Effect of Alcohol Consumption Habits on Early Arterial Aging in Subjects with Metabolic Syndrome and Elevated Serum Uric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluation of the Study Population
2.3. Statistical Analysis
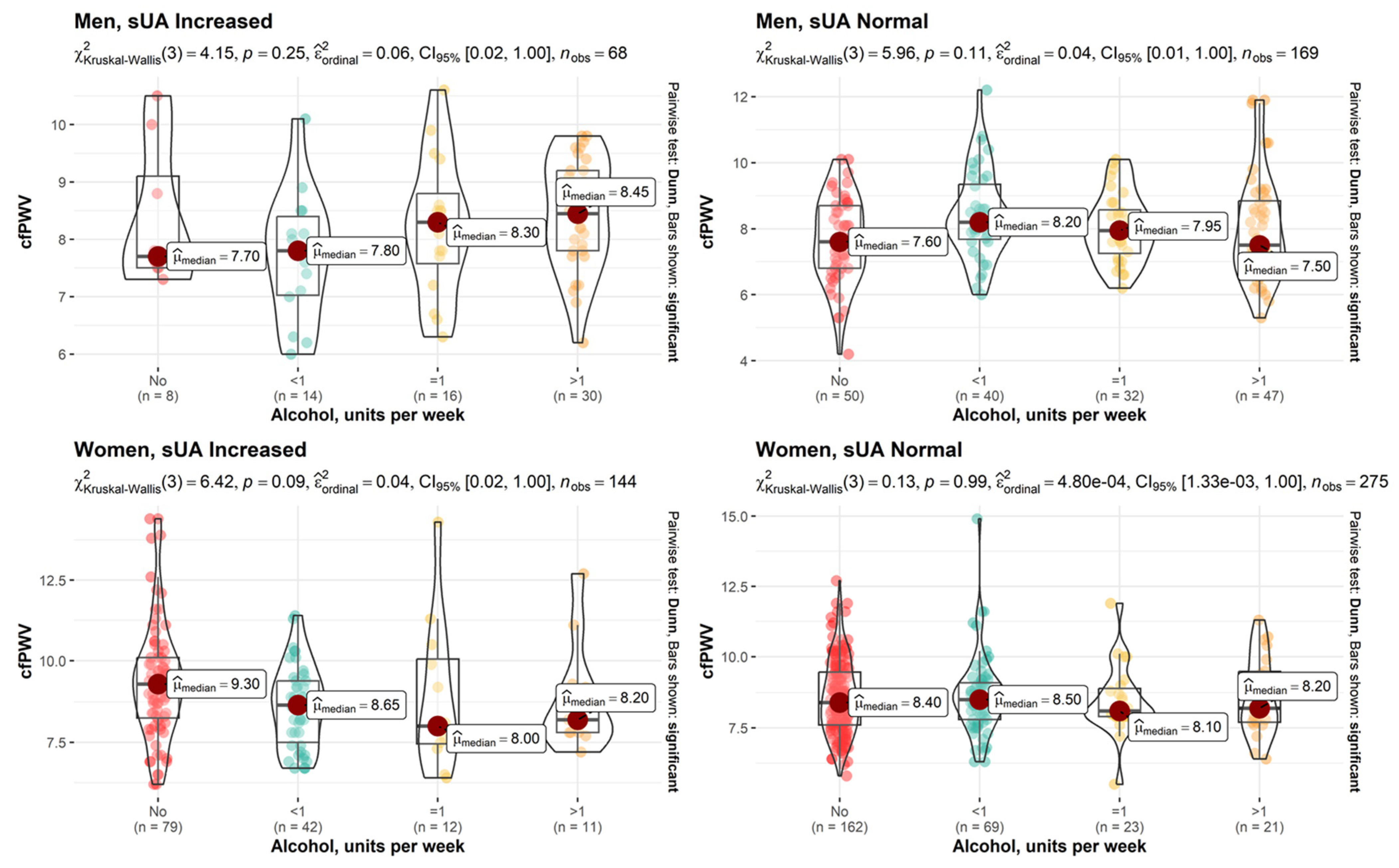
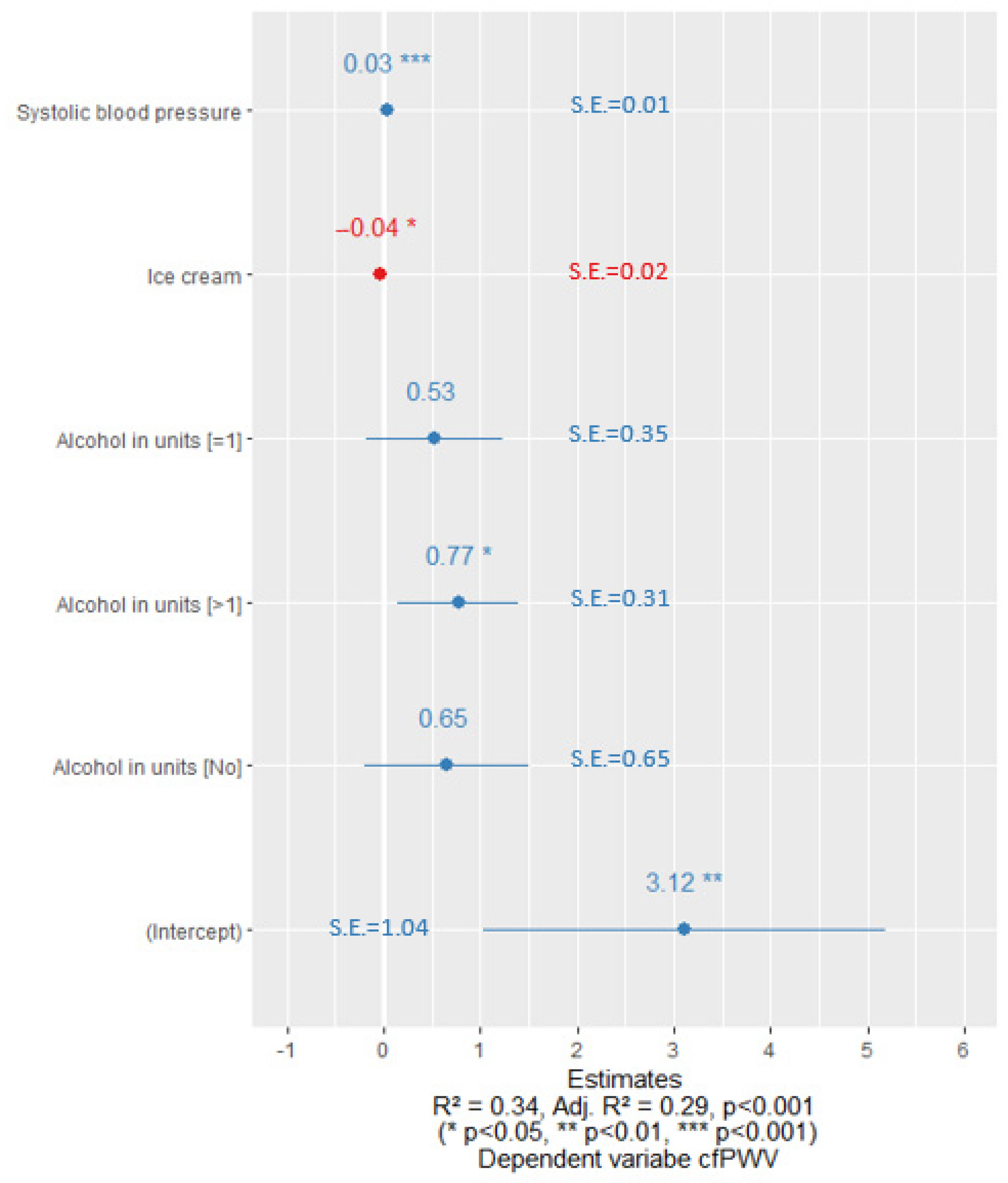
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Cachón-Suárez, I.; Garcia-Martín, C.; Short, A.; Bataller, R.; Muga, R. Prevalence and Associations of Metabolic Syndrome in Patients with Alcohol Use Disorder. Sci. Rep. 2022, 12, 2625. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Prejbisz, A.; Kuryłowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic Syndrome—A New Definition and Management Guidelines. Arch. Med. Sci. 2022, 18, 1133–1156. [Google Scholar] [CrossRef]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160. [Google Scholar] [CrossRef]
- Nagahama, K.; Inoue, T.; Kohagura, K.; Ishihara, A.; Kinjo, K.; Ohya, Y. Hyperuricemia Predicts Future Metabolic Syndrome: A 4-Year Follow-up Study of a Large Screened Cohort in Okinawa, Japan. Hypertens. Res. 2014, 37, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.; Shao, X.; Guo, J.; Liu, X.; Liu, A.; Zhang, Y.; Wang, H.; Li, B.; Deng, K.; et al. Association of Uric Acid with Metabolic Syndrome in Men, Premenopausal Women and Postmenopausal Women. Int. J. Environ. Res. Public Health 2014, 11, 2899–2910. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Choi, H.K. The Role of Diet in Hyperuricemia and Gout. Curr. Opin. Rheumatol. 2021, 33, 135–144. [Google Scholar] [CrossRef]
- Lubawy, M.; Formanowicz, D. High-Fructose Diet–Induced Hyperuricemia Accompanying Metabolic Syndrome–Mechanisms and Dietary Therapy Proposals. Int. J. Environ. Res. Public Health 2023, 20, 3596. [Google Scholar] [CrossRef]
- Li, K.; Li, K.; Yao, Q.; Shui, X.; Zheng, J.; He, Y.; Lei, W. The Potential Relationship of Coronary Artery Disease and Hyperuricemia: A Cardiometabolic Risk Factor. Heliyon 2023, 9, e16097. [Google Scholar] [CrossRef] [PubMed]
- Reustle, A.; Torzewski, M. Role of P38 MAPK in Atherosclerosis and Aortic Valve Sclerosis. Int. J. Mol. Sci. 2018, 19, 3761. [Google Scholar] [CrossRef] [PubMed]
- Kırça, M.; Oğuz, N.; Çetin, A.; Uzuner, F.; Yeşilkaya, A. Uric Acid Stimulates Proliferative Pathways in Vascular Smooth Muscle Cells through the Activation of P38 MAPK, P44/42 MAPK and PDGFRβ. J. Recept. Signal Transduct. 2017, 37, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.; Zhang, M.; Zhou, C.; Lin, N. Uric Acid Enhances PKC-Dependent ENOS Phosphorylation and Mediates Cellular ER Stress: A Mechanism for Uric Acid-Induced Endothelial Dysfunction. Int. J. Mol. Med. 2016, 37, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric Acid Stimulates Vascular Smooth Muscle Cell Proliferation and Oxidative Stress via the Vascular Renin–Angiotensin System. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.; Elsaid, K.A. Protein Phosphatase 2A Regulates Xanthine Oxidase-Derived ROS Production in Macrophages and Influx of Inflammatory Monocytes in a Murine Gout Model. Front. Pharmacol. 2022, 13, 1033520. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xie, Y.; Hao, Z.; Bi, H.; Liu, Y.; Yang, X.; Xia, Y. Protective Effect of Uric Acid on Ox-LDL-Induced HUVECs Injury via Keap1-Nrf2-ARE Pathway. J. Immunol. Res. 2021, 2021, 5151168. [Google Scholar] [CrossRef] [PubMed]
- Lubawy, M.; Blacha, A.; Nowicki, M.; Deja, T.; Wałkowski, K.; Formanowicz, D. Ghrelin and Leptin among Patients with Urolithiasis with Concomitant Hyperuricemia and Metabolic Syndrome. Biomedicines 2023, 11, 285. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Y.; Shi, Y.; Song, N.; Fang, Y.; Ding, X. Long-Term Drinking Behavior Change Patterns and Its Association with Hyperuricemia in Chinese Adults: Evidence from China Health and Nutrition Survey. BMC Public Health 2022, 22, 1230. [Google Scholar] [CrossRef]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Alcohol Intake and Risk of Incident Gout in Men: A Prospective Study. Lancet 2004, 363, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Neves, M.C.; Freire, M.G. Towards the Use of Adsorption Methods for the Removal of Purines from Beer. Molecules 2021, 26, 6460. [Google Scholar] [CrossRef]
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural Compounds Lower Uric Acid Levels and Hyperuricemia: Molecular Mechanisms and Prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Mellinger, J.L. Epidemiology of Alcohol Use and Alcoholic Liver Disease. Clin. Liver Dis. 2019, 13, 136–139. [Google Scholar] [CrossRef]
- Rovira, P.; Belian, G.; Ferreira-Borges, C.; Kilian, C.; Neufeld, M.; Tran, A.; Štelemėkas, M.; Rehm, J. Alcohol Taxation, Alcohol Consumption and Cancers in Lithuania: A Case Study. Nord. Stud. Alcohol Drugs 2022, 39, 25–37. [Google Scholar] [CrossRef] [PubMed]
- NTAKD. Psichoaktyvios Medžiagos: Tendencijos ir Pokyčiai 2022. Narkotikų, Tabako ir Alkoholio Kontrolės Departamentas. 2022. Available online: https://ntakd.lrv.lt/uploads/ntakd/documents/files/2022%20metinis%20(galutinis).pdf (accessed on 21 June 2023).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-L.; Kim, S.-H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, T.S.; Gissler, M.; Merikukka, M.; Tuomikoski, P.; Ylikorkala, O. Sex Differences in Age-Related Cardiovascular Mortality. PLoS ONE 2013, 8, e63347. [Google Scholar] [CrossRef]
- Čypienė, A.; Gimžauskaitė, S.; Rinkūnienė, E.; Jasiūnas, E.; Rugienė, R.; Kazėnaitė, E.; Ryliškytė, L.; Badarienė, J. The Association between Water Consumption and Hyperuricemia and Its Relation with Early Arterial Aging in Middle-Aged Lithuanian Metabolic Patients. Nutrients 2023, 15, 723. [Google Scholar] [CrossRef]
- Redon, P.; Maloberti, A.; Facchetti, R.; Redon, J.; Lurbe, E.; Bombelli, M.; Mancia, G.; Grassi, G. Gender-Related Differences in Serum Uric Acid in Treated Hypertensive Patients from Central and East European Countries: Findings from the Blood Pressure Control Rate and Cardiovascular Risk ProfilE Study. J. Hypertens. 2019, 37, 380–388. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Zhu, B.; Zhang, H.; Zhang, X.; Ding, X. Demographic, Regional and Temporal Trends of Hyperuricemia Epidemics in Mainland China from 2000 to 2019: A Systematic Review and Meta-Analysis. Glob. Health Action 2021, 14, 1874652. [Google Scholar] [CrossRef]
- Capuano, V.; Marchese, F.; Capuano, R.; Torre, S.; Iannone, A.G.; Capuano, E.; Lamaida, N.; Sonderegger, M.; Capuano, E. Hyperuricemia as an Independent Risk Factor for Major Cardiovascular Events: A 10-Year Cohort Study from Southern Italy. J. Cardiovasc. Med. 2017, 18, 159. [Google Scholar] [CrossRef]
- Singh, G.; Lingala, B.; Mithal, A. Sat0405 Gout and Hyperuricemia in the Us: Prevalence and Trends. Ann. Rheum. Dis. 2019, 78 (Suppl. S2), 1290. [Google Scholar] [CrossRef]
- Global Prevalence of Hyperuricemia: A Systematic Review of Population-Based Epidemiological Studies. ACR Meeting Abstracts. Available online: https://acrabstracts.org/abstract/global-prevalence-of-hyperuricemia-a-systematic-review-of-population-based-epidemiological-studies/ (accessed on 21 June 2023).
- Choi, H.K.; McCormick, N.; Lu, N.; Rai, S.K.; Yokose, C.; Zhang, Y. Population Impact Attributable to Modifiable Risk Factors for Hyperuricemia. Arthritis Rheumatol. 2020, 72, 157–165. [Google Scholar] [CrossRef]
- Lima, W.G.; Martins-Santos, M.E.S.; Chaves, V.E. Uric Acid as a Modulator of Glucose and Lipid Metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef]
- Towiwat, P.; Li, Z.-G. The association of vitamin C, alcohol, coffee, tea, milk and yogurt with uric acid and gout. Int. J. Rheum. Diseases 2015, 18, 495–501. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/1756-185X.12622?casa_token=k4MyxlA4Z-4AAAAA%3Alr6Vz6HTarMO8JoRaFE0MzlyIksj8V2pgcMshF64Zi3hA3_axMDq1V7HHCLx41fnTzCztUuEKpgS (accessed on 21 June 2023). [CrossRef] [PubMed]
- Wang, M.; Jiang, X.; Wu, W.; Zhang, D. A Meta-Analysis of Alcohol Consumption and the Risk of Gout. Clin. Rheumatol. 2013, 32, 1641–1648. [Google Scholar] [CrossRef]
- Teramura, S.; Yamagishi, K.; Umesawa, M.; Hayama-Terada, M.; Muraki, I.; Maruyama, K.; Tanaka, M.; Kishida, R.; Kihara, T.; Takada, M.; et al. Risk Factors for Hyperuricemia or Gout in Men and Women: The Circulatory Risk in Communities Study (CIRCS). J. Atheroscler. Thromb. 2023, 30, 63907. [Google Scholar] [CrossRef]
- Del Giorno, R.; Maddalena, A.; Bassetti, S.; Gabutti, L. Association between Alcohol Intake and Arterial Stiffness in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 1207. [Google Scholar] [CrossRef] [PubMed]
- Alcohol Consumption—Oficialiosios Statistikos Portalas. Available online: https://osp.stat.gov.lt/en/lietuvos-gyventoju-sveikata-2020/alkoholio-vartojimas (accessed on 21 June 2023).
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice: Developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies with the Special Contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]

| Variable | Men (N = 241) | Women (N = 420) | Overall | ||||
|---|---|---|---|---|---|---|---|
| sUA Elevated (N = 70) | sUA Normal (N = 171) | p-Value | sUA Elevated (N = 144) | sUA Normal (N = 276) | p-Value | ||
| Age, years | <0.05 | 0.51 | |||||
| Mdn [Q1, Q3] | 46.0 [43.0, 48.0] | 48.0 [44.0, 51.0] | 58.0 [54.0, 61.0] | 58.0 [54.0, 61.0] | 54.0 [49.0, 59.0] | ||
| BMI, kg/m2 | <0.05 | <0.05 | |||||
| Mdn [Q1, Q3] | 31.7 [30.1, 35.1] | 30.5 [28.1, 32.8] | 33.6 [30.1, 37.5] | 30.5 [27.4, 33.6] | 31.2 [28.4, 34.3] | ||
| Circumference of waist, cm | <0.001 | 0.06 | |||||
| Mdn [Q1, Q3] | 109 [104, 113] | 105 [102, 110] | 105 [97.0, 112] | 97.0 [91.0, 105] | 103 [95.0, 109] | ||
| SBP, mmHg | 0.05 | 0.04 | |||||
| Mdn [Q1, Q3] | 139 [128, 148] | 133 [126, 142] | 137 [130, 146] | 135 [123, 145] | 135 [126, 145] | ||
| sUA, µmol/L | <0.05 | <0.05 | |||||
| Mdn [Q1, Q3] | 481 [457, 520] | 366 [328, 393] | 397 [378, 447] | 291 [259, 324] | 352 [298, 404] | ||
| LDL-cholesterol, mmol/L | 0.37 | 0.36 | |||||
| Mdn [Q1, Q3] | 3.81 [3.14, 4.28] | 3.60 [2.92, 4.25] | 3.67 [3.06, 4.36] | 3.89 [3.01, 4.83] | 3.74 [2.98, 4.49] | ||
| TG, mmol/L | 0.44 | <0.001 | |||||
| Mdn [Q1, Q3] | 2.39 [1.59, 2.83] | 1.82 [1.26, 2.53] | 1.79 [1.37, 2.43] | 1.52 [1.07, 2.03] | 1.73 [1.24, 2.39] | ||
| Fasting glucose, mmol/L | 0.04 | 0.39 | |||||
| Mdn [Q1, Q3] | 5.98 [5.61, 6.51] | 6.03 [5.63, 6.49] | 6.13 [5.79, 6.94] | 6.00 [5.66, 6.54] | 6.06 [5.66, 6.60] | ||
| hs-CRP, mg/L | 0.09 | <0.001 | |||||
| Mdn [Q1, Q3] | 1.99 [1.16, 3.91] | 1.46 [0.783, 2.35] | 2.61 [1.15, 4.62] | 1.46 [0.810, 2.76] | 1.60 [0.880, 3.10] | ||
| Creatinine, μmol/L | <0.001 | <0.001 | |||||
| Mdn [Q1, Q3] | 81.5 [77.0, 91.0] | 77.0 [71.5, 84.0] | 68.0 [62.0, 73.0] | 65.0 [60.0, 70.0] | 70.0 [63.0, 78.0] | ||
| Diabetes | 0.92 | 0.07 | |||||
| Yes | 11.0 (15.7%) | 26.0 (15.2%) | 38.0 (26.4%) | 52.0 (18.8%) | 127 (19.2%) | ||
| No | 59.0 (84.3%) | 145 (84.8%) | 106 (73.6%) | 224 (81.2%) | 534 (80.8%) | ||
| Variable | Men (N = 241) | Women (N = 420) | Overall | ||||
|---|---|---|---|---|---|---|---|
| sUA Elevated (N = 70) | sUA Normal (N = 171) | p-Value | sUA Elevated (N = 144) | sUA Normal (N = 276) | p-Value | ||
| Alcohol, units per week | 0.012 | 0.83 | |||||
| <1 | 14 (20.6%) | 40 (23.7%) | 42 (29.2%) | 69 (25.1%) | 165 (25.2%) | ||
| 1 | 16 (23.5%) | 32 (18.9%) | 12 (8.33%) | 23 (8.36%) | 83 (12.7%) | ||
| >1 | 30 (44.1%) | 47 (27.8%) | 11 (7.64%) | 21 (7.64%) | 109 (16.6%) | ||
| No | 8 (11.8%) | 50 (29.6%) | 79 (54.9%) | 162 (58.9%) | 299 (45.6%) | ||
| Missing | 2 (2.9%) | 2 (1.2%) | 0 (0%) | 1 (0.4%) | 5 (0.8%) | ||
| Variable | Men (N = 241) | Women (N = 420) | Overall | ||||
|---|---|---|---|---|---|---|---|
| sUA Elevated (N = 70) | sUA Normal (N = 171) | p-Value | sUA Elevated (N = 144) | sUA Normal (N = 276) | p-Value | ||
| cfPWV, m/s | 0.14 | 0.004 | |||||
| Mdn (Q1, Q3) | 8.15 (7.53, 8.98) | 8.00 (7.00, 8.75) | 8.90 (7.80, 9.90) | 8.40 (7.70, 9.33) | 8.40 (7.60, 9.30) | ||
| MBP, mmHg | 0.05 | 0.519 | |||||
| Mdn (Q1, Q3) | 101 (95.0, 109) | 99.0 (93.0, 106) | 99.0 (94.0, 106) | 99.0 (91.8, 106) | 99.0 (93.0, 106) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čypienė, A.; Gimžauskaitė, S.; Rinkūnienė, E.; Jasiūnas, E.; Laucevičius, A.; Ryliškytė, L.; Badarienė, J. Effect of Alcohol Consumption Habits on Early Arterial Aging in Subjects with Metabolic Syndrome and Elevated Serum Uric Acid. Nutrients 2023, 15, 3346. https://doi.org/10.3390/nu15153346
Čypienė A, Gimžauskaitė S, Rinkūnienė E, Jasiūnas E, Laucevičius A, Ryliškytė L, Badarienė J. Effect of Alcohol Consumption Habits on Early Arterial Aging in Subjects with Metabolic Syndrome and Elevated Serum Uric Acid. Nutrients. 2023; 15(15):3346. https://doi.org/10.3390/nu15153346
Chicago/Turabian StyleČypienė, Alma, Silvija Gimžauskaitė, Egidija Rinkūnienė, Eugenijus Jasiūnas, Aleksandras Laucevičius, Ligita Ryliškytė, and Jolita Badarienė. 2023. "Effect of Alcohol Consumption Habits on Early Arterial Aging in Subjects with Metabolic Syndrome and Elevated Serum Uric Acid" Nutrients 15, no. 15: 3346. https://doi.org/10.3390/nu15153346
APA StyleČypienė, A., Gimžauskaitė, S., Rinkūnienė, E., Jasiūnas, E., Laucevičius, A., Ryliškytė, L., & Badarienė, J. (2023). Effect of Alcohol Consumption Habits on Early Arterial Aging in Subjects with Metabolic Syndrome and Elevated Serum Uric Acid. Nutrients, 15(15), 3346. https://doi.org/10.3390/nu15153346






