Machine Learning Prediction of Iron Deficiency Anemia in Chinese Premenopausal Women 12 Months after Sleeve Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Surgical Techniques
2.3. Definitions of Anemia and Iron Deficiency Anemia
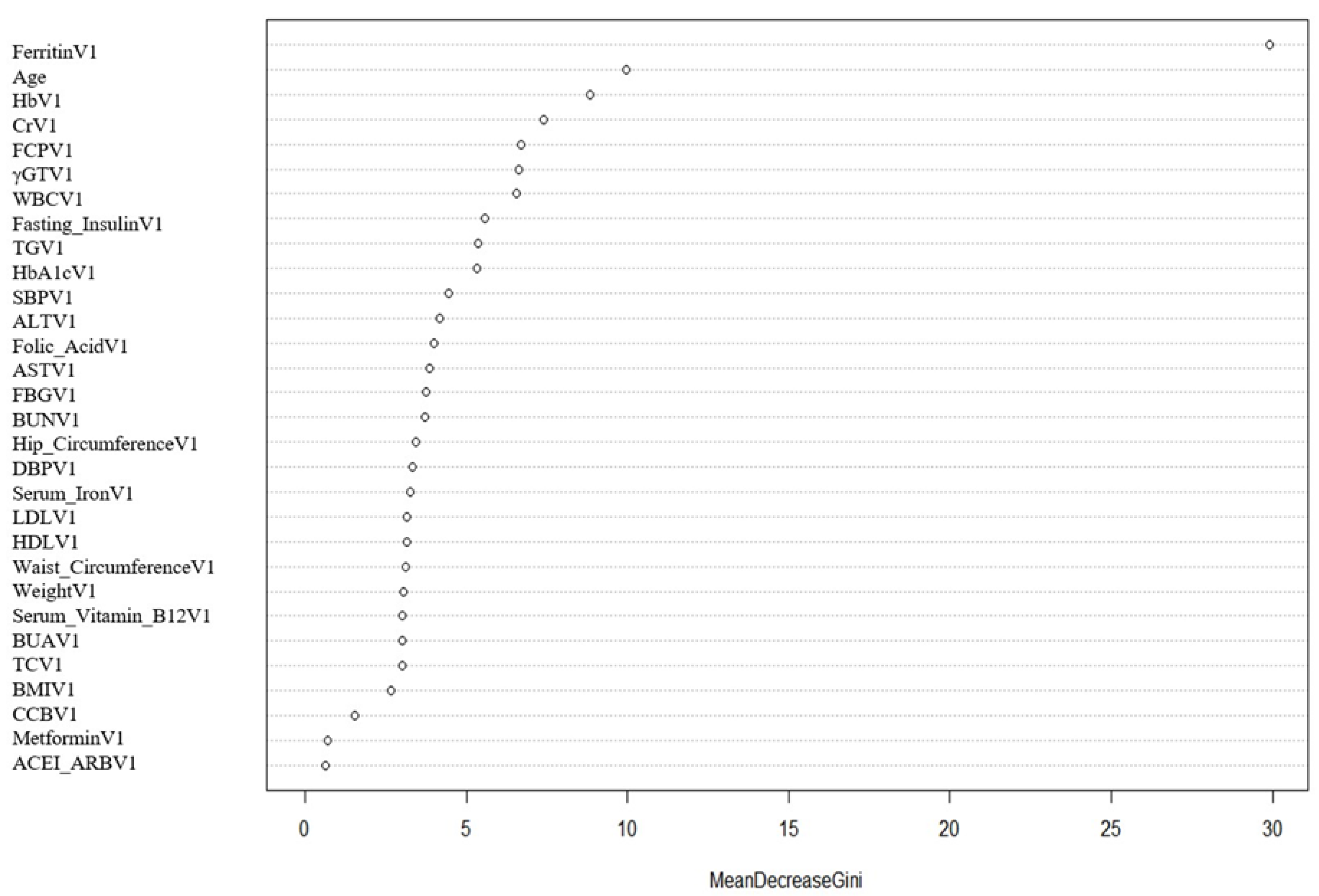
2.4. Data Pre-Processing and Feature Selection
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Subjects
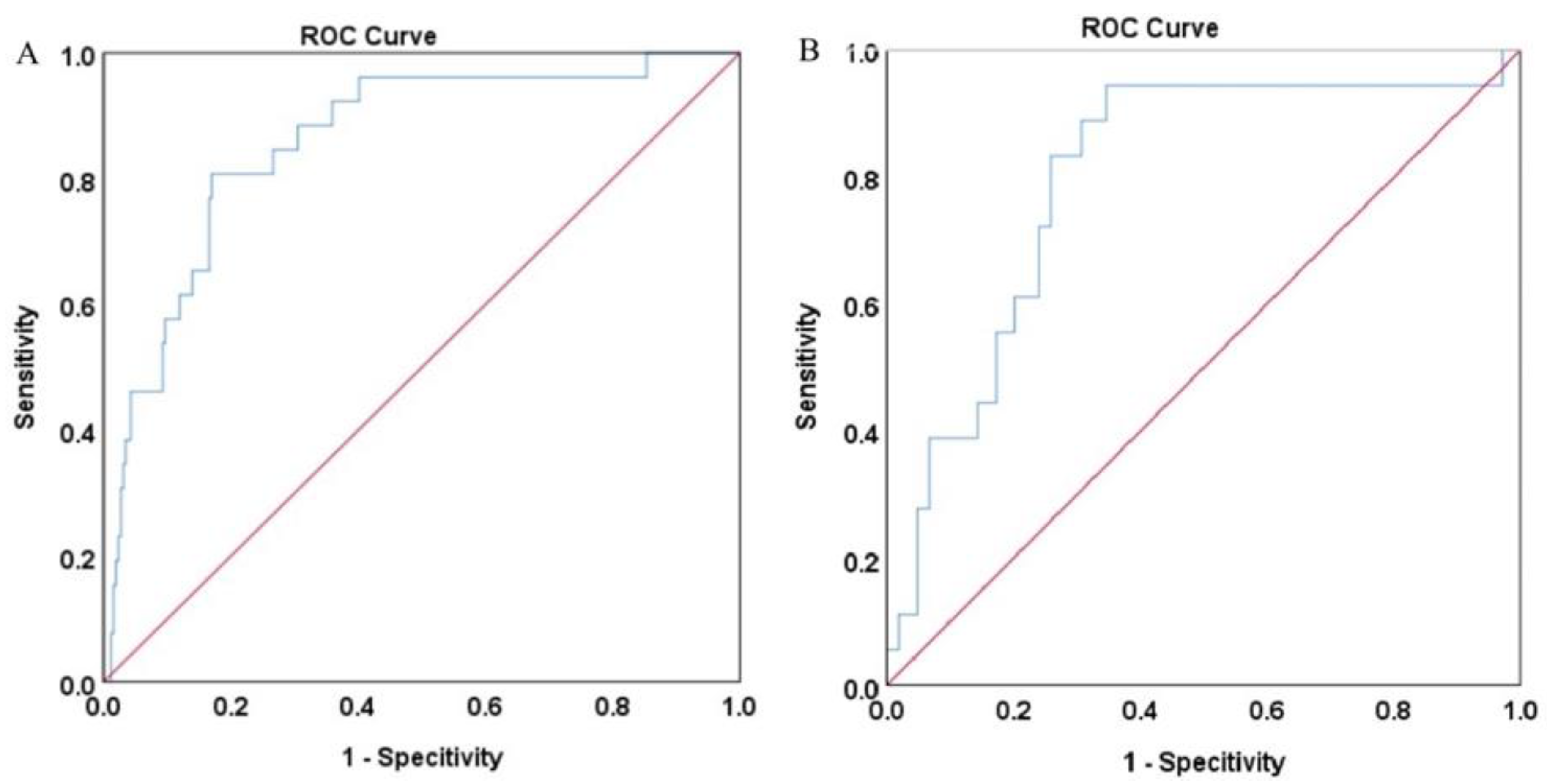
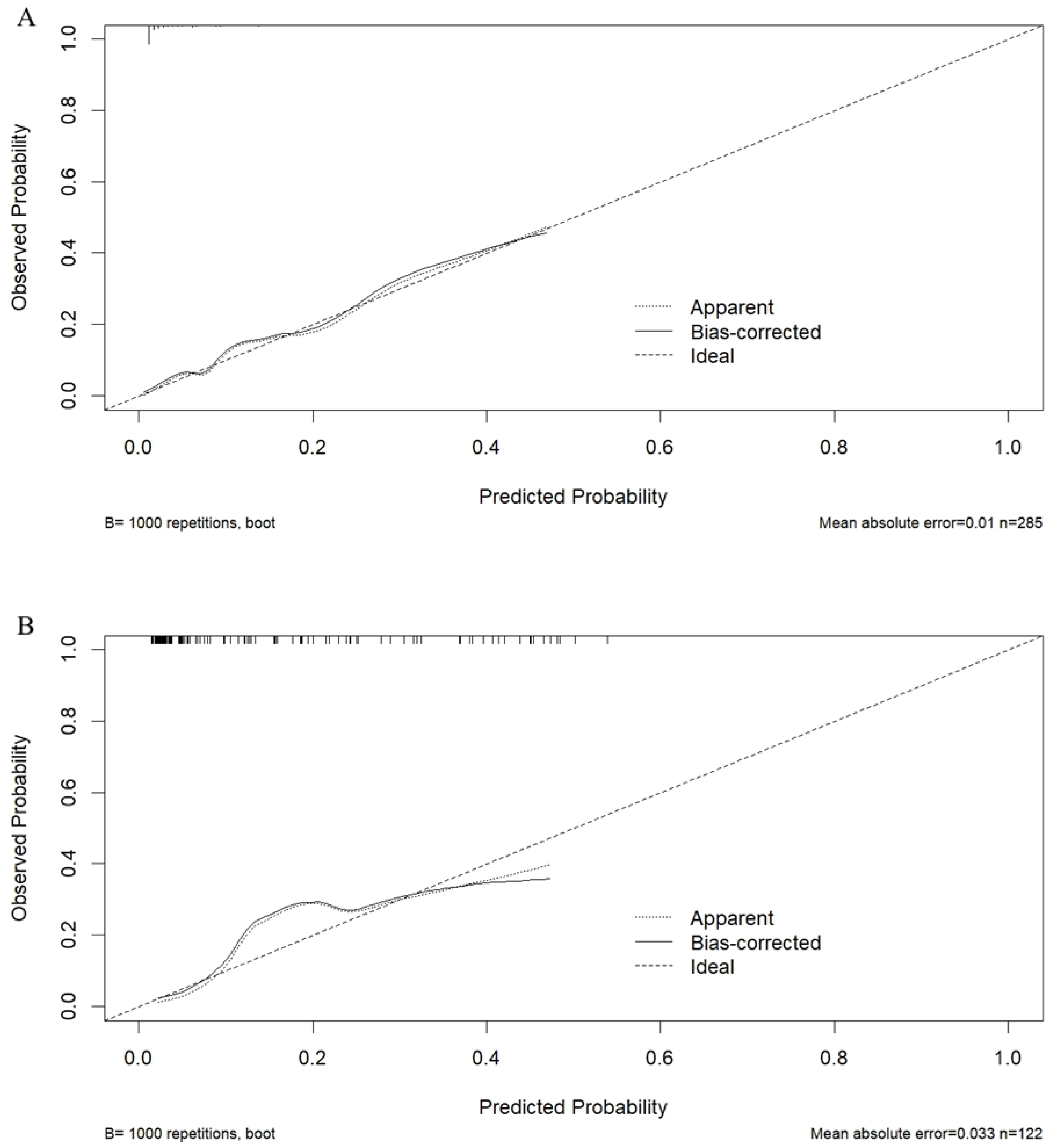
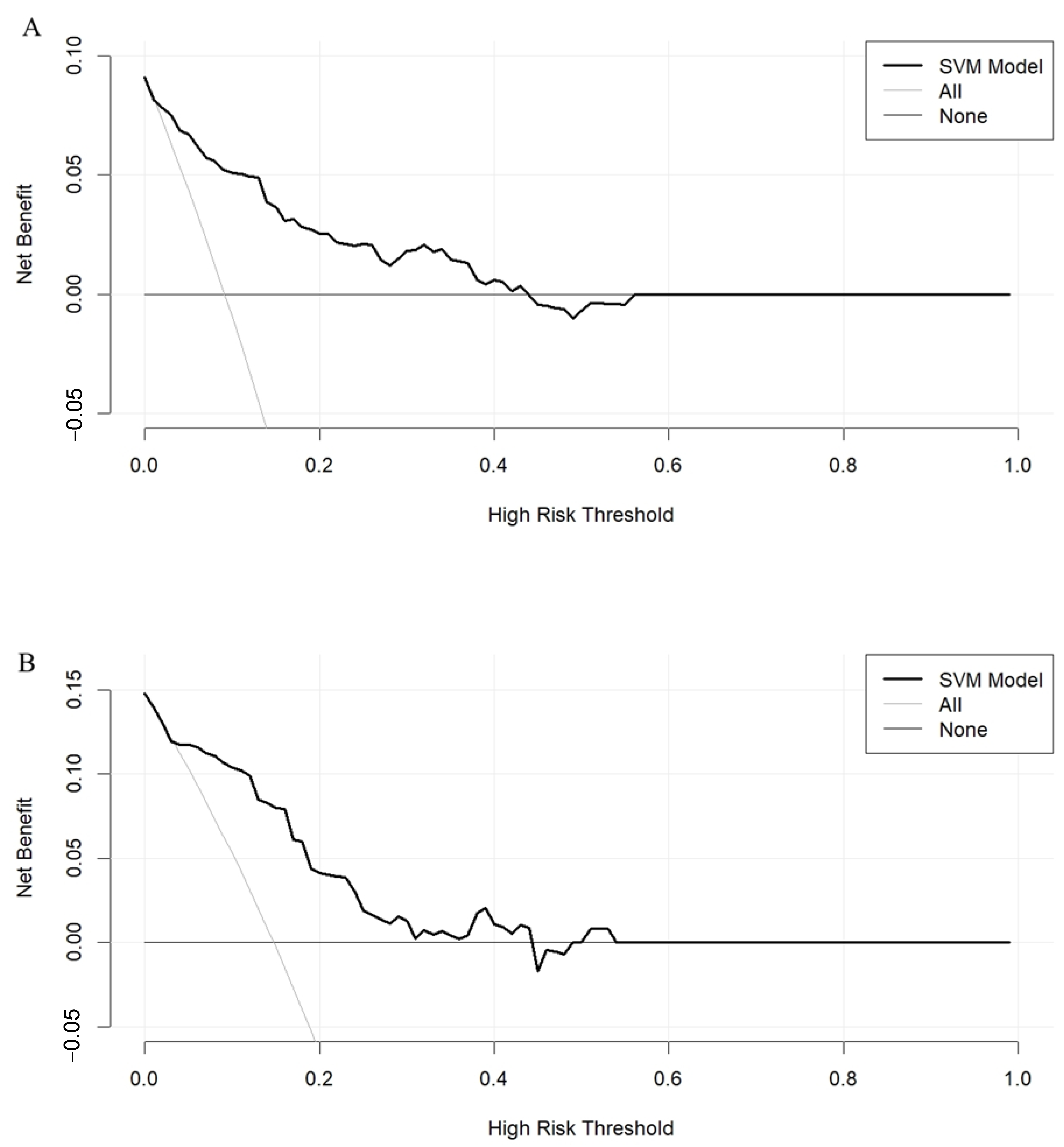
3.2. The SVM Model Construction and Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron deficiency and bariatric surgery. Nutrients 2013, 5, 1595–1608. [Google Scholar] [CrossRef]
- Obinwanne, K.M.; Fredrickson, K.A.; Mathiason, M.A.; Kallies, K.J.; Farnen, J.P.; Kothari, S.N. Incidence, treatment, and outcomes of iron deficiency after laparoscopic Roux-en-Y gastric bypass: A 10-year analysis. J. Am. Coll. Surg. 2014, 218, 246–252. [Google Scholar] [CrossRef]
- Decker, G.A.; Swain, J.M.; Crowell, M.D.; Scolapio, J.S. Gastrointestinal and nutritional complications after bariatric surgery. Am. J. Gastroenterol. 2007, 102, 2571–2580, quiz 81. [Google Scholar] [CrossRef]
- Levi, M.; Rosselli, M.; Simonetti, M.; Brignoli, O.; Cancian, M.; Masotti, A.; Pegoraro, V.; Cataldo, N.; Heiman, F.; Chelo, M.; et al. Epidemiology of iron deficiency anaemia in four European countries: A population-based study in primary care. Eur. J. Haematol. 2016, 97, 583–593. [Google Scholar] [CrossRef]
- Yu, H.; Du, R.; Zhang, N.; Zhang, M.; Tu, Y.; Zhang, L.; Bao, Y.; Han, J.; Zhang, P.; Jia, W. Iron-Deficiency Anemia After Laparoscopic Roux-en-Y Gastric Bypass in Chinese Obese Patients with Type 2 Diabetes: A 2-Year Follow-Up Study. Obes. Surg. 2016, 26, 2705–2711. [Google Scholar] [CrossRef]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Cohen, R.; Morton, J.; Ghaferi, A.; Higa, K.; Ottosson, J.; Pattou, F.; et al. Bariatric-Metabolic Surgery Utilisation in Patients with and without Diabetes: Data from the IFSO Global Registry 2015–2018. Obes. Surg. 2021, 31, 2391–2400. [Google Scholar] [CrossRef]
- Knight, T.; D’Sylva, L.; Moore, B.; Barish, C.F. Burden of Iron Deficiency Anemia in a Bariatric Surgery Population in the United States. J. Manag. Care Spec. Pharm. 2015, 21, 946–954. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Guo, X.; Xu, R.; Wang, Y.; Liu, M. Machine learning prediction of symptomatic intracerebral hemorrhage after stroke thrombolysis: A cross-cultural validation in Caucasian and Han Chinese cohort. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221129380. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning-Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Khera, R.; Haimovich, J.; Hurley, N.C.; McNamara, R.; Spertus, J.A.; Desai, N.; Rumsfeld, J.S.; Masoudi, F.A.; Huang, C.; Normand, S.L.; et al. Use of Machine Learning Models to Predict Death after Acute Myocardial Infarction. JAMA Cardiol. 2021, 6, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Sokolovska, N.; Liu, Y.; Comaneshter, D.S.; Vinker, S.; Pecht, T.; Poitou, C.; Oppert, J.M.; Bouillot, J.L.; Genser, L.; et al. The advanced-DiaRem score improves prediction of diabetes remission 1 year post-Roux-en-Y gastric bypass. Diabetologia 2017, 60, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Sriprasert, I.; Pakrashi, T.; Kimble, T.; Archer, D.F. Heavy menstrual bleeding diagnosis and medical management. Contracept. Reprod. Med. 2017, 2, 20. [Google Scholar] [CrossRef]
- Straatman, J.; Verhaak, T.; Demirkiran, A.; Harlaar, N.J.; Cense, H.A.; Jonker, F.H.W.; Dutch Audit for Treatment of Obesity (DATO) Research Group. Risk factors for postoperative bleeding in bariatric surgery. Surg. Obes. Relat. Dis. 2022, 18, 1057–1065. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Di, J.; Wang, C.; Zhang, P.; Han, X.; Liu, W.; Zhang, H. The middle-term result of laparoscopic sleeve gastrectomy in Chinese obesity patients in a single hospital, with the review of literatures and strategy for gastric stenosis. Ann. Transl. Med. 2018, 6, 479. [Google Scholar] [CrossRef]
- Bermejo, F.; García-López, S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J. Gastroenterol. 2009, 15, 4638–4643. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Gowanlock, Z.; Lezhanska, A.; Conroy, M.; Crowther, M.; Tiboni, M.; Mbuagbaw, L.; Siegal, D.M. Iron deficiency following bariatric surgery: A retrospective cohort study. Blood Adv. 2020, 4, 3639–3647. [Google Scholar] [CrossRef]
- Kwon, Y.; Ha, J.; Lee, Y.H.; Kim, D.; Lee, C.M.; Kim, J.H.; Park, S. Comparative risk of anemia and related micronutrient deficiencies after Roux-en-Y gastric bypass and sleeve gastrectomy in patients with obesity: An updated meta-analysis of randomized controlled trials. Obes. Rev. 2022, 23, e13419. [Google Scholar] [CrossRef]
- Nie, Y.; Tian, Z.; Wang, P.; Liu, B.; Zhang, N.; Zhou, B.; Wang, S.; Hei, X.; Meng, H. Prevalence of anemia and related nutrient deficiencies after sleeve gastrectomy: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13516. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell FEJr Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- McCracken, E.; Wood, G.C.; Prichard, W.; Bistrian, B.; Still, C.; Gerhard, G.; Rolston, D.; Benotti, P. Severe anemia after Roux-en-Y gastric bypass: A cause for concern. Surg. Obes. Relat. Dis. 2018, 14, 902–909. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, T.S.; Lee, W.J.; Lin, Y.C.; Lee, C.K.; Liew, P.L. Predictors of anemia after bariatric surgery using multivariate adaptive regression splines. Hepatogastroenterology 2012, 59, 1378–1380. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Elazary, R.; Sherf-Dagan, S.; Weiss, R.; Levin, G.; Rottenstreich, M.; Sakran, N.; Rottenstreich, A. Factors Associated with the Development of Anemia During Pregnancy After Sleeve Gastrectomy. Obes. Surg. 2020, 30, 3884–3890. [Google Scholar] [CrossRef]
- Bailly, L.; Schiavo, L.; Sebastianelli, L.; Fabre, R.; Pradier, C.; Iannelli, A. Anemia and Bariatric Surgery: Results of a National French Survey on Administrative Data of 306,298 Consecutive Patients Between 2008 and 2016. Obes. Surg. 2018, 28, 2313–2320. [Google Scholar] [CrossRef]
- Wang, T.Y.; Huang, H.H.; Hsieh, M.S.; Chen, C.Y. Risk of anemia in morbidly obese patients after bariatric surgery in Taiwan. World J. Diabetes 2020, 11, 447–458. [Google Scholar] [CrossRef]
- Chung, J.O.; Park, S.Y.; Cho, D.H.; Chung, D.J.; Chung, M.Y. Anemia is inversely associated with serum C-peptide concentrations in individuals with type 2 diabetes. Medicine 2018, 97, e11783. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Ikeda-Taniguchi, M.; Takahashi, K.; Shishido, K.; Honda, H. Total iron binding capacity is a predictor for muscle loss in maintenance hemodialysis patients. Clin. Exp. Nephrol. 2022, 26, 583–592. [Google Scholar] [CrossRef]
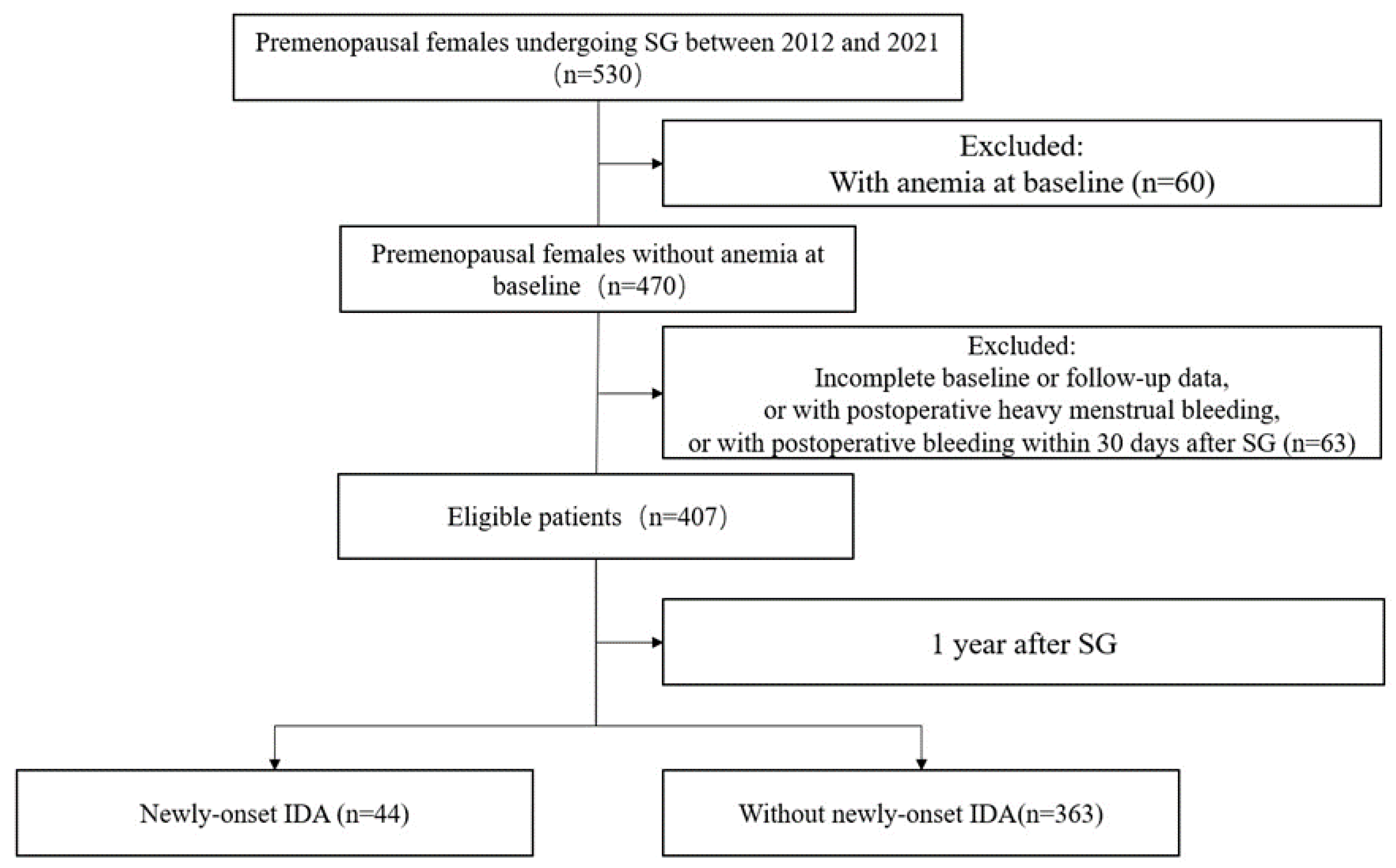

| All Patients (n = 407) | Training Set (n = 285) | Validation Set (n = 122) | |
|---|---|---|---|
| Median follow-up (months) | 12 (7, 13) | 12 (7, 13) | 12 (7, 13) |
| Newly onset IDA (N, %) | 44 (10.8) | 26 (9.1) | 18 (14.8) |
| T2DM (N, %) | 146 (35.9) | 107 (37.5) | 39 (32.0) |
| Hypertension (N, %) | 96 (23.6) | 69 (24.2) | 27 (22.1) |
| Age (y) | 31.0 (26.0, 36.0) | 31.0 (26.5, 36.0) | 31.0 (25.8, 37.0) |
| Weight (kg) | 100.0 (89.0, 113.5) | 100.0 (89.4, 113.0) | 100.5 (88.9, 114.5) |
| BMI (kg/m2) | 37.0 (33.3, 41.3) | 36.7 (33.4, 41.5) | 37.5 (33.3, 41.0) |
| Waist circumference (cm) | 112.0 (103.0, 123.0) | 112.0 (103.0, 123.0) | 112.0 (103.4, 124.0) |
| Hip circumference (cm) | 116.0 (108.0,124.0) | 116.0 (108.0, 124.0) | 117.0 (108.8, 123.0) |
| SBP (mmHg) | 130.0 (120.0, 140.0) | 129.0 (120.0, 140.0) | 130.0 (120.0, 139.3) |
| DBP (mmHg) | 84.0 (78.0, 92.0) | 85.0 (78.0, 94.0) | 84.0 (77.8, 90.0) |
| WBC (×109/L) | 7.9 (6.7, 9.3) | 8.0 (7.0, 9.4) | 7.7 (6.6, 9.1) |
| Hb (g/L) | 136.0 (130.0, 142.0) | 137.0 (130.5, 143.0) | 135.0 (129.0, 140.3) |
| ALT (U/L) | 34.0 (22.0, 63.0) | 35.0 (22.0, 60.5) | 33.5 (22.0, 64.3) |
| AST (U/L) | 24.0 (17.0, 38.0) | 23.0 (18.0, 41.0) | 24.0 (16.8, 35.3) |
| γ-GT (U/L) | 34.0 (23.0, 53.0) | 35.0 (23.0, 58.0) | 34.0 (23.0, 46.3) |
| BUN (mmol/L) | 4.6 (3.9, 5.5) | 4.6 (3.9, 5.5) | 4.6 (3.8, 5.4) |
| Cr (μmol/L) | 54.0 (47.9, 61.0) | 54.0 (47.7, 61.0) | 54.0 (48.0, 60.2) |
| BUA (μmol/L) | 385.0 (329.0, 445.0) | 389.0 (327.5, 450.5) | 379.5 (333.8, 432.0) |
| TC (mmol/L) | 5.1 (4.5, 5.9) | 5.2 (4.5, 5.8) | 5.1 (4.5, 5.9) |
| TG (mmol/L) | 1.5 (1.1, 2.2) | 1.5 (1.2, 2.2) | 1.4 (1.1, 2.2) |
| HDL-c (mmol/L) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) |
| LDL-c (mmol/L) | 3.2 (2.7, 3.8) | 3.2 (2.7, 3.8) | 3.2 (2.7, 3.8) |
| FPG (mmol/L) | 5.6 (5.0, 7.0) | 5.6 (5.0, 7.2) | 5.6 (5.0, 6.6) |
| HbA1c (%) | 5.8 (5.4, 6.8) | 5.9 (5.4, 6.9) | 5.7 (5.4, 6.6) |
| Fasting insulin (mIU/L) | 27.1 (18.8, 40.3) | 26.5 (18.2, 41.2) | 28.3 (20.4, 37.1) |
| Fasting C-peptide (ng/mL) | 4.0 (3.2, 5.0) | 3.9 (3.2, 5.0) | 4.1 (3.2, 4.9) |
| Serum folic acid (μg/L) | 7.2 (4.7, 10.4) | 6.9 (4.7, 10.3) | 7.8 (4.7, 10.9) |
| Serum vitamin B12 (ng/L) | 530.9 (410.1, 669.8) | 531.0 (409.0, 660.8) | 527.8 (409.6, 683.4) |
| Serum iron (μmol/L) | 14.4 (11.5, 18.8) | 14.6 (11.7, 19.0) | 14.1 (11.0, 18.5) |
| Ferritin (ng/mL) | 106.8 (60.0, 175.4) | 109.8 (60.5, 174.9) | 103.7 (57.1, 176.2) |
| Antidiabetic agents (N, %) | |||
| Sulfonylurea | 16 (3.9) | 12 (4.2) | 4 (3.3) |
| Metformin | 69 (17.0) | 47 (16.5) | 22 (18.0) |
| α-glucosidase inhibitors | 18 (4.4) | 14 (4.9) | 4 (3.3) |
| Thiazolidinediones | 7 (1.7) | 4 (1.4) | 3 (2.5) |
| DPP-IV inhibitors | 5 (1.2) | 4 (1.4) | 1 (0.8) |
| SGLT-2 inhibitors | 7 (1.7) | 3 (1.1) | 4 (3.3) |
| Insulin | 28 (6.9) | 20 (7.0) | 8 (6.6) |
| GLP-1RAs | 13 (3.2) | 10 (3.5) | 3 (2.5) |
| Antihypertensive agents (N, %) | |||
| ACEI/ARB | 34 (8.4) | 23 (8.1) | 11 (9.0) |
| β-blockers | 12 (2.9) | 8 (2.8) | 4 (3.3) |
| CCB | 34 (8.4) | 22 (7.7) | 12 (9.8) |
| Diuretics | 4 (1.0) | 2 (0.7) | 2 (1.6) |
| Lipid-lowering agents (N, %) | |||
| Statins | 7 (1.7) | 3 (1.1) | 4 (3.3) |
| Fibrates | 8 (2.0) | 5 (1.8) | 3 (2.5) |
| Normal (n = 259) | Newly Onset IDA (n = 26) | p between Groups | |
|---|---|---|---|
| T2DM (N, %) | 98 (37.8) | 9 (34.6) | 0.746 |
| Hypertension (N, %) | 60 (23.2) | 9 (34.6) | 0.194 |
| Age (y) | 31.0 (26.0, 36.0) | 33.5 (29.5, 36.0) | 0.191 |
| Weight (kg) | 101.5 (90.0, 113.0) | 94.7 (87.1, 113.8) | 0.279 |
| BMI (kg/m2) | 36.9 (33.3, 41.8) | 35.9 (33.3, 40.2) | 0.440 |
| Waist circumference (cm) | 113.0 (103.0, 123.0) | 110.0 (101.8, 121.5) | 0.549 |
| Hip circumference (cm) | 117.0 (108.0, 124.0) | 112.0 (108.3, 121.5) | 0.295 |
| SBP (mmHg) | 130.0 (120.0, 140.0) | 122.5 (113.0, 132.8) | 0.032 |
| DBP (mmHg) | 86.0 (78.0, 94.0) | 80.0 (72.3, 90.5) | 0.182 |
| WBC (×109/L) | 8.1 (7.0, 9.5) | 7.5 (6.0, 8.5) | 0.048 |
| Hb (g/L) | 137.0 (131.0, 144.0) | 131.0 (125.0,142.0) | 0.021 |
| ALT (U/L) | 35.0 (23.0, 61.0) | 32.0 (17.5, 63.8) | 0.513 |
| AST (U/L) | 24.0 (18.0, 41.0) | 22.0 (17.5, 46.8) | 0.636 |
| γ-GT (U/L) | 35.0 (24.0, 59.0) | 27.0 (21.0, 53.3) | 0.075 |
| BUN (mmol/L) | 4.7 (4.0, 5.5) | 4.3 (3.6, 5.3) | 0.101 |
| Cr (μmol/L) | 54.5 (48.0, 62.0) | 49.5 (46.0, 55.2) | 0.006 |
| BUA (μmol/L) | 391.0 (331.0, 455.0) | 326.0 (303.0, 412.5) | 0.005 |
| TC (mmol/L) | 5.2 (4.5, 5.8) | 5.1 (4.5, 6.4) | 0.940 |
| TG (mmol/L) | 1.5 (1.1, 2.2) | 1.7 (1.2, 2.5) | 0.471 |
| HDL-c (mmol/L) | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.4) | 0.975 |
| LDL-c (mmol/L) | 3.2 (2.7, 3.8) | 3.3 (2.8, 3.7) | 0.755 |
| FPG (mmol/L) | 5.6 (5.0, 7.3) | 5.4 (4.9, 7.2) | 0.712 |
| HbA1c (%) | 6.0 (5.4, 7.1) | 5.5 (5.3, 6.3) | 0.016 |
| Fasting insulin (mIU/L) | 27.5 (18.8, 42.0) | 17.6 (13.8, 28.5) | 0.013 |
| Fasting C-peptide (ng/mL) | 4.0 (3.3, 5.1) | 3.2 (2.4, 4.2) | 0.009 |
| Serum folic acid (μg/L) | 6.8 (4.6, 10.3) | 8.1 (5.2, 10.5) | 0.540 |
| Serum vitamin B12 (ng/L) | 531.0 (410.1, 657.5) | 551.0 (405.3, 740.3) | 0.411 |
| Serum iron (μmol/L) | 14.8 (11.8, 18.9) | 12.3 (8.2, 20.5) | 0.104 |
| Ferritin (ng/mL) | 115.6 (71.3, 196.5) | 41.9 (29.0, 67.1) | <0.001 |
| Antidiabetic agents (N, %) | |||
| Sulfonylurea | 12 (4.6) | 0 (0) | 0.610 |
| Metformin | 44 (17.0) | 3 (11.5) | 0.662 |
| α-glucosidase inhibitors | 14 (5.4) | 0 (0) | 0.625 |
| Thiazolidinediones | 4 (1.5) | 0 (0) | 1.000 |
| DPP-IV inhibitors | 4 (1.5) | 0 (0) | 1.000 |
| SGLT-2 inhibitors | 3 (1.2) | 0 (0) | 1.000 |
| Insulin | 18 (6.9) | 2 (7.7) | 1.000 |
| GLP-1RAs | 9 (3.5) | 1 (3.8) | 1.000 |
| Antihypertensive agents (N, %) | |||
| ACEI/ARB | 20 (7.7) | 3 (11.5) | 0.762 |
| β-blockers | 6 (2.3) | 2 (7.7) | 0.337 |
| CCB | 20 (7.7) | 2 (7.7) | 1.000 |
| Diuretics | 1 (0.4) | 1 (3.8) | 0.434 |
| Lipid-lowering agents (N, %) | |||
| Statins | 2 (0.8) | 1 (3.8) | 0.648 |
| Fibrates | 5 (1.9) | 0 (0) | 1.000 |
| Variables | Importance |
|---|---|
| Ferritin | 0.86 |
| Hb | 0.69 |
| Age | 0.68 |
| Fasting C-peptide | 0.64 |
| Cr | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Du, R.; Han, X.; Zhu, W.; Peng, D.; Tu, Y.; Han, J.; Bao, Y.; Yu, H. Machine Learning Prediction of Iron Deficiency Anemia in Chinese Premenopausal Women 12 Months after Sleeve Gastrectomy. Nutrients 2023, 15, 3385. https://doi.org/10.3390/nu15153385
Pan Y, Du R, Han X, Zhu W, Peng D, Tu Y, Han J, Bao Y, Yu H. Machine Learning Prediction of Iron Deficiency Anemia in Chinese Premenopausal Women 12 Months after Sleeve Gastrectomy. Nutrients. 2023; 15(15):3385. https://doi.org/10.3390/nu15153385
Chicago/Turabian StylePan, Yunhui, Ronghui Du, Xiaodong Han, Wei Zhu, Danfeng Peng, Yinfang Tu, Junfeng Han, Yuqian Bao, and Haoyong Yu. 2023. "Machine Learning Prediction of Iron Deficiency Anemia in Chinese Premenopausal Women 12 Months after Sleeve Gastrectomy" Nutrients 15, no. 15: 3385. https://doi.org/10.3390/nu15153385
APA StylePan, Y., Du, R., Han, X., Zhu, W., Peng, D., Tu, Y., Han, J., Bao, Y., & Yu, H. (2023). Machine Learning Prediction of Iron Deficiency Anemia in Chinese Premenopausal Women 12 Months after Sleeve Gastrectomy. Nutrients, 15(15), 3385. https://doi.org/10.3390/nu15153385





