlled trial. PLoS ONE 2021, 16, e0246460. [Google Scholar] [CrossRef]
- Labib, J.R.; Ibrahem, S.K.; Ismail, M.M.; El Fatah, S.A.A.; Sedrak, A.S.; Attia, M.A.S.; El-Hanafi, H.M.; Kamel, M.H. Vitamin D supplementation and improvement of pneumonic children at a tertiary pediatric hospital in Egypt: A randomized controlled trial. Medicine 2021, 100, e25011. [Google Scholar] [CrossRef]
- Alpcan, A.; Tursun, S.; Kandur, Y. Vitamin D levels in children with COVID-19: A report from Turkey. Epidemiol. Infect. 2021, 149, e180. [Google Scholar] [CrossRef]
- Loeb, M.; Dang, A.D.; Thiem, V.D.; Thanabalan, V.; Wang, B.; Nguyen, N.B.; Tran, H.T.M.; Luong, T.M.; Singh, P.; Smieja, M.; et al. Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influ. Other Respir. Viruses 2019, 13, 176–183. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, R.-R.; Yan, Z.-X.; Yi, W.-X.; Yue, B. Correlation of serum vitamin A, D, and E with recurrent respiratory infection in children. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8133–8138. [Google Scholar]
- Kuang, L.; Liang, Z.; Wang, C.; Lin, T.; Zhang, Y.; Zhu, B. Serum 25-Hydroxy Vitamin D Levels in Children with Acute Respiratory Infections Caused by Respiratory Virus or Atypical Pathogen Infection. Nutrients 2023, 15, 1486. [Google Scholar] [CrossRef]
- Golan-Tripto, I.; Loewenthal, N.; Tal, A.; Dizitzer, Y.; Baumfeld, Y.; Goldbart, A. Vitamin D deficiency in children with acute bronchiolitis: A prospective cross-sectional case- control study. BMC Pediatr. 2021, 21, 211. [Google Scholar] [CrossRef]
- Alakaş, Y.; Celiloğlu, C.; Tolunay, O.; Matyar, S. The Relationship between Bronchiolitis Severity and Vitamin D Status. J. Trop. Pediatr. 2021, 67, fmab081. [Google Scholar] [CrossRef]
- Vo, P.; Koppel, C.; Espinola, J.A.; Mansbach, J.M.; Celedón, J.C.; Hasegawa, K.; Bair-Merritt, M.; Camargo, C.A. Vitamin D Status at the Time of Hospitalization for Bronchiolitis and Its Association with Disease Severity. J. Pediatr. 2018, 203, 416–422.e1. [Google Scholar] [CrossRef]
- Toivonen, L.; Hasegawa, K.; Ajami, N.J.; Celedón, J.C.; Mansbach, J.M.; Petrosino, J.F.; Camargo, C.A., Jr. Circulating 25-hydroxyvitamin D, nasopharyngeal microbiota, and bronchiolitis severity. Pediatr. Allergy Immunol. 2018, 29, 877–880. [Google Scholar] [CrossRef]
- Hueniken, K.; Aglipay, M.; Birken, C.S.; Parkin, P.C.; Loeb, M.B.; Thorpe, K.; Dai, D.W.H.; Laupacis, A.; Mamdani, M.; Mazzulli, T.; et al. Effect of High-Dose Vitamin D Supplementation on Upper Respiratory Tract Infection Symptom Severity in Healthy Children. Pediatr. Infect. Dis. J. 2019, 38, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Kun, J.; Silei, Y.; Sun, C.; Wenyan, H. Reduced serum 25(OH)D is closely related to bronchial mucus plug formation in children with mycoplasma pneumonia: A prospective cohort study. Front. Public Health 2023, 11, 1099683. [Google Scholar] [CrossRef]
- Oktaria, V.; Danchin, M.; Triasih, R.; Soenarto, Y.; Bines, J.E.; Ponsonby, A.-L.; Clarke, M.W.; Graham, S.M. The incidence of acute respiratory infection in Indonesian infants and association with vitamin D deficiency. PLoS ONE 2021, 16, e0248722. [Google Scholar] [CrossRef] [PubMed]
- Oktaria, V.; Triasih, R.; Graham, S.M.; Bines, J.E.; Soenarto, Y.; Clarke, M.W.; Lauda, M.; Danchin, M. Vitamin D deficiency and severity of pneumonia in Indonesian children. PLoS ONE 2021, 16, e0254488. [Google Scholar] [CrossRef]
- Dele Akeredolu, F.; Akuse, R.M.; Mado, S.M.; Yusuf, R. Relationship Between Serum Vitamin D Levels and Acute Pneumonia in Children Aged 1–59 Months in Nigeria. J. Trop. Pediatr. 2021, 67, fmaa101. [Google Scholar] [CrossRef] [PubMed]
- Bayramoğlu, E.; Akkoç, G.; Ağbaş, A.; Akgün, Ö.; Yurdakul, K.; Duru, H.N.S.; Elevli, M. The association between vitamin D levels and the clinical severity and inflammation markers in pediatric COVID-19 patients: Single-center experience from a pandemic hospital. Eur. J. Pediatr. 2021, 180, 2699–2705. [Google Scholar] [CrossRef]
- Doğan, A.; Dumanoğlu Doğan, İ.; Uyanık, M.; Köle, M.T.; Pişmişoğlu, K. The Clinical Significance of Vitamin D and Zinc Levels with Respect to Immune Response in COVID-19 Positive Children. J. Trop. Pediatr. 2022, 68, fmac072. [Google Scholar] [CrossRef]
- Jaybhaye, A.P.; Sangle, A.L.; Ugra, D.; Chittal, R.Y. A Hospital-Based Study of Vitamin D Levels in Children With Recurrent Respiratory Infections. Cureus 2022, 14, e27864. [Google Scholar] [CrossRef]
- Cariolou, M.; Cupp, M.A.; Evangelou, E.; Tzoulaki, I.; Berlanga-Taylor, A.J. Importance of vitamin D in acute and critically ill children with subgroup analyses of sepsis and respiratory tract infections: A systematic review and meta-analysis. BMJ Open 2019, 9, e027666. [Google Scholar] [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C.; et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern. Child Nutr. 2020, 16, e12987. [Google Scholar] [CrossRef]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef]
- Anitua, E.; Tierno, R.; Alkhraisat, M.H. Current opinion on the role of vitamin D supplementation in respiratory infections and asthma/COPD exacerbations: A need to establish publication guidelines for overcoming the unpublished data. Clin. Nutr. 2022, 41, 755–777. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, P.; Goyal, J.P.; Thakur, C.; Choudhary, P.; Meena, J.; Charan, J.; Singh, K.; Gupta, A. Vitamin D supplementation in childhood asthma: A systematic review and meta-analysis of randomised controlled trials. ERJ Open Res. 2021, 8, 00662–2021. [Google Scholar] [CrossRef]
- Fang, Q.; Wu, Y.; Lu, J.; Zheng, H. A meta-analysis of the association between vitamin D supplementation and the risk of acute respiratory tract infection in the healthy pediatric group. Front. Nutr. 2023, 10, 1188958. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, K.; Şen, V. Is vitamin D deficiency a risk factor for COVID-19 in children? Pediatr. Pulmonol. 2020, 55, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Seeing the Forest by Looking at the Trees: How to Interpret a Meta-Analysis Forest Plot. Global Spine J. 2021, 11, 614–616. [Google Scholar] [CrossRef]
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- RETINA Study Group; Chang, Y.; Phillips, M.R.; Guymer, R.H.; Thabane, L.; Bhandari, M.; Chaudhary, V. The 5 min meta-analysis: Understanding how to read and interpret a forest plot. Eye 2022, 36, 673–675. [Google Scholar] [CrossRef]
- Kim, J.; Kaufman, J.S.; Bang, H. Graphing Ratio Measures on Forest Plot. J. Am. Coll. Cardiol. 2018, 71, 585–586. [Google Scholar] [CrossRef]
- Barde, M.P.; Barde, P.J. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect. Clin. Res. 2012, 3, 113–116. [Google Scholar] [CrossRef]
- Andrade, C. Understanding the Difference Between Standard Deviation and Standard Error of the Mean, and Knowing When to Use Which. Indian J. Psychol. Med. 2020, 42, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Noja, G.G.; Istodor, A.V.; Moise, G.; Leretter, M.; Rusu, L.C.; Marza, A.M.; Mederle, A.O. Individual Characteristics as Prognostic Factors of the Evolution of Hospitalized COVID-19 Romanian Patients: A Comparative Observational Study between the First and Second Waves Based on Gaussian Graphical Models and Structural Equation Modeling. J. Clin. Med. 2021, 10, 1958. [Google Scholar] [CrossRef]
- Mihai, C.M.; Chisnoiu, T.; Cambrea, C.S.; Frecus, C.E.; Mihai, L.; Balasa, A.L.; Stroe, A.Z.; Gogu, A.E.; Docu Axelerad, A. Neurological manifestations found in children with multisystem inflammatory syndrome. Exp. Ther. Med. 2022, 23, 261. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.D.; Galvão Azevedo, L.M.G.; de Almeida Oliveira, T.D.A.; da Mota Santana, J.D.M. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef]
- Ghasemian, R.; Shamshirian, A.; Heydari, K.; Malekan, M.; Alizadeh-Navaei, R.; Ebrahimzadeh, M.A.; Ebrahimi Warkiani, M.; Jafar-Pour, H.; Razavi Bazaz, S.; Rezaei Shahmirzadi, A.; et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14675. [Google Scholar] [CrossRef] [PubMed]
- Banack, H.R.; Bea, J.W.; Kaufman, J.S.; Stokes, A.; Kroenke, C.H.; Stefanick, M.L.; Beresford, S.A.; Bird, C.E.; Garcia, L.; Wallace, R.; et al. The Effects of Reverse Causality and Selective Attrition on the Relationship Between Body Mass Index and Mortality in Postmenopausal Women. Am. J. Epidemiol. 2019, 188, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.L.; Rees, J.R.; Peacock, J.L.; Mott, L.A.; Amos, C.I.; Bostick, R.M.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Burke, C.A.; et al. Genetic Variants in CYP2R1, CYP24A1, and VDR Modify the Efficacy of Vitamin D3Supplementation for Increasing Serum 25-Hydroxyvitamin D Levels in a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2014, 99, E2133–E2137. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Griffin, G.; Kenny, R.A. Perspective: Vitamin D deficiency and COVID-19 severity—Plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2021, 289, 97–115. [Google Scholar] [CrossRef]
- Ashique, S.; Gupta, K.; Gupta, G.; Mishra, N.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Dureja, H.; Zacconi, F.; Oliver, B.G.; et al. Vitamin D-A prominent immunomodulator to prevent COVID-19 infection. Int. J. Rheum. Dis. 2023, 26, 13–30. [Google Scholar] [CrossRef]
- Bergman, P.; Lindh, A.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in COVID-19 infection in pediatric population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef]
- Waldron, M.; Nonnecke, B.; Nishida, T.; Horst, R.; Overton, T. Effect of Lipopolysaccharide Infusion on Serum Macromineral and Vitamin D Concentrations in Dairy Cows. J. Dairy Sci. 2003, 86, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Feketea, G.; Vlacha, V.; Bocsan, I.C.; Vassilopoulou, E.; Stanciu, L.A.; Zdrenghea, M. Vitamin D in Corona Virus Disease 2019 (COVID-19) Related Multisystem Inflammatory Syndrome in Children (MIS-C). Front. Immunol. 2021, 12, 648546. [Google Scholar] [CrossRef] [PubMed]
- Balan, K.V.; Babu, U.S.; Godar, D.E.; Calvo, M.S. Vitamin D and respiratory infections in infants and toddlers: A nutri-shine perspective. In Handbook of Vitamin D in Human Health; Springer: Berlin/Heidelberg, Germany, 2013; Volume 4, pp. 276–297. [Google Scholar] [CrossRef]
- Manaseki-Holland, S.; Qader, G.; Isaq Masher, M.; Bruce, J.; Zulf Mughal, M.; Chandramohan, D.; Walraven, G. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: A randomized controlled trial. Trop. Med. Int. Health 2010, 15, 1148–1155. [Google Scholar] [CrossRef]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Vitamin D. Available online: https://ods.od.nih.gov/factsheets/VitaminD-Consumer/#h2 (accessed on 27 June 2023).
- Vitamin D and Your Health. Available online: https://www.healthdirect.gov.au/vitamin-d-and-your-health#:~:text=The%20Australian%20government%20publishes%20recommended,of%20vitamin%20D%20per%20day (accessed on 27 June 2023).
- NHS. Vitamin D. nhs.uk. 19 November 2021. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/#:~:text=Children%20from%20the%20age%20of,of%20vitamin%20D%20a%20day (accessed on 27 June 2023).
- Kimball, S.M.; Mirhosseini, N.; Holick, M.F. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Dermato-Endocrinol. 2017, 9, e1300213. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]

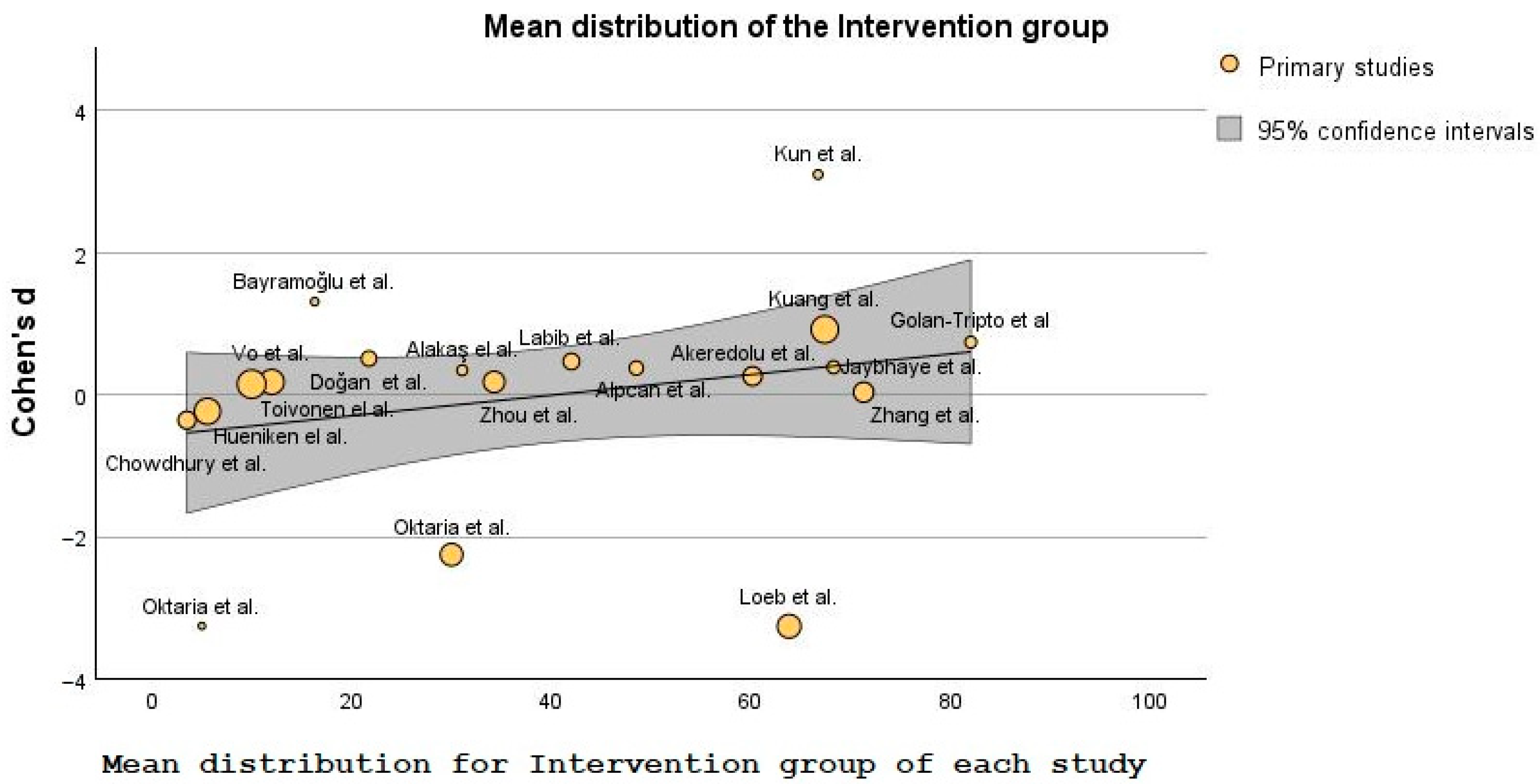
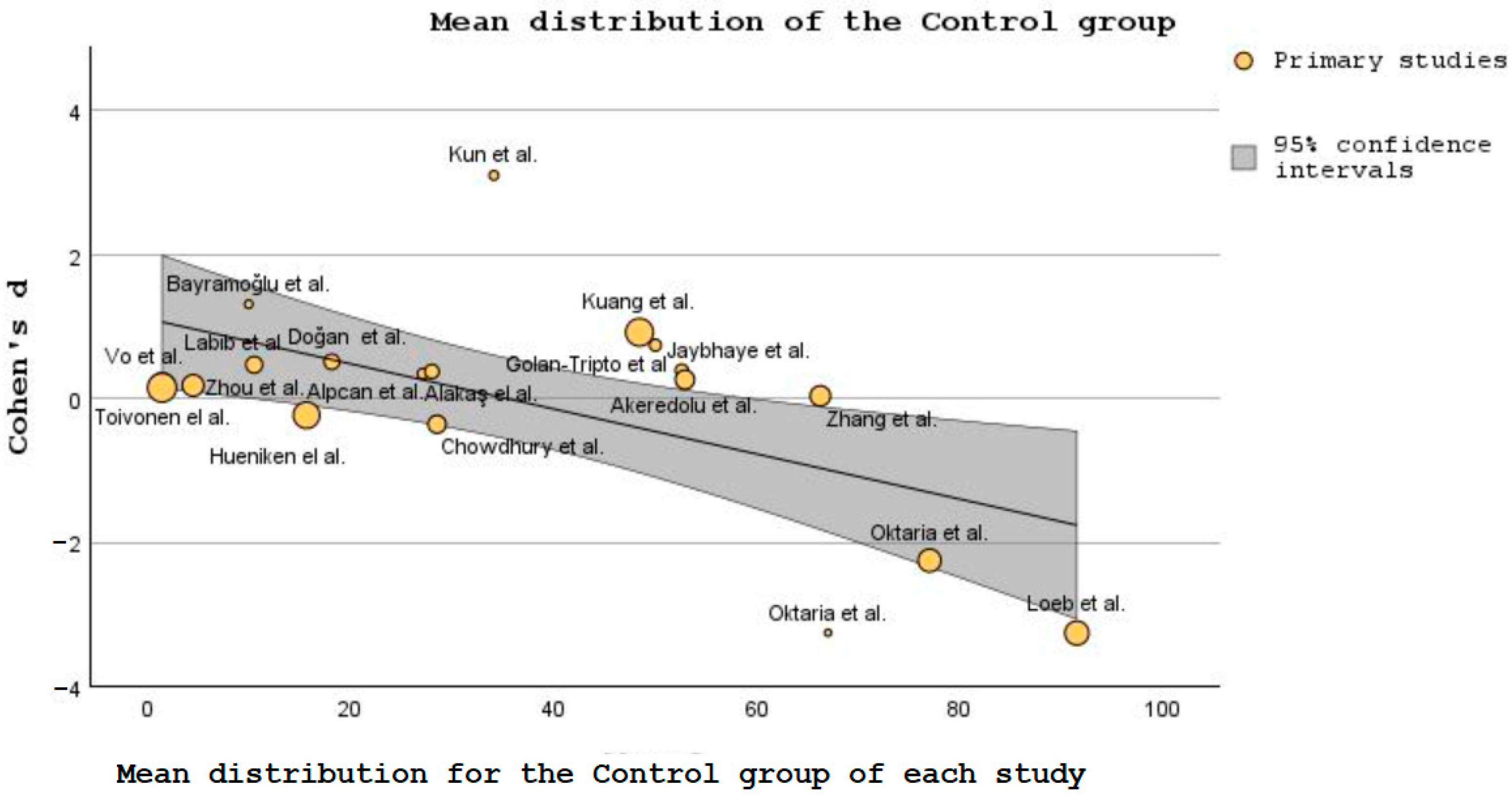
| Study | Study Design | Pico Framework | Results of the Study | Conclusion | VIT. D Supplementation |
|---|---|---|---|---|---|
| Zhou et al. [47] | RCT | Participants: In total, 169 children were included in the low-dose vitamin D group and 164 in the high-dose group (aged 3–12 months old) Intervention: Infants were randomly assigned to low-dose or high-dose vitamin D3 (VD3) groups, with children in each group receiving drops orally for 4 months (one drop containing 400 IU of vitamin D) Comparison: The two groups were compared for the suitability of administering vitamin D to prevent the development of seasonal influenza. Outcome: To assess vitamin D’s safety and clinical effectiveness in preventing influenza A. | In total, 78 (46%) and 43 (26%) influenza A infections occurred in the groups receiving low and high vitamin D doses, respectively. When comparing the two sets of children, there was a significant difference when it comes to the median durations of fever (22.18 ∓ 36.77 vs. 35.20 ∓ 47.53), cough (1.42∓2.57 vs. 3.43 ∓ 4.38), and wheeze (0.99 ∓ 1.82 vs. 2.83 ∓ 3.69) in the high dose and the low dose group, respectively. | A dose of 1200 IU 25(OH)D is suitable for lowering the risk of Influenza A. This conclusion is backed up by the rapid rate of disease improvement, lower viremia, and fast recovery. In addition, an increased dose of VD is probably safe for patients in this age group. | YES |
| Chowdhury et al. [48] | RCT | Participants: In total, 97 vs. 100 children included in the experimental and the placebo group, respectively (2–59 months old with severe pneumonia). Intervention: Depending on the group, children received either 20,000 IU per mL of vitamin D3 (a high dose or miglyol oil 812. Children could not tell the difference between these two substances, as they looked and tasted the same. Comparison: Children received a vitamin D3 doze according to their age, as follows: 20,000 IU for those under 6 months, 50,000 IU for children 6 to 12 months, 100,000 IU for the remaining ones 13 to 59 months old, and those in the placebo group received miglyol oil. Upon receiving the mega dose, all children included in the study received 10,000 IU doses for 4 days or until discharge from the hospital.Outcome: To evaluate the necessity of 25(OH)D3 supplementation in children admitted to the hospital with severe cases of pneumonia. | On admission to the hospital, the serum VD level was measured, and around half of the children from each group were VDD (52% the intervention group and 49% in the placebo group). Children with severe pneumonia from the 1st group seemed to recover faster (72 h pneumonia resolution time) when compared with the control group (88 h resolution time). The same trend was also noticed regarding hypoxemia, as children in the VD group were hypoxic around 24 h, while those in the 2nd group were hypoxic 48 h. A small difference was also noticed in the period needed for the full recovery of children. The VD group needed around 4 days, while the placebo group needed 5 days. | Even though there were some differences between the two groups, the authors concluded that the time needed for improving tachypnea, chest in-drawing, or the overall resolution of severe pneumonia, irrespective of VD status, were not that great. Also, not-so-great differences were registered regarding the number of days until hospital discharge of children from the two groups. A maintenance dosage of VD after an age-specific megadose was shown to not statistically vary from the other two intervention groups. | NO |
| Labib et al. [49] | RCT | Participants: In total, 191 children (1 month to 12 years old) were put into two groups, 93 in the experimental group and 98 in the control group. All children were either with VDI or VDD and were diagnosed with pneumonia. Intervention: A dose of 100,000 IU of VD3 (intravenous), or placebo. Comparison: To ensure that every participant was evaluated before being discharged, children were assessed for seven days following vitamin D treatment, especially on the day when vitamin D concentration in the blood reached their peak. Outcome: To evaluate the development and recovery time of the children. | Seven days after the injection with the high dose of VD3, the experimental group showed higher serum concentration levels. In this group, the percentage of patients who were VDI or VDD on the 1st day had significantly reduced by the last day (with 11.8% on the 1st day and 52.7% on the 7th day). Also, most of the children in the intervention group (93.5%) of the supplementation group registered normal PaO2/FiO2 values, from 300 to 360 (day 1 and day 7), compared with 340 to 345 (day 1 and day 7) in the intervention group and placebo group, respectively. | It was shown that a high dose of VD administration is indeed related to a decreased risk of mortality, shortened recovery time, better PaO2/FiO2, and lower qSOFA scores in pneumonic children with high VDD. Seven days following the intervention, most of the vitamin D group’s PaO2/FiO2 readings returned to normal, showing a reduction in the severity of lung damage in the children receiving mechanical ventilation (MV). | YES |
| Alpcan et al. [50] | RCT | Participants: In total, 155 pediatric patients (75 in the intervention group and 80 in the control group) with mild or moderate COVID-19 disease (1 month to 17 years old). Intervention: To determine vitamin D serum level and other biological analyses. Comparison: To compare the blood results between the groups. Outcome: To compare the VD levels of pediatric patients identified with COVID-19. | The average blood level of 25(OH)D concentration was lower in the 1st group than the control group (21.5 vs. 28.0), and the percentage of VDD patients was higher in the COVID-19 group than 2nd group (44% vs. 17.5%). The mean C-reactive protein (CRP) level was higher in the vitamin D-deficient children (9.6 vs. 4.5). Still the hospitalization days were similar for both groups, with a statistical difference of only r = 0.724. | Parallel examination of different blood results showed an essential relation between VD level and leukocyte, lymphocyte, and thrombocyte count and no negative association with age and duration of hospitalization. The regression analysis suggests an inverse relation between low vitamin D concentration and the risk of developing respiratory infections. | YES |
| Loeb et al. [51] | Participants: Two groups totaling 1300 healthy children (3 to 17 years of age) were divided between two groups, each with 650 children (vitamin D and placebo). Intervention: Those included in the study were randomized, half of them receiving 7 drops of vitamin D (0.028 mL per drop—14,000 U/week) every week for 8 months, while those arbitrarily receiving were administered 7 placebo drops (0.028 mL per drop) for the same period. Comparison: Children in the two groups were compared for signs and symptoms of influenza two times every week over a period of one year. Outcome: To analyze RT-PCR-confirmed infection with influenza and PCR-confirmed non-influenza respiratory viruses. | In total, 7.7% (50) of children in the intervention group were confirmed by RT-PCR with influenza A or B, while only 1% less (6.6%, or 43 children) were RT-PCR confirmed with the same virus. Additionally, regarding other viruses, 146 patients (22.5%) from the VD group acquired non-influenza infections, compared with 185 patients (28.5%) in the placebo group. Thus, 25 hydroxyvitamin D’s had some effect in reducing all respiratory infections, 177 of the total 650 (27.2%) in the VD group compared with 209 in the 2nd group (32.2%). | The conclusions of this study suggest that 25 hydroxy cholecalciferol supplementations did not reduce the incidence of influenza A or B virus but reduced in a small percentage the other respiratory infections. Therefore, it can be presumed that VD supplements can have some influence in reducing viral respiratory infections. Still the authors conclude that it is not enough to recommend the routine administration of it to the general pediatric population. | NO | |
| Zhang et al. [52] | RCT | Participants: In total, 422 youngsters were included in the intervention group and 100 in the control group were observed for recurrent respiratory infections (RRIs). Intervention: Serum levels of 25 (OH) D2, and vitamin A, and E were analyzed. Comparison: The study group was further separated into two subgroups, with an active group of 222 children, and a stable group of 200, along with other 100 healthy children and they were all compared for their risk of developing RRIs. Outcome: To analyze if there is any connection between the different vitamins (A, D and E) and RRIs among the pediatric population. | The study found that the relationship between vitamins A and D was positive in children with RRIs (r = 0.945 for vit. A and r = 0.988 for vit. D). The relationship between vitamin E and vitamin D was also positive in those children, with r = 0.959 for vitamin E (vitamin D having an r = 0.988). | Deficiency in the three vitamins relates to RRIs in children. Thus, supplementing vitamins A, D, and E via dietary changes is advantageous for the faster recovery of the patients. | YES |
| Kuang et al. [53] | RCT | Participants: In total, 948 children aged 0 to 16 years, divided as follows: 295 with ARTIs, 17 with co-infection and 636 healthy children. Intervention: PCR or (RT)-PCR was used to examine oropharyngeal samples from patients, detecting the existence of viruses or unusual infections. Comparison: The study compared the results of the PCR and RT-PCR of the intervention group and the healthy group. Outcome: To determine if there is a connection between the vitamin D status in children with ARTI due to viruses or other unusual pathogens. | Of the total 295 children infected with a single virus, more than half (58.98%) were VDD (>50.0 nmol/L), together with a large percentage of the 17 children infected with more than one virus (76.47%). However, regarding the ARTIs and the co-infected sub-groups, the differences in the VD levels were not great. | The study showed that VDD is associated with developing ARTIs with typical or atypical pathogens. Thus, the recovery of ARTIs may be correlated with serum 25(OH)D concentrations. | YES |
| Golan-Tripto et al. [54] | RCT | Participants: In total, 127 patients less than 24 months were divided into an acute bronchiolitis group (80 children) and a group of 47 children with other non-respiratory illnesses. Intervention: Blood samples were collected to determine if the children were either VDD (<50 nmol/L), VDI (≥50 and <75 nmol/L), or sufficient (≥75 nmol/L). Comparison: The vitamin D status of the children was compared against the severity of bronchiolitis and length of hospital stay. Outcome: To analyze if there is a connection between the 25(OH)D concentration in very young patients diagnosed with acute bronchiolitis compared to other infections not related to respiratory tract. | In this study, more than half of the children from each group received vitamin D supplementation (66% and 60% in the bronchiolitis group and control groups, respectively). In the bronchiolitis group, blood 25(OH)D concentration was considerably lower when compared with the control group. The statistics showed a median of 28 vs. 50 nmol/L, respectively. VDI happened more likely in the bronchiolitis group, according to the regression model used by the authors. | Even though the authors did not observe any association between 25(OH)D concentrations and bronchiolitis severity, they noticed that children in acute bronchiolitis group displayed lower vitamin D levels than children from the 2nd group. Also, they required a longer recovery period, measured in the length of days spent in the hospital. | YES |
| Alakaş et al. [55] | RCT | Participants: In total, 182 children with acute bronchiolitis between 1 month and 2 years of age were divided into a group of 73 children (40%) with severe cases of disease admitted in the ICU department, and 109 children (60%) in the typical patient ward. Intervention: An amount of 2 mL of blood was collected for vitamin D examination. This was done on the same day as admission to the hospital, along with other regular blood analyses. Comparison: The vitamin D concentration of those admitted in the ICU department as opposed to those that did not need intensive care. Outcome: To assess if VD is linked to the symptoms and outcome of acute bronchiolitis in very young patients. | When comparing the progress to the ICU department, the study shows that 25 (34.2%) of children whose VD levels were normal (73), 29 (39.7%) who were VDD, and 19 (26%) who were VDI needed intensive care. The trend is similar with the 109 patients admitted to the normal patient ward. Therefore, 70 (64.2%) children had sufficient VD concentration, 25 (22.9%) and 14 (12.8%) were VDD and VDI, respectively. | A significantly higher likelihood of VDD or VDI was found in patients diagnosed with bronchiolitis that needed intensive care compared to those in normal patient wards. Hence, vitamin D deficiency is indeed correlated with severe bronchiolitis. | YES |
| Vo et al. [56] | Prospective cohort study | Participants: In total, 1016 infants under 12 months hospitalized with bronchiolitis were divided into 3 groups, depending on blood 25(OH)D levels: 298 children (<20 ng/mL), 352 with vit. D values of 20–29.9 ng/mL, and 366 children having serum concentrations of ≥30 ng/mL. Intervention: Blood was collected within 24 h of patients being admitted to the hospital. Using a standardized approach, RT-PCR was also performed, as well as a nasopharyngeal aspirate. Comparison: The number of patients needing ICU department, as well as longer hospital length of stay (LOS) was compared between the two groups. Outcome: To evaluate if there is a connection between the circulating VD status at the start of the illness and the severity among infants diagnosed with bronchiolitis. | Compared to those with VD values of ≥30 ng/mL, children with <20 ng/mL had a greater probability of needing ICU and a longer LOS. Patients at the lowest end of circulating 25(OH)D concentration had longer LOS than those with the highest tertile, but neither unadjusted nor adjusted models found a relationship between the free 25-OHD concentration and the clinical outcome of bronchiolitis. | This research concluded that values of <20 ng/mL put infants at risk of needing intensive care and longer hospital days; thus, this vitamin plays an essential role in the outcome of bronchiolitis. The observed correlation between total 25(OH)D concentration and bronchiolitis severity, rather than bioavailable or free 25(OH)D, shows that total concentration may be a more accurate indicator of ARTI. | YES |
| Toivonen et al. [57] | Multicenter prospective cohort study | Participants: In total, 1005 infants under 12 months hospitalized with bronchiolitis were distributed into two groups. The lower serum concentration of 25OHD included 498 patients, and the higher serum concentration had 507 patients. Intervention: In the first 24 h of hospital admission, staff collected blood and nasopharyngeal airway samples. Comparison: The lower vitamin D level group (<26.5 ng/mL) and the higher one (≥26.5 ng/mL) were compared against the days of ICU stay. Outcome: To analyze the possible 25(OH)D—ICU admission relation and use of mechanical ventilation (such as CPAP or intubation) during hospitalization. | 25(OH)D and nasopharyngeal microbiota profiles appear to be related to the risk of needing intensive care, as patients at the lower spectrum of 25(OH)D levels had a higher risk of ICU (24.5%) when it comes to Hemophilus when compared with the higher-level group (16.2%). In contrast, there were no significant associations among those with higher 25(OH)D concentrations. | The study concludes that circulating 25OHD concentrations are associated with certain nasopharyngeal metabolomic signatures, like increased pro-inflammatory lipids, which may function as mediators in the microbiome-host interactions in the airways. | YES |
| Hueniken et al. [58] | RCT | Participants: In total, 703 children between 1 and 5 years of age with upper respiratory tract infections (URTIs) were divided into a group that received a high-dose supplementation of vitamin D (349 children) and a group that received the standard dose (354 children). Intervention: Oral VD doses of 2000 IU/day or 400 IU/day were randomly administered to children in the study for one winter season. Comparison: The two groups were compared for the risk of developing URTIs. Outcome: To analyze the effects of vitamin D concentration of 2000 IU/day on outpatient or emergency department (ED) visits correlated with antibiotic prescriptions for URTI. | The incidence rate ratio of the high supplementation group did not reduce the severity of the URTIs symptoms (IRR = 0.97), together with the frequency of ED and outpatients’ visits (IRR = 1.17 and IRR = 1.16, respectively). The IRR of antibiotic prescriptions for the two groups had similar results, of only 1.02. | This research concluded that high doses of VD in wintertime did not lessen the severity of the symptoms in URTI compared to the normal dosage for children, as the American Academy of Pediatrics (AAP) advised. High-dose vitamin D supplementation also did not reduce the incidence of ED visits, outpatient doctor visits, or antibiotic prescriptions. | NO |
| Kun et al. [59] | Prospective cohort study | Participants: In total, 175 children with Mycoplasma pneumoniae (MPP) were divided into two groups according to the existence of bronchial mucus plugs (BMP). Thus, 73 children were included in a BMP group and 102 in a non-mucus group. Intervention: Fiberoptic bronchoscopy of the mucus secretions was performed on patients where it was blocking the respiratory tract, and for the rest of the children where they could not be easily removed, doctors used a cell brush or biopsy forceps. Comparison: The two groups of patients were compared by looking at 25(OH)D concentrations, IL8, and other inflammatory markers. Outcome: To evaluate any associations between these specific blood tests and clinical characteristics in the pediatric population with mycoplasma pneumonia (MPP). | 25(OH)D, IL8, neutrophils and other inflammatory cells were lower in the non-BMP group when compared with the BMP group. On the other hand, the relation between the blood VD and the other inflammatory markers tested here appeared to be inversely related. | According to this research, vitamin D could be used as a marker in diagnosing children with MPP. Young patients with MPP often have vitamin D insufficiency, and its levels are strongly correlated with the presence of inflammatory cells and the severity of the illness. Children with MPP and BMPs had serum 25(OH)D concentrations lower than those without MPP and BMPs. | YES |
| Oktaria et al. [60] | Prospective cohort study | Participants: In total, 422 pregnant women and their subsequent babies were observed for ARTI episodes for a period of 12 months following delivery. Intervention: At birth, newborns were tested for their VD levels, and a follow-up occurred 6 months later. Comparison: Blood results of children’s vitamin D taken 6 months apart were analyzed against the incidence of ARTIs. Outcome: To observe the incidence of pneumonia concerning VDD for the 1st year of life. | Within the selected sample of patients, 1601 ARTIs incidents were found to have occurred over 1 year. In total, 96 and 7 pneumonia cases matched the WHO criteria for pneumonia and severe pneumonia, respectively (8.7% of the total). Nearly all newborns (96%) had at least one instance of ARI that was not pneumonia. The 9 to 12 months age group had the greatest pneumonia frequency, whereas newborns under 3 months had the lowest. For non-pneumonia and pneumonia illnesses, the median length of full symptom resolution was 7 days and 15 days. Between those with VDD and non-VDD at birth, there was no difference in the prevalence of the 1st episode of pneumonia when compared with the 2nd one within 6 months, as well as other incidences of pneumonia from 6 to 12 months. It appears as if these events were unrelated to vitamin D status at 6 months of age. | VD status of newborns, influenced by the mother’s exposure to the sun, influenced very little the occurrence rate of pneumonia in the first 6 months of life. Still, the overall frequency rate was not considerably changed by the status of vitamin D (whether at birth of after 6 months). Reducing VDD at birth by giving supplements to mothers or increased sun exposure during pregnancy has very little potential to reduce ARTIs. | NO |
| Oktaria et al. [61] | Cross-sectional study | Participants: In total, 127 hospitalized patients diagnosed with pneumonia (2 to 59 months old) whose vitamin D concentrations were measured. The study sample was divided between those who had VDI or VDD (25 children), and those whose levels were normal (102 children). Intervention: Doctors measured the total VD2 and VD3 using liquid chromatography [tandem mass spectrometry. Comparison: The two groups were analyzed, comparing the vitamin D concentration and their risk of developing pneumonia. Outcome: To observe if there is any correlation linking vitamin D and symptom severity in these patients. | Infants younger than 6 months needed longer LOS in the hospital (more than 5 days). However, this seemed to be more related to the low birth weight and poor nutritional status on admission, which were also risk factors for hypoxemia. | 1/5 children in this study group were VDD. Still, the authors concluded that this status was not associated with the severity of pneumonia, and with the presence of other danger signs, such as hypoxemia or the quantity of oxygen required. Also, the 25(OH)D concentration was unrelated to the hospitalization duration. | NO |
| Akeredolu et al. [62] | Cross-sectional study | Participants: In total, 270 children were put into a group for those with pneumonia (135), and another one with 135 apparently healthy children. The children were aged 1 to 59 months. Intervention: Vitamin D serum levels were measured for both groups. Comparison: The two groups were compared for their vitamin D concentration. Outcome: To assess the suitable intervention of vitamin D supplementation in preventing acute pneumonia in children younger than 5 years of age. | Statistics in this study showed a difference between the means of the serum concentration (52.14 vs. 60.91) for the 1st group when compared to the control group. Also, most children in the pneumonia group (91.1%) were VDI (with VD concentration of less than 75.0 nmol/L) when compared to the healthy children, that were almost 20% less (71.9%). | This study supports the link between vitamin D and acute pneumonia in pediatric population. To lower children’s risk of pneumonia, the authors advise that techniques and treatments, including supplementation of VD, better nutrition (food that includes vitamin D), and additional measures regarding increasing children’s vitamin D status should be seriously examined. | YES |
| Bayramoğlu et al. [63] | RCT | Participants: In total, 103 COVID-19-positive children between 1 and 18 years of age were put into 3 groups: asymptomatic patients (29); patients with mild infections (40); and those with severe infections (34). Patients in the last group had pneumonia confirmed by physical examination and X-ray/CT Intervention: The infection with the virus was confirmed by PCR, with sample collected through a nasopharyngeal swab. Comparison: The 3 groups (asymptomatic, mild, and severe) were compared against their vitamin D concentration. Outcome: To assess the symptoms severity and inflammatory markers in the pediatric population during COVID-19 concerning the VD status. | Compared to the mild and asymptomatic groups, the 3rd group showed lower lymphocyte counts and significantly C reactive protein, lymphocyte count, pro-calcitonin, fibrinogen, and D-dimers, which are signs of inflammation. Some children were also VDI, and the ratio was 70.6% in the severe group. The VD level had a positive relation with the lymphocyte count (r = 0.375), and an inverse association with age (r = −0.496), CRP (r = −0.309), and fibrinogen levels (r = −0.381). | This study revealed an association between insufficient concentrations of 25(OH)D and the evolution of the COVID-19 infection. The 3rd group had worse vitamin D levels and higher inflammatory cells. Although vitamin D’s effects on the immune system are important as the evidence suggests that appropriate 25OH vitamin D concentration assists the body’s ability to fight off viral and bacterial infections and minimize inflammation. | YES |
| Doğan et al. [64] | Prospective cohort study | Participants: In total, 176 children aged 1–18 years divided into two groups: one with 88 children with COVID-19 disease and the other with 88 healthy children. Intervention: Electrochemiluminescence immunoassay was used to measure the serum VD. Comparison: The 25(OH)D concentrations of the groups were compared in relation to the severity of the infection. Outcome: To evaluate if there is a connection between the severity of the COVID-19 infection and this vitamin. | When looking at the two sets of children, COVID-19-positive patients’ mean blood 25 hydroxy cholecalciferol levels were lower (11.73) than those of the other group (18.14). Also, the study shows a significant link between VD and Zn values in the two groups (r = 0.245). | Since the two assessment markers (vitamin D and Zn) showed a connection with the infection, being higher in healthy children, the authors recommend supplementing of the two as a supportive treatment for COVID-19 infection. | YES |
| Jaybhaye et al. [65] | RCT | Participants: In total, 108 children with RRIs (both inpatients or outpatients) aged from six months to 15 years in one group and 55 healthy children of the same age group who visited the hospital throughout the research period for immunizations and regular checkups, as the control group. Intervention: The serum 25-hydroxyvitamin D collected from venous blood from patients and controls was analyzed. Comparison: The intervention group was compared with the control one Outcome: To evaluate whether the RRIs and 25 hydroxyvitamin D are connected. | Essentially, all children with RRIs had less than normal vitamin D levels. Around 75% of these patients had VDI, and the rest had VDD. With a p-value of less than 0.001, these variations in 25(OH)D status of the two groups were statistically significant. | Comparatively, to controls, children with recurrent respiratory infections had a significantly high rate of vitamin D insufficiency. Children with recurrent respiratory infections should be managed with an evaluation of their vitamin D level. | YES |
| Study | Study Design | Pico Framework | Results of the Study | Conclusion | VIT. D Supplementation |
|---|---|---|---|---|---|
| Cariolou et al. [66] | Systematic review and meta-analysis of observational studies | Participants: In total, 52 studies that included 7434 children from 15 countries hospitalized with acute or critical conditions (sepsis and respiratory tract infections). Intervention: Serum level of vitamin D was analyzed against the RTIs. Comparison: The patients were either with a VDI status (<50 nmol/L) (3473, which accounts for 47.0%) or with a sufficient VD status. Outcome: To evaluate the prevalence of VDD in children with acute or critical conditions. | Of the total number of children in the study, almost half (47%) were VDD with less than 50 nmol/L, with a joint incidence rate estimation of 54.6% (48.5% to 60.6%). After ignoring studies with a small number of patients, the authors found out that the frequency of infections was comparable to the above findings, with 51.5%. | As 2463 young patients were at risk of mortality, this review (that included 18 cohort studies) revealed that this probability was linked with VDD status. Thus, a lower mortality rate can be achieved if children with VDD receive supplementation doses to normalize their VD level. | YES |
| Hong et al. [67] | Prospective birth cohort study | Participants: In total, 2244 infants monitored from birth to 6 months of age divided into four groups according to the frequency of VD supplementation Intervention: For the first 6 months of life, infants received every day a 400 to 600 IU dose of 25(OH)D. Comparison: The frequency of supplementation was from 0 to 5 to 7 days per week. Outcome: To assess the first episode of pediatrician-diagnosed RTI, or to determine if, in the first 6 months, children had no RTI events. | Infants without vitamin D supplements had their first RTI episode on average 60 days after birth, whereas those receiving supplements had it when they were older than 6 months. According to the authors, the risk ratios in the supplementing group that took supplements 5–7 days per week were 0.46 and 0.18, respectively, showing an inverse association between supplementation periods of time and the risk for various respiratory tract infections that would need hospitalization. | Infants who received vitamin D supplements in the first six months of life saw the onset of the first RTI later than those who did not. For all children without supplements, the median time until their first RTI was 60 days. For exclusively breastfed newborns, it was 36 days and for formula-fed infants it was 90 days. | YES |
| Pham et al. [68] | Systematic review and meta-analysis of observational studies | Participants: In total, 14 studies were included in a meta-analysis evaluating the ARTIs risk, and a further 5 were analyzed for severity of symptoms. Intervention: Blood 25 hydroxycholecalciferol was measured. Comparison: Blood levels were compared with the risk of developing RTIs. Outcome: To assess the risk and severity of ARTI in children with VDI or VDD. | When comparing the lowest to the highest 25(OH)D category, the pooled odds ratios were 1.83 and 2.46, respectively, showing an inverse relationship between 25(OH)D and risk and development of ARTIs. The likelihood of infections rose by 1.02 for every 10 nmol/L of 25(OH)D drop in serum. This was a straightforward trend, as and the risk of ARTIs increased most dramatically when the VD concentration was below 37.5 nmol/L. | According to the research, vitamin D significantly reduces the likelihood and severity of ARTIs, particularly in those with low concentrations. Still, finding the ideal concentration or the level below which supplementation would be beneficial proved difficult. | YES |
| Anitua et al. [69] | Systematic review and meta-analysis of observational studies | Participants: In total, 65 studies were deemed eligible, with a total of 50,554 participants. Intervention: 25(OH)D was determined from blood samples Comparison: The results were compared with the risk of developing RTIs. Outcome: To assess the risk and severity of ARTI in children with VDI or VDD. | Many studies stated that vitamin D supplementation reduced the risk of respiratory infections among all participants. Consequently, the protective effect of vitamin D was only substantial when taken regularly. | According to incidence count data, this meta- concluded that the incidence of upper and lower respiratory tract infections was noticeably decreased by vitamin D prevention. | YES |
| Kumar et al. [70] | Systematic review and meta-analysis of RCTs | Participants: In total, 18 RCTs totaling 1579 participants were included in this study. Intervention: Supplementing with vitamin D in addition to the treatment for asthma Comparison: Placebo groups were compared to the intervention groups Outcome: To assess the risks and severity of asthma in children in relation to their vitamin D status. | This meta-analysis included randomized control trials with VDI children whose levels were below 20 nmol/L. However, information on asthma aggravation was only given by one research, where vitamin D supplementation had no detectable impact on any of the outcomes that were reported. Other results did not significantly alter in any way. Using the fixed-effect model, the authors conducted a sensitivity analysis for results with heterogeneity of less than 50%. The conclusion was that these groups had no difference in any of the outcomes. | Vitamin D supplementation had no noticeable impact on asthma episodes needing systemic corticosteroids, according to the collective RCTs included in this research. Furthermore, there was no obvious change in the proportion of children experiencing asthma episodes. Therefore, vitamin D does not lessen the need for hospital stays and urgent care visits. There is little evidence to support the idea that vitamin D supplementation may be protective in children with asthma. | NO |
| Fang et al. [71] | Systematic review and meta-analysis of observational studies | Participants: Four trials totaling 13.367 participants examined the effectiveness of vitamin D in avoiding ARTIs. Intervention: The concentration of vitamin D was determined. Comparison: Infection rates between the VD groups and control ones. Outcome: To find out if there is any association between a higher dose of vitamin D and the incidence and outcome of ARTIs in children. | With 1.596 participants, two studies investigated the effectiveness of higher vs. regular supplementation doses of 25(OH)D when it comes to preventing ARTIs. There were few obvious differences between the two groups, and the included trials lacked substantial heterogeneity. Because the influenza A infection was reported in many studies utilizing different treatment arms, only that illness could be the subject of a meta-analysis. Compared to the lower dose of the VD group, the higher one had some decline in influenza A rates. | Investigating the effectiveness of vitamin D supplementation in preventing these illnesses produced mixed findings. According to this research, vitamin D administration offers no noticeable advantage over a placebo in terms of considerably lowering ARTI rates, but it did show some improvement when it came to the Influenza A virus. Furthermore, a high amount of vitamin D had no advantages over administering the recommended quantity. Additionally, in most infections recorded, the frequencies of various viral infections were equally independent of the regimen or organism that caused the illness. | NO |
| Life Stage | Recommended Amount |
|---|---|
| <12 months | 10 mcg (400 IU) |
| Children 1 to 13 years | 15 mcg (600 IU) |
| Teens 14 to18 years | |
| Adults 19 to 70 years | |
| Adults ≥71 years | 20 mcg (800 IU) |
| Pregnant and breastfeeding women | 15 mcg (600 IU) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolae, M.; Mihai, C.M.; Chisnoiu, T.; Balasa, A.L.; Frecus, C.E.; Mihai, L.; Lupu, V.V.; Ion, I.; Pantazi, A.C.; Nelson Twakor, A.; et al. Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children. Nutrients 2023, 15, 3430. https://doi.org/10.3390/nu15153430
Nicolae M, Mihai CM, Chisnoiu T, Balasa AL, Frecus CE, Mihai L, Lupu VV, Ion I, Pantazi AC, Nelson Twakor A, et al. Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children. Nutrients. 2023; 15(15):3430. https://doi.org/10.3390/nu15153430
Chicago/Turabian StyleNicolae, Maria, Cristina Maria Mihai, Tatiana Chisnoiu, Adriana Luminita Balasa, Corina Elena Frecus, Larisia Mihai, Vasile Valeriu Lupu, Irina Ion, Alexandru Cosmin Pantazi, Andreea Nelson Twakor, and et al. 2023. "Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children" Nutrients 15, no. 15: 3430. https://doi.org/10.3390/nu15153430
APA StyleNicolae, M., Mihai, C. M., Chisnoiu, T., Balasa, A. L., Frecus, C. E., Mihai, L., Lupu, V. V., Ion, I., Pantazi, A. C., Nelson Twakor, A., Andrusca, A., Cambrea, C. S., Arghir, I. A., Lupu, A., & Arghir, O. C. (2023). Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children. Nutrients, 15(15), 3430. https://doi.org/10.3390/nu15153430






