Effectiveness of Whey Protein Supplementation during Resistance Exercise Training on Skeletal Muscle Mass and Strength in Older People with Sarcopenia: A Systematic Review and Meta-Analysis
Abstract
:1. Background
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategies
2.5. Study Selection
2.6. Data Collection Process
2.7. Risk of Bias
2.8. Statistical Methods
2.9. Grading of Recommendation, Assessment, Development, and Evaluation
3. Results
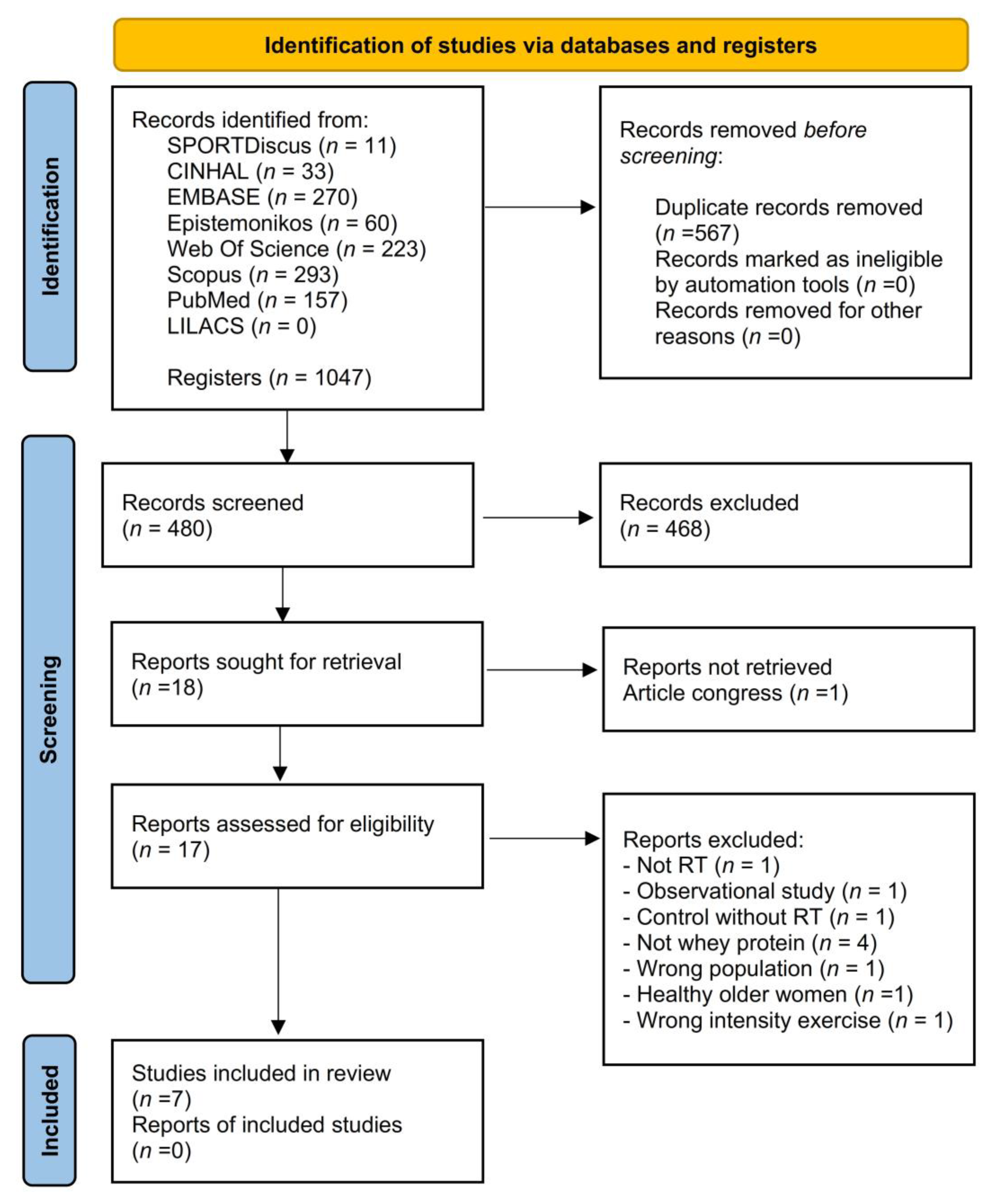
3.1. Study Selection
3.2. Study Characteristics
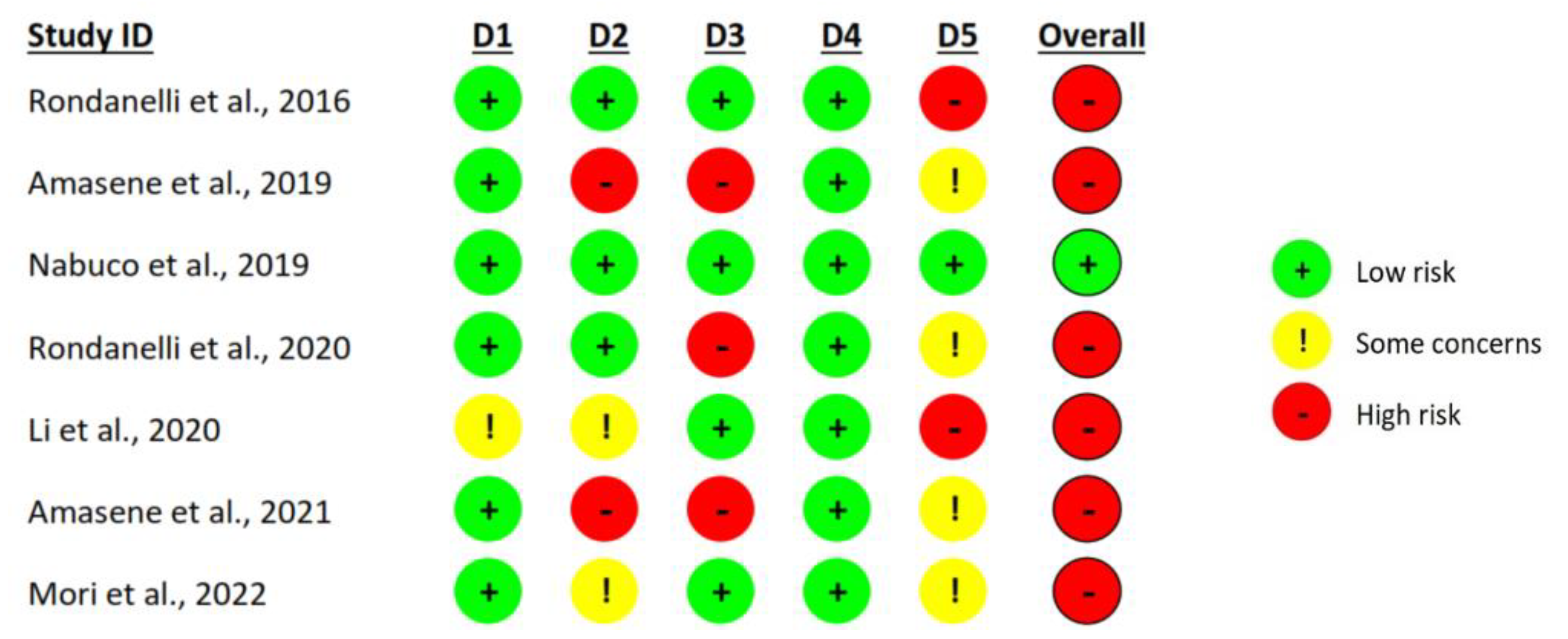
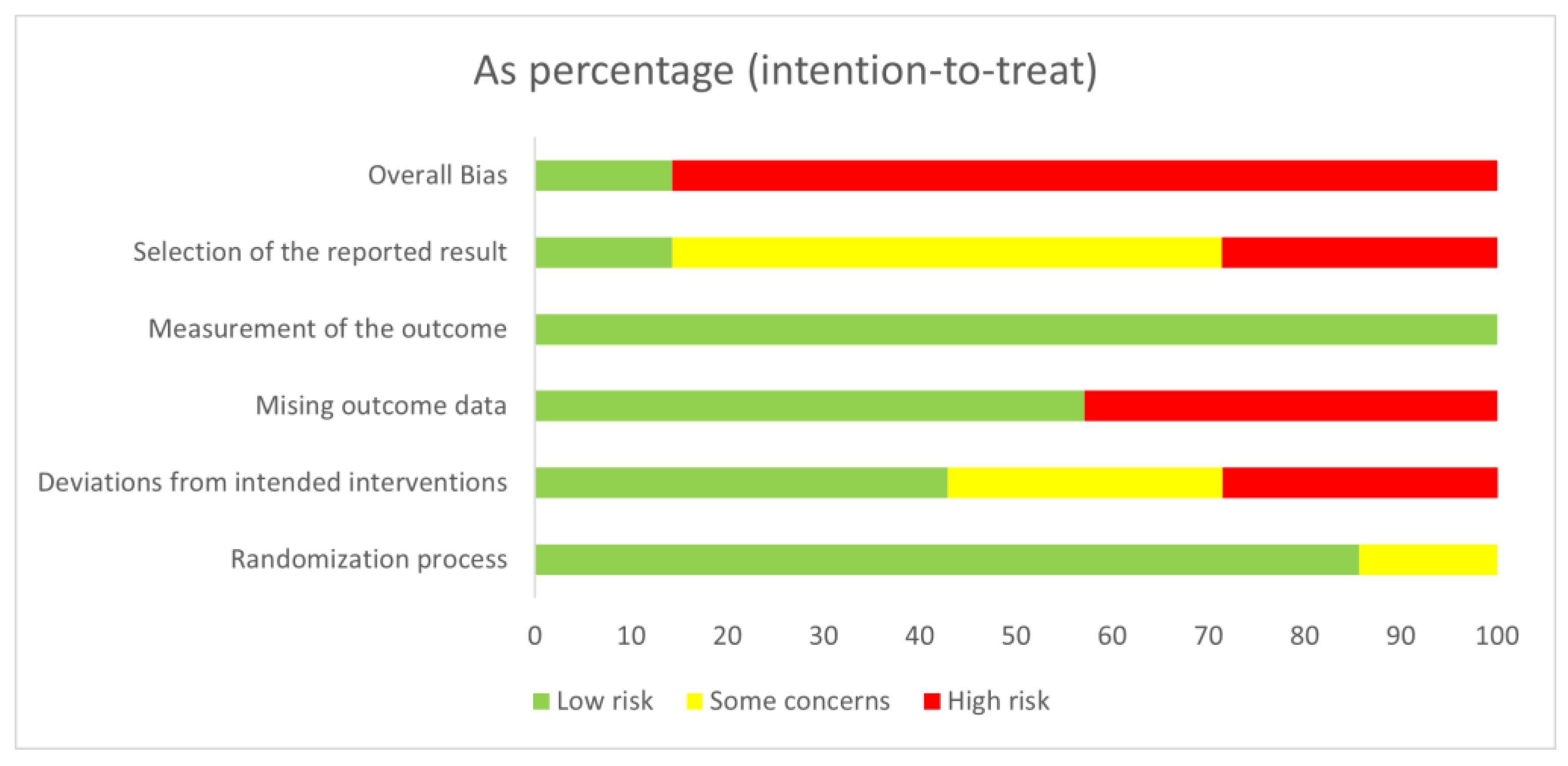
3.3. Risk of Bias Assessment
3.4. Synthesis of Results
3.4.1. Skeletal Muscle Mass: Appendicular Muscle Index
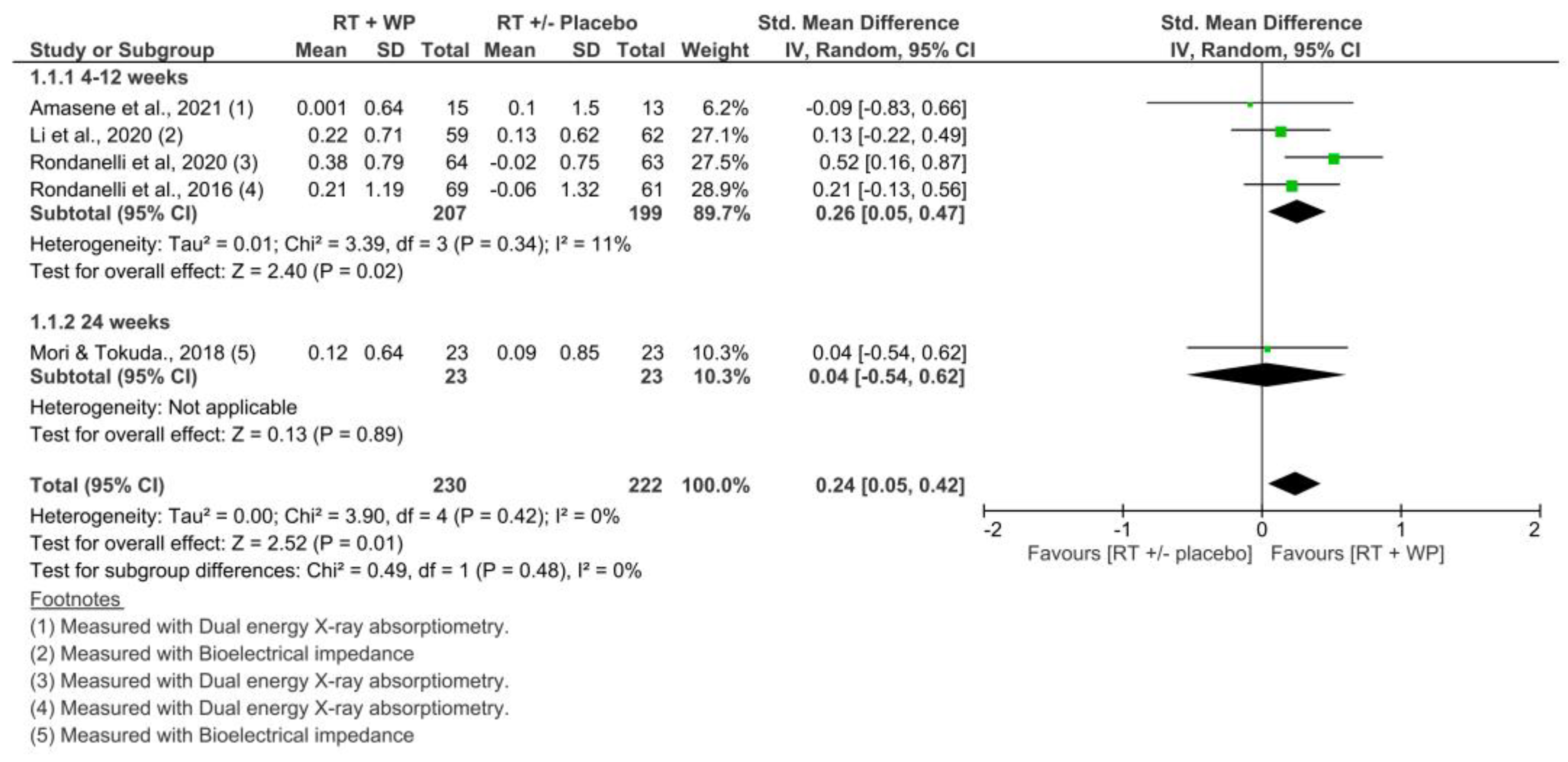
3.4.2. Skeletal Muscle Mass: Appendicular Muscle Mass
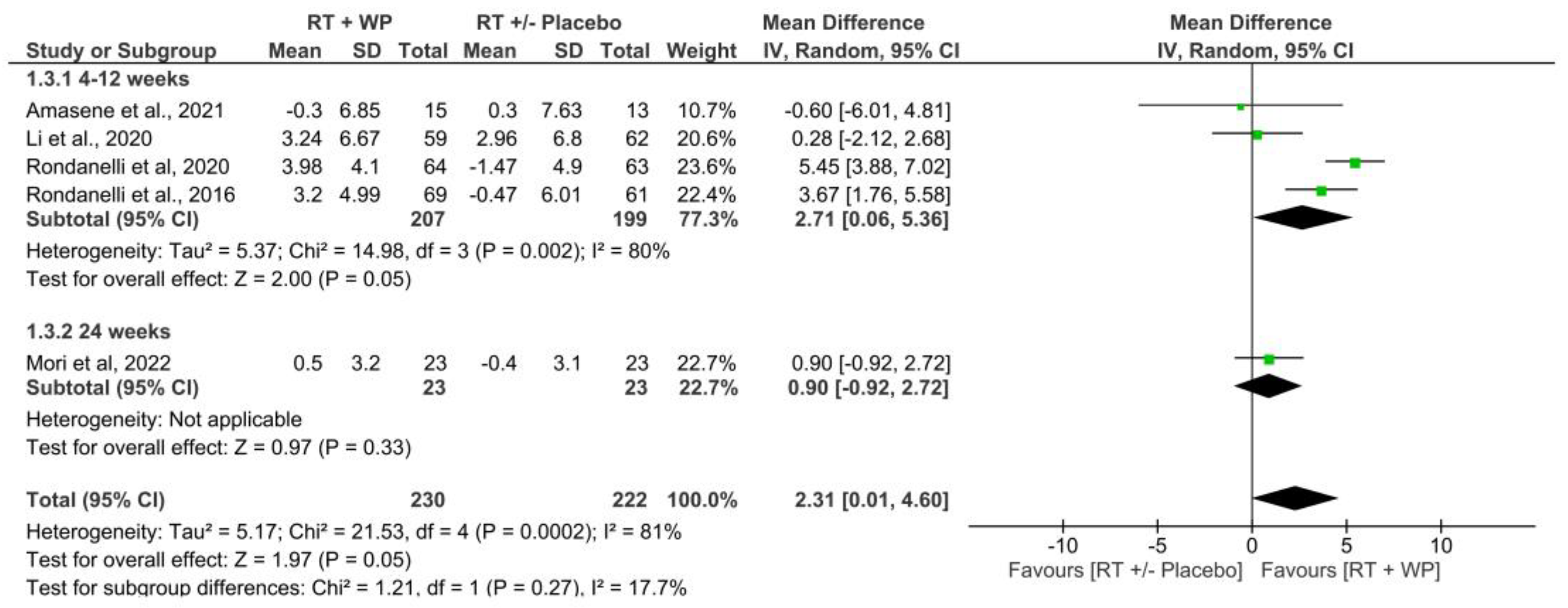
3.4.3. Muscle Strength
3.4.4. Physical Performance
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Bustos, A.; Rodríguez-Sánchez, B.; Carnicero-Carreño, J.A.; Sepúlveda-Loyola, W.; Garcia-Garcia, F.J.; Rodríguez-Mañas, L. Healthcare cost expenditures associated to frailty and sarcopenia. BMC Geriatr. 2022, 22, 747. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Tieland, M.; Verdijk, L.B.; Leenders, M.; Dirks, M.L.; van Loon Luc, J.C. There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J. Am. Med. Dir. Assoc. 2015, 16, 400–411. [Google Scholar] [CrossRef]
- Cannataro, R.; Cione, E.; Bonilla, D.A.; Cerullo, G.; Angelini, F.; D’Antona, G. Strength training in elderly: An useful tool against sarcopenia. Front. Sports Act. Living 2022, 4, 950949. [Google Scholar] [CrossRef]
- Prevett, C.; Moncion, K.; Phillips, S.M.; Richardson, J.; Tang, A. Role of Resistance Training in Mitigating Risk for Mobility Disability in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 2023–2035. [Google Scholar] [CrossRef]
- Lu, L.; Mao, L.; Feng, Y.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 708. [Google Scholar] [CrossRef]
- Carneiro, M.A.S.; Franco, C.M.C.; Silva, A.L.; Castro-E-Souza, P.; Kunevaliki, G.; Izquierdo, M.; Cyrino, E.S.; Padilha, C.S. Resistance exercise intervention on muscular strength and power, and functional capacity in acute hospitalized older adults: A systematic review and meta-analysis of 2498 patients in 7 randomized clinical trials. Geroscience 2021, 43, 2693–2705. [Google Scholar] [CrossRef]
- Marzuca-Nassr, G.N.; Alegría-Molina, A.; SanMartín-Calísto, Y.; Artigas-Arias, M.; Huard, N.; Sapunar, J.; Salazar, L.A.; Verdijk, L.B.; van Loon, L.J.C. Muscle Mass and Strength Gains Following Resistance Exercise Training in Older Adults 65–75 Years and above 85 Years. Int. J. Sport Nutr. Exerc. Metab. 2023. ahead of print. [Google Scholar]
- Cuyul-Vásquez, I.; Berríos-Contreras, L.; Soto-Fuentes, S.; Hunter-Echeverría, K.; Marzuca-Nassr, G.N. Effects of resistance exercise training on redox homeostasis in older adults. A systematic review and meta-analysis. Exp. Gerontol. 2020, 138, 111012. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Gao, Y.; Qin, L.; Feng, H.; Tan, H.; Chen, Q.; Peng, L.; Wu, I.X.Y. Comparative effectiveness of non-pharmacological interventions for frailty: A systematic review and network meta-analysis. Age Ageing 2023, 52, afad004. [Google Scholar] [CrossRef]
- Adjetey, C.; Karnon, B.; Falck, R.S.; Balasubramaniam, H.; Buschert, K.; Davis, J.C. Cost-effectiveness of exercise versus multimodal interventions that include exercise to prevent falls among community-dwelling older adults: A systematic review and meta-analysis. Maturitas 2023, 169, 16–31. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.Y.; Zhao, Y. Efficacy of Exercise on Muscle Function and Physical Performance in Older Adults with Sarcopenia: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8212. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, R.; Song, G.; Teng, J.; Shen, S.; Fu, X.; Yan, Y.; Liu, C. The Effect of Resistance Training on the Rehabilitation of Elderly Patients with Sarcopenia: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15491. [Google Scholar] [CrossRef]
- Mende, E.; Moeinnia, N.; Schaller, N.; Weiß, M.; Haller, B.; Halle, M.; Siegrist, M. Progressive machine-based resistance training for prevention and treatment of sarcopenia in the oldest old: A systematic review and meta-analysis. Exp. Gerontol. 2022, 163, 111767. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [Green Version]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.P.; Mazidi, M.; García, C.R.; Lane, K.E.; Jafari, A.; Butler, T.; de Heredia, F.P.; Davies, I.G. Protein interventions augment the effect of resistance exercise on appendicular lean mass and handgrip strength in older adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 115, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E.P.; Memelink, R.G.; Verlaan, S.; Wolfe, R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.Y.; Chang, H.Y.; Huang, Y.C.; Liu, C.W. Effect of Whey Protein Supplementation in Postmenopausal Women: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4210. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 1st ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J Phys Ther Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Santesso, N.; Glenton, C.; Dahm, P.; Garner, P.; Akl, E.A.; Alper, B.; Brignardello-Petersen, R.; Carrasco-Labra, A.; De Beer, H.; Hultcrantz, M.; et al. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 2020, 119, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef]
- Amasene, M.; Cadenas-Sanchez, C.; Echeverria, I.; Sanz, B.; Alonso, C.; Tobalina, I.; Irazusta, J.; Labayen, I.; Besga, A. Effects of Resistance Training Intervention along with Leucine-Enriched Whey Protein Supplementation on Sarcopenia and Frailty in Post-Hospitalized Older Adults: Preliminary Findings of a Randomized Controlled Trial. JCM 2021, 11, 97. [Google Scholar] [CrossRef]
- Amasene, M.; Besga, A.; Echeverria, I.; Urquiza, M.; Ruiz, J.R.; Rodriguez-Larrad, A.; Aldamiz, M.; Anaut, P.; Irazusta, J.; Labayen, I. Effects of Leucine-Enriched Whey Protein Supplementation on Physical Function in Post-Hospitalized Older Adults Participating in 12-Weeks of Resistance Training Program: A Randomized Controlled Trial. Nutrients 2019, 11, 2337. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Cui, M.; Yu, K.; Zhang, X.-W.; Li, C.-W.; Nie, X.-D.; Wang, F. Effects of nutrition supplementation and physical exercise on muscle mass, muscle strength and fat mass among sarcopenic elderly: A randomized controlled trial. Appl. Physiol. Nutr. Metab. 2021, 46, 494–500. [Google Scholar] [CrossRef]
- Nabuco, H.C.G.; Tomeleri, C.M.; Fernandes, R.R.; Sugihara, P., Jr.; Cavalcante, E.F.; Cunha, P.M.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2019, 32, 88–95. [Google Scholar] [CrossRef]
- Mori, H.; Tokuda, Y. De-Training Effects Following Leucine-Enriched Whey Protein Supplementation and Resistance Training in Older Adults with Sarcopenia: A Randomized Controlled Trial with 24 Weeks of Follow-Up. J. Nutr. Health Aging 2022, 26, 994–1002. [Google Scholar] [CrossRef]
- Chang, M.C.; Choo, Y.J. Effects of Whey Protein, Leucine, and Vitamin D Supplementation in Patients with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 521. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals with Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B.; et al. Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zou, L.; Chen, S.-T.; Bae, J.H.; Kim, D.Y.; Liu, X.; Song, W. Effects and Moderators of Exercise on Sarcopenic Components in Sarcopenic Elderly: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 649748. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J.C. Anabolic Resistance of Muscle Protein Synthesis with Aging. Exerc. Sport Sci. Rev. 2013, 41, 169. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Holwerda, A.M.; Phillips, S.M.; van Loon, L.J.C. What is the Optimal Amount of Protein to Support Post-Exercise Skeletal Muscle Reconditioning in the Older Adult? Sports Med. 2016, 46, 1205–1212. [Google Scholar] [CrossRef]
- Verlaan, S.; Maier, A.B.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults—The PROVIDE study. Clin. Nutr. 2018, 37, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Lin, C.C.; Shih, M.H.; Chen, C.D.; Yeh, S.L. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: An open-label, parallel-group study. Clin. Nutr. 2021, 40, 1323–1329. [Google Scholar] [CrossRef]
- Kemmler, W.; von Stengel, S.; Kohl, M.; Rohleder, N.; Bertsch, T.; Sieber, C.C.; Freiberger, E.; Kob, R. Safety of a Combined WB-EMS and High-Protein Diet Intervention in Sarcopenic Obese Elderly Men. Clin. Interv. Aging 2020, 15, 953–967. [Google Scholar] [CrossRef]
- Fuchs, C.J.; Kuipers, R.; Rombouts, J.A.; Brouwers, K.; Schrauwen-Hinderling, V.B.; Wildberger, J.E.; Verdijk, L.B.; van Loon, L.J. Thigh muscles are more susceptible to age-related muscle loss when compared to lower leg and pelvic muscles. Exp. Gerontol. 2023, 175, 112159. [Google Scholar] [CrossRef]
- Talar, K.; Hernández-Belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-Ibáñez, J. Benefits of Resistance Training in Early and Late Stages of Frailty and Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Clin. Med. 2021, 10, 1630. [Google Scholar] [CrossRef]
| Author and Year | Population Characteristics | Intervention Characteristics | Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample | Groups | Sarcopenia Diagnosis | Training Level and Comorbidities | Time Intervention and Context | Groups | Supplement | RET | Outcome Measure | Control | Exp | |||
| Control | Exp | Control | Exp | ||||||||||
| Rondanelli et al., 2016 [34] | N = 130 ≥65 years | N = 61 A = 80.2 ± 8.5 M = 24 W = 37 | N = 69 A = 80.7 ± 6.2 M = 29 W = 40 | DEXA < 7.26 kg/m2 for M and <5.5 kg/m2 for W. | NT. With comorbidities (NR). Without physical or cognitive impairment. | Twelve weeks. Outpatient in hospital, 5 days/week. | CG = RET + Placebo EG = RET + WP | A total of 32 g isocaloric maltodextrin once a day at 12:00 pm. | A total of 22 g WP enriched with 2.5 g essential Vitamin D and AA once a day at 12:00 pm. | CG and EG = 20 min per day; Strengthen: chair exercise, toe raises, heel, knee. Knee flexion and extension exercises used weights of 0.50 and 1.50 kg. Resistance bands were used for leg extension, knee flexion, and bicep curls. Balance and Gait exercises, tandem stance, and a tandem walk, each type of exercise 8 times, BORG Intensity 12–14. | SMM: RSMM with DEXA (kg/m2) MS: Handgrip with hand dynamometer (kg) | SMM (Δ) = −0.06 (0.21, 0.90) MS (Δ) = −0.47 (−1.07, 0, 12) | SMM (Δ) = 0.21 (0.07, 0.35) # * MS (Δ) = 3.20 (2.23, 4.18) # * |
| Amasene M. et al., 2019 [37] | N = 28 >70 years | N = 13 A = 81.7 ± 6.45 M = 6 W = 7 | N = 15 A = 82.9 ± 5.59 M = 8 W = 7 | EWGSOP | NT. With comorbidities (NR), without physical or cognitive impairment. | Twelve weeks. Inpatient and outpatient in hospital. Two non-consecutive days/week. | CG = RET + Placebo EG = RET + WP | Placebo with maltodextrin and lemon-flavored hydroxyethylcellulose after each training session. | A total of 20 g WP isolate, enriched with 3 g lemon flavor leucine, after each training session. | CG and EG = 60 min per day. Adapted based on 1RM and then gradually increased the load until reaching 70% of 1RM. Strengthening limbs, 2 set x exercise, load and RM vary by participant; exercises were also practiced to improve dynamic balance. | MS: Handgrip with hand dynamometer (kg/body mass) PP: SPPB total score | MS (post) = 0.3 (0.09) PP (post) = 10.3 (1.89) # | MS (post) = 0.4 (0.09) PP (post) = 11.3 (0.96) # |
| Nabuco H. et al., 2019 [39] | N = 26 ≥60 years | N = 13 A = 70.1 ± 3.9 W = 13 | N = 13 A = 68.0 ± 4.2 W = 13 | DEXA. Assessed body fat mass 35% combined with ALST less than <15.02 kg. | NT. Obesity, HT, DM or HLP. Without physical or cognitive alteration. | Twelve weeks. Outpatient at university, 3 alternate days/week. | CG = RET + Placebo EG = RET + WP | Placebo, after each training session. Maltodextrin only on training days. | A total of 35 g hydrolyzed WP after each training session. Only on training days. | CG and EG = Alternate conventional RET, 3 sets of 8–12 repetitions, loads adjusted individually for each exercise according to their abilities. Exercises: chest press, horizontal leg press, seated row, knee extension, preacher curl (free weights), leg curl, triceps pushdown, and seated calf raise. | MS1: Knee extension (kg). MS2: Chest press (kg) MS3: Preacher curl (kg). PP = 10-m walk test (s) | MS1 (post) = 53.4 ± 9.4 # MS2 (post) = 42.8 ± 7.1# MS3 (post) = 21.5 ± 2.9 # PP (post) = 6.8 ± 0.6 # | MS1 (post) = 51.8 # 10.9 # MS2 (post) = 44.8 ± 8.6 # MS3 (post) = 23.7 ± 4.3 # PP (post) = 6.9 ± 0.8 # |
| Rondanelli et al., 2020 [35] | N = 127 ≥65 years | N = 63 A = 81 ± 5 M = 17 W = 46 | N = 64 A = 80 ± 7 M = 26 W = 38 | EWGSOP 2010. | NT. With OA, COPD, STROKE, fracture, surgery. without physical or cognitive impairment. | Eight weeks. Outpatient in hospital, 5 days/week. | CG = RET + Placebo EG = RET + WP | Isocaloric formula 40 g flavored powder with maltodextrins. Twice a day, once at breakfast and once in the afternoon. | A total of 20 g WP enriched with 2.8 g leucine, 9 g carbohydrates, 3 g fat, 800 IU vitamin D, a mixture of vitamins, 500 mg calcium, and fibers. Twice a day, once at breakfast and once in the afternoon. | CG and EG = RET (Borg 12–14); 20 min, increased by intensity exercises up to 30 min. RET, Strengthening (5–10 min) toe raises, heel raises, knee raises, seated knee extensions, standing hip flexions, and lateral leg raises; weight-bearing ankle exercises with weights ranging from 0.5 to 1.5 kg; Resistance band leg extensions and hip flexions; double arm curls, and bicep curls. Balance, walking (5–10 min), single-leg stands, tandem stands, multi-directional weight shifts, tandem walk. | SMM1: AMM with DEXA (g). SMM2: RSMM with DEXA (kg/m2). MS: Handgrip with dynamometer (kg) PP1: SPPB total score PP2: Walk speed with 4 m test (m/s) PP3: TUG | SMM1 (Δ) = −69.4 (−843.7, 704.9) # SMM2 (Δ) = −0.02 (−0.35, 0.32) MS (Δ) = −1.47 (−2.01, −0.92) # PP1 (Δ) = 0.33 (0.19, 0.46) # PP2 (Δ) = 0.06 (0.043, 0.08) # PP3 (Δ) = −0.76 (−1.07, −0.44) | SMM1 (Δ) = 949.8 (783.7, 1115.8) # * SMM2 (Δ) = 0.38 (0.31, 0.44) # * MS (Δ) = 3.98 (3.20, 4.75) # * PP1 (Δ) = 2.6 (2.23, 2.98) # * PP2 (Δ) = 0.06 (0.43, 0.08) # PP3 (Δ) = 2.95 (2.41, 3.49) # * |
| Li Z. et al., 2020 [38] | N = 169 ≥60 years | CG1 = 51 A = 70 ± 3 M = 22 W = 29 CG2 = 37 A = 73 ± 5 M = 14 W = 23 CG3 = 48 A = 72 ± 6 M = 12 W = 21 | N = 59 EG = 33 A = 71.52 ± 5.28 M = 22 W = 37 | AWGS 2014. | NT. With DM, HT or HLP. | Twelve weeks. Outpatient in two hospital centers, 3 alternate days/week. | CG1 = WP CG2 = RET CG3 = usual care EG = RET + WP | Without supplementation or placebo. | A total of 10 g WP 3 times/day with food. EPA (300 mg), DHA (200 mg), and vitamin D3 (250 IU) in capsules, with 2 capsules × 2 times a day, 30 min after breakfast and dinner. | CG2 and EG = 30 min + 60 min walk GE = Strengthening (20 min) and slow walking (5 min) 8RM focused on limbs using dumbbells and sandbags. Outdoor activity refers to a one-hour walk with sun exposure 3 days/week on the days opposite resistance training. The speed should be more than 800 steps in 10 min. | SMM1: AMM with M-BIA (kg). SMM2: RSMM with M-BIA (kg/m2). MS: Handgrip with hand dynamometer (kg) | SMM1 (post) = 15.00 ± 3.00 SMM2 (post) = 6.09 ± 0.73 HS (post) = 23.62 ± 5.83 | SMM1(post) = 16.21 ± 3.59 * SMM2 (post) = 6.32 ± 0.84 * HS (post) = 24.83 ± 6.26 * |
| Amasene et al., 2021 [37] | N = 41 ≥70 years | N = 20 A = 81.2 ± 6.14 W = 20 | N = 21 A = 82.9 ± 5.67 W = 21 | EWGSOP 2018. | NT. With comorbidities, without physical or cognitive impairment. | Twelve weeks. Inpatient and outpatient in hospital. Two non-consecutive. | CG = RET. EG = RET + WP | Placebo with maltodextrin and lemon-flavored hydroxyethylcellulose after each training session. | A total of 20 g isolate WP enriched with 3 g leucine once daily post-exercise. | CG and EG = Supervised training, 60 min per day. Adapted based on 1RM and then gradually increased the load until reaching 70% of 1RM. | SMM1: AMM with DEXA (kg). SMM2: RSMM with DEXA (kg/m2). MS: Handgrip with hand dynamometer (kg) PP: SPPB total score | SMM1 (post) = 18.5 ± 3.6 SMM2 (post) = 7.5 ± 1.16 MS (post) = 24.5 ± 7.32 PP (post) = 10.3 ± 1.89 # | SMM1 (post) = 17.3 ± 2.78 SMM2 (post) = 6.9 ± 0.66 MS (post) = 26.6 ± 6.50 PP (post) = 11.3 ± 0.96 # |
| Mori et al., 2022 [40] | N = 70 ≥65 years | CG1 = 23 A = 77.6 ± 5.2 M = 4 W = 19 CG2 = 24 A = 77.8 ± 4.5 M = 16 W = 8 | EG = 23 A = 77.7 ± 3.3 M = 3 W = 20 | AWGS 2014. | NT. With comorbidities (NR), without physical or cognitive impairment. | Twenty-four weeks. Outpatient in hospital, 2 days/week. | CG1 = RET CG2 = WP EG = RET + WP | Without supplementation or placebo. | A total of 11 g of protein, which contains 160 kcal of energy, 2.2 g of fat, 24 g of carbohydrates, and 2300 mg of leucine per serving. It was used after 3 h of lunch. | CG1 and EG = 30–40 min. Elastic resistance band exercises, resistance exercises with body weight load 50–70% of 1RM, 2–3 sets. | SMM: RSMM with M-BIA (kg/m2). MS1: Handgrip with hand dynamometer (kg) MS2: Knee extension with hand-held dynamometer (kg) PP: Usual walking speed (m/s) | SMM (post) = 5.39 ± 0.92 # MS1 (post) = 16.8 ± 3.0 MS2 (post) = 14.6 ± 5.9 # PP (post) = 1.03 ± 0.27 | SMM (post) = 5.51 ± 0.66 # MS1 (post) = 17.6 ± 3.4 # MS2 (post) = 14.6 ± 3.0 # PP (post) = 1.03 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuyul-Vásquez, I.; Pezo-Navarrete, J.; Vargas-Arriagada, C.; Ortega-Díaz, C.; Sepúlveda-Loyola, W.; Hirabara, S.M.; Marzuca-Nassr, G.N. Effectiveness of Whey Protein Supplementation during Resistance Exercise Training on Skeletal Muscle Mass and Strength in Older People with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3424. https://doi.org/10.3390/nu15153424
Cuyul-Vásquez I, Pezo-Navarrete J, Vargas-Arriagada C, Ortega-Díaz C, Sepúlveda-Loyola W, Hirabara SM, Marzuca-Nassr GN. Effectiveness of Whey Protein Supplementation during Resistance Exercise Training on Skeletal Muscle Mass and Strength in Older People with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(15):3424. https://doi.org/10.3390/nu15153424
Chicago/Turabian StyleCuyul-Vásquez, Iván, José Pezo-Navarrete, Cristina Vargas-Arriagada, Cynthia Ortega-Díaz, Walter Sepúlveda-Loyola, Sandro Massao Hirabara, and Gabriel Nasri Marzuca-Nassr. 2023. "Effectiveness of Whey Protein Supplementation during Resistance Exercise Training on Skeletal Muscle Mass and Strength in Older People with Sarcopenia: A Systematic Review and Meta-Analysis" Nutrients 15, no. 15: 3424. https://doi.org/10.3390/nu15153424
APA StyleCuyul-Vásquez, I., Pezo-Navarrete, J., Vargas-Arriagada, C., Ortega-Díaz, C., Sepúlveda-Loyola, W., Hirabara, S. M., & Marzuca-Nassr, G. N. (2023). Effectiveness of Whey Protein Supplementation during Resistance Exercise Training on Skeletal Muscle Mass and Strength in Older People with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients, 15(15), 3424. https://doi.org/10.3390/nu15153424







