Does the Micronutrient Molybdenum Have a Role in Gestational Complications and Placental Health?
Abstract
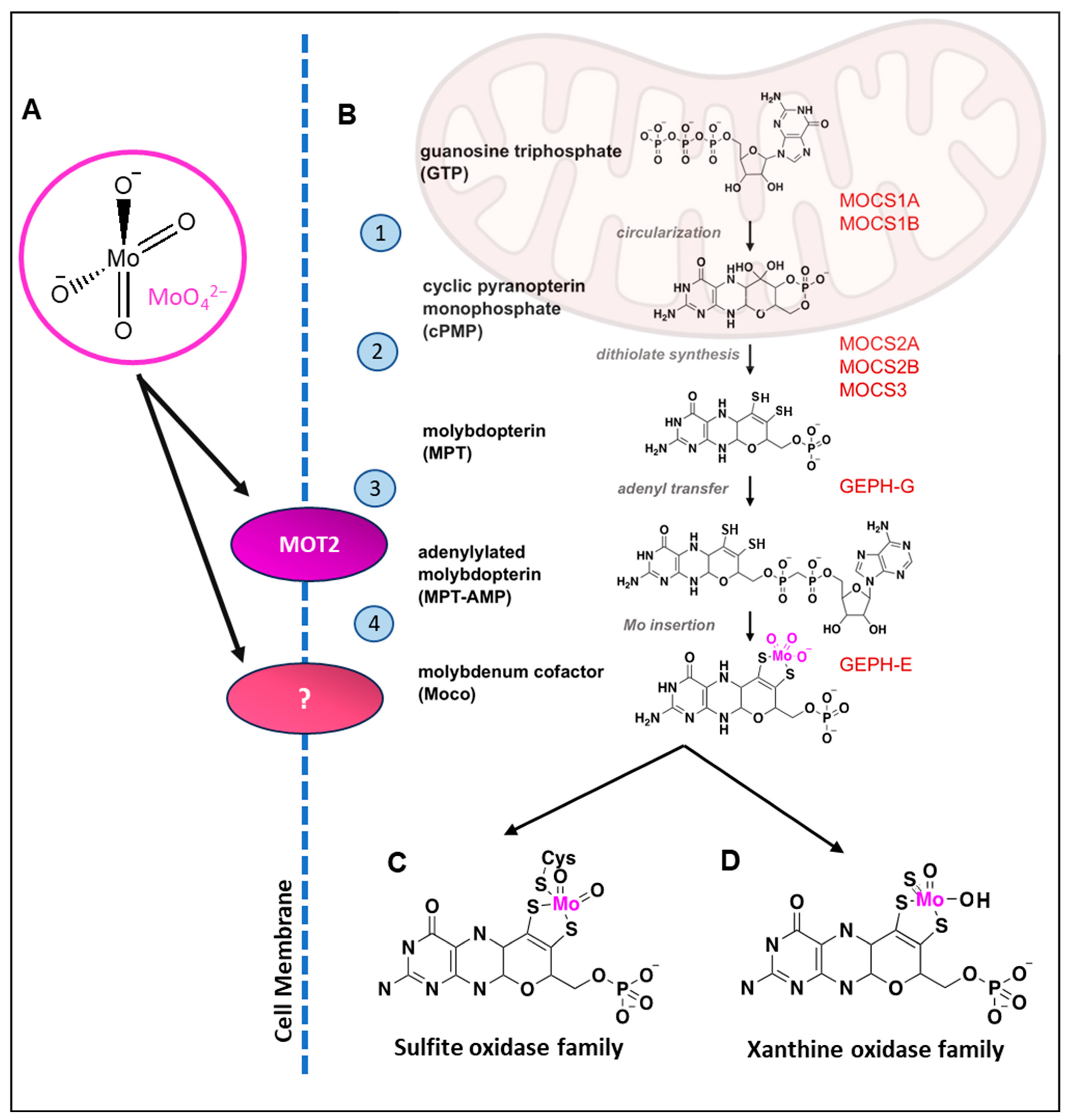
:1. Introduction
2. Molybdenum: Ubiquitous Yet Unknown
2.1. Discovery of Molybdenum and Chemical Versatility
2.2. Bioavailability and Intake of Molybdenum
2.3. Current Nutritional Parameters
2.4. Rare, But Present: Molybdenum Deficiency and Overexposure
2.5. Gaps in the Research—Molybdenum in Pregnancy
3. Molybdenum Exposure during Pregnancy and Disease
3.1. The (Understandable) Overexposure Bias
3.2. Molybdenum as a Mediator of Hyperglycaemia and Hyperlipidemia, and Its Role in GDM
3.3. Molybdenum and Antioxidant Activity
3.4. Molybdenum and Foetal Development
| Authors | Method of Detection | Population Number | Population Details | Matrix | Country | Results |
|---|---|---|---|---|---|---|
| Al-Saleh et al., 2004 [102] | AAS | 39 healthy non-obese subjects | Late third trimester (39.2 + 0.3 weeks) | Blood/serum | Kuwait | Significant positive correlation was found between maternal and umbilical cord levels of Mo, and placental weight. Maternal blood Mo did not correlate with birth weight. |
| Goodrich et al., 2019 [147] | ID LC-MS/MS | 56 healthy subjects | First trimester | Spot urine | US | No association found between urinary Mo in 1st trimester and 2nd trimester foetal biometrics or birth weight at term. |
| Kim et al., 2018 [150] | ICP-MS | 390 subjects (99 preterm birth, 291 control) | Early third trimester (median 26 weeks) | Spot urine | US | No significant association found between urinary Mo and risk of preterm birth. |
| Kim et al., 2020 [149] | ICP-MS | 390 healthy subjects | Early third trimester (median 26 weeks) | Urine | US | Positive association between urinary Mo and foetal z scores, inc. femur length (significant), abdominal circumference and head circumference. |
| Kot et al., 2019 [14] | ICP-AAS | 83 healthy subjects | Late third trimester (39 ± 1.8 weeks) | Umbilical, placental and foetal membrane tissue | Poland | Negative association found between placental Mo concentration and placental width. |
| McAlpine et al., 2019 [137] | ICP-MS | 127 subjects (89 used supplements, 38 did not) | Late second and early third trimester, 180–210 days gestation (25–30 weeks) | Fasting serum | Australia | No significant association found between Mo in serum or dietary levels, and the incidence of negative outcomes (low birthweight infants, pre-term birth, hypertensive disorders). |
| McKeating et al., 2021 [146] | ICP-MS | <128 subjects, crossover (10 with SGA, 18 with low placental weight, 13 preterm birth, 87 controls) | Second trimester (18 weeks) | Plasma, urine | Australia | Plasma (but not urine) Mo concentrations were significantly lower in pregnancies with low placental weight in comparison to controls. Plasma Se:Mo ratio had 87.3% predictive capability for determining placental weight. |
| McKeating et al., 2021 [151] | ICP-MS | 328 samples (38 who developed PE, 91 who delivered with SGA, 193 healthy controls) | Late third trimester (36 weeks) | Plasma | Australia | No significant differences found between plasma Mo of controls, and negative outcome (pre-eclampsia and small-for-gestational-age) groups at 36 weeks. An increase in plasma Mo was noted with PE outcome (non-significant). |
| Shirai et al., 2010 [152] | ICP-MS | 78 healthy subjects | Variable collection (9–40 weeks) | Spot urine | Japan | No significant association found between urinary Mo and birth size. |
| Zhao et al., 2021 [148] | ICP-MS | 220 subjects | Second trimester (24.9 ± 0.8 weeks) | Spot urine | China | Negative association found between urinary Mo levels and foetal AC (significant) and EFW during the second trimester of pregnancy. |
| Authors | Method of Detection | Population Number | Population Details | Matrix | Country | Results |
|---|---|---|---|---|---|---|
| Liu et al., 2020 [156] | ICP-MS | 332 subjects (166 with NTD-affected pregnancies, and 166 controls) | Collection across first, second and third trimesters | Umbilical cord tissue | China | Median concentrations of Mo in umbilical cord tissue were significantly higher in controls than NTD-affected pregnancies. Mo concentration was significantly correlated with gestation in NTD cases. No relationship between Mo and NTD risk observed when adjusting for confounders. |
| Ovayolu et al., 2019 [157] | ICP-MS | 75 subjects (36 with NTD-affected pregnancies, and 39 controls) | Second trimester (NTD: 21.6 ± 6.6 weeks, controls: 19.6 ± 2.4 weeks) | Amniotic fluid | Turkey | Mean concentrations of Mo in amniotic fluid were significantly higher in controls than NTD-affected pregnancies. |
| Tian et al., 2021 [154] | ICP-MS | 750 subjects (273 with NTD-affected pregnancies, and 477 controls) | Collection across first, second and third trimesters | Serum | China | Median concentrations of Mo in maternal serum were significantly higher in controls than NTD-affected pregnancies. Mo was found to have a significant protective effect against NTDs. |
| Tian et al., 2022 [158] | ICP-MS | 213 subjects (99 with NTD-affected pregnancies, 114 controls) | Collection across first, second and third trimesters | Serum | China | No significant difference between median concentrations of Mo in maternal serum from healthy controls and NTD-affected pregnancies. |
| Yan et al., 2017 [155] | ICP-MS | 452 subjects (191 with NTD-affected pregnancies, 261 controls) | First trimester (4 weeks preconception to 8 weeks post) | Hair | China | Mo concentrations in maternal hair samples from healthy controls were significantly higher than in NTD-affected pregnancies, and specifically spina bifida-affected pregnancies. When adjusting for confounders, Mo hair content was inversely associated with NTD risk. Mo deficiency was associated with increased risk of NTD subtypes anencephaly and spina bifida. |
| Yin et al., 2020 [159] | ICP-MS | 1001 subjects (408 with NTD-affected pregnancies, and 593 controls) | Collection across first, second and third trimesters | Placental tissue | China | Median concentrations of Mo in placental tissue were significantly higher in NTD-affected pregnancies in comparison to controls. Mo concentrations were significantly higher in females than males. Increased Mo concentrations were associated with higher risk of NTDs in multivariable logistic regression model, but was close to null in BKMR model. |
4. The Role of Molybdoenzymes in Physiology
| Molybdoenzyme | Tissue Locations | Cellular Compartments | Substrates (Not Exhaustive) | Products (Not Exhaustive) | Associated Reactive Species | Pathologies | Pregnancy-Related Research |
|---|---|---|---|---|---|---|---|
| Aldehyde Oxidase | liver, gut, lungs, brain, adipose tissue, skin, placenta [17,20] | Cytosol [17] | organic aldehydes, azaheterocycles, exogenous toxins (e.g., vanillin), nitrite, prodrugs [17,20] | carboxylic acid, lactams, vanillic acid, nitric oxide [17,20,145] | O2•−, H2O2 [169] | Implicated in development of obesity, cancer, lateral sclerosis and ageing [20]. | Gene expression increased in placental tissue of stillbirth and late-term pregnancy; associated with increased lipid peroxidation in late-term and stillbirth placentae [170]. |
| Xanthine Oxidoreductase | liver, gut, lungs, kidney, breast, placenta [54,171,172] | Cytosol, Peroxisomes [171] | hypoxanthine, xanthine, nitrite [171] | xanthine, uric acid, nitric oxide [145] | O2•−, H2O2 [56,169] | Excess uric acid leads to gout, hyperuricemia; deficiency of enzyme leads to excess xanthine and potential renal failure [52,53]. | Increased expression and activity of XOR found in placentae (invasive cytotrophoblasts) of pre-eclamptic women [173]. Hyperuricemia associated with pre-eclampsia and GDM [174]. |
| Sulfite Oxidase | liver, kidney, heart, skeletal muscle, brain, placenta [175] | Mitochondria (intermembrane space) [176] | sulfite, nitrite | sulfate, nitric oxide [177] | O2•−, H2O2 in plant SO [178,179] | Deficiency leads to toxic sulfite accumulation and brain damage, neurological abnormalities, myoclonic seizures, and neonatal or early-childhood mortality [50,180]. | Deficiency leads to toxic sulfite accumulation and brain damage, neurological abnormalities, myoclonic seizures and neonatal or early-childhood mortality following uneventful pregnancies. Brain damage begins in utero [180]. |
| Mitochondrial Amidoxime Component 1 & 2 | liver, kidney, adipose tissue [181] | mitochondria (outer mitochondrial membrane), Peroxisomes (mARC2) [182,183] | nitrite; N-hydroxylated compounds/prodrugs [183] | nitric oxide; corresponding nucleoside/drug [183,184,185] | Not explored | Implicated in lipid metabolism and fatty liver disease [186], mARC1 deficiency was found to lower blood cholesterol levels and protect against cirrhosis [187]. | mARC1 was detected in both adult and foetal livers; mARC2 protein was only present in adult liver [181]. |
4.1. Molybdoenzyme Associations with Redox Status in Pregnancy
4.1.1. Aldehyde Oxidase
4.1.2. Xanthine Oxidoreductase
4.1.3. Sulfite Oxidase
4.2. Mitochondrial Amidoxime-Reducing Component 1 and 2, and Lipogenesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy: Review articles. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Salam, R.A.; Das, J.K.; Bhutta, Z.A. Impact of intrauterine growth restriction on long-term health. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Damhuis, S.E.; Ganzevoort, W.; Gordijn, S.J. Abnormal fetal growth: Small for gestational age, fetal growth restriction, large for gestational age: Definitions and epidemiology. Obstet. Gynecol. Clin. 2021, 48, 267–279. [Google Scholar] [CrossRef]
- Nirupama, R.; Divyashree, S.; Janhavi, P.; Muthukumar, S.; Ravindra, P. Preeclampsia: Pathophysiology and management. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101975. [Google Scholar] [CrossRef]
- Chu, A.H.; Godfrey, K.M. Gestational diabetes mellitus and developmental programming. Ann. Nutr. Metab. 2020, 76, 4–15. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [Green Version]
- McKeating, D.R.; Fisher, J.J.; Perkins, A.V. Elemental metabolomics and pregnancy outcomes. Nutrients 2019, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Mehri, A. Trace elements in human nutrition (II)—An update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar]
- Parisi, F.; Di Bartolo, I.; Savasi, V.; Cetin, I. Micronutrient supplementation in pregnancy: Who, what and how much? Obstet. Med. 2019, 12, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Stojsavljević, A.; Rovčanin, M.; Rovčanin, B.; Miković, Ž.; Jeremić, A.; Perović, M.; Manojlović, D. Human biomonitoring of essential, nonessential, rare earth, and noble elements in placental tissues. Chemosphere 2021, 285, 131518. [Google Scholar] [CrossRef] [PubMed]
- Cerrillos, L.; Fernández, R.; Machado, M.J.; Morillas, I.; Dahiri, B.; Paz, S.; Gonzalez-Weller, D.; Gutiérrez, A.; Rubio, C.; Hardisson, A.; et al. Placental levels of metals and associated factors in urban and sub-urban areas of Seville (Spain). J. Trace Elem. Med. Biol. 2019, 54, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Malinowski, W.; Szymański, S.; Mularczyk, M.; Tomska, N.; Rotter, I. Interactions between 14 Elements in the Human Placenta, Fetal Membrane and Umbilical Cord. Int. J. Environ. Res. Public Health 2019, 16, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in pregnancy and lactation: A review article. J. Clin. Diagn. Res. JCDR 2017, 11, QE01–QE05. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. Cell biology of molybdenum. Biofactors 2009, 35, 429–434. [Google Scholar] [CrossRef]
- Terao, M.; Garattini, E.; Romão, M.J.; Leimkühler, S. Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes. J. Biol. Chem. 2020, 295, 5377–5389. [Google Scholar] [CrossRef] [Green Version]
- Llamas, A.; Chamizo-Ampudia, A.; Tejada-Jimenez, M.; Galvan, A.; Fernandez, E. The molybdenum cofactor enzyme mARC: Moonlighting or promiscuous enzyme? Biofactors 2017, 43, 486–494. [Google Scholar] [CrossRef]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Qiao, Y.; Maiti, K.; Sultana, Z.; Fu, L.; Smith, R. Inhibition of vertebrate aldehyde oxidase as a therapeutic treatment for cancer, obesity, aging and amyotrophic lateral sclerosis. Eur. J. Med. Chem. 2020, 187, 111948. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Kutyreff, C.J.; Lai, J.; Yan, Y.; Barnhart, T.E.; Yu, B.; Im, H.-J.; Kang, L.; Cho, S.Y. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 2018, 9, 5421. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhao, T.; Liu, M.; Chen, Q.; Yang, Y.; Zhang, J.; Wang, S.; Zhu, X.; Zhang, H.; Huang, Q. Ultra-small molybdenum-based nanodots as an antioxidant platform for effective treatment of periodontal disease. Front. Bioeng. Biotechnol. 2022, 10, 1042010. [Google Scholar] [CrossRef] [PubMed]
- Ale-Ebrahim, M.; Eidi, A.; Mortazavi, P.; Tavangar, S.M.; Tehrani, D.M. Hepatoprotective and antifibrotic effects of sodium molybdate in a rat model of bile duct ligation. J. Trace Elem. Med. Biol. 2015, 29, 242–248. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Al-Ebrahim, M.; Rohani, A.H.; Mortazavi, P. Protective effects of sodium molybdate on carbon tetrachloride-induced hepatotoxicity in rats. J. Trace Elem. Med. Biol. 2011, 25, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A. Molybdenum nutriture in humans. J. Evid.-Based Integr. Med. 2011, 16, 164–168. [Google Scholar] [CrossRef]
- Lunk, H.-J.; Hartl, H. Discovery, properties and applications of molybdenum and its compounds. ChemTexts 2017, 3, 13. [Google Scholar] [CrossRef]
- Tejada-Jiménez, M.; Galván, A.; Fernández, E. Algae and humans share a molybdate transporter. Proc. Natl. Acad. Sci. USA 2011, 108, 6420–6425. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. The molybdenum cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, S.J.; Mendel, R.-R.; Schwarz, G. Molybdenum cofactor biology, evolution and deficiency. Biochim. Biophys. Acta (BBA)-Mol. Cell. Res. 2021, 1868, 118883. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R.; Schwarz, G. Molybdenum cofactor biosynthesis in plants and humans. Coord. Chem. Rev. 2011, 255, 1145–1158. [Google Scholar] [CrossRef]
- Mendel, R.R.; Kruse, T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta (BBA)-Mol. Cell. Res. 2012, 1823, 1568–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hänzelmann, P.; Schwarz, G.; Mendel, R.R. Functionality of alternative splice forms of the first enzymes involved in human molybdenum cofactor biosynthesis. J. Biol. Chem. 2002, 277, 18303–18312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallmeyer, B.; Schwarz, G.; Schulze, J.; Nerlich, A.; Reiss, J.; Kirsch, J.; Mendel, R. The neurotransmitter receptor-anchoring protein gephyrin reconstitutes molybdenum cofactor biosynthesis in bacteria, plants, and mammalian cells. Proc. Natl. Acad. Sci. USA 1999, 96, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Stallmeyer, B.; Drugeon, G.; Reiss, J.; Haenni, A.; Mendel, R. Human molybdopterin synthase gene: Identification of a bicistronic transcript with overlapping reading frames. Am. J. Hum. Genet. 1999, 64, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Mayr, S.J.; Röper, J.; Schwarz, G. Alternative splicing of the bicistronic gene molybdenum cofactor synthesis 1 (MOCS1) uncovers a novel mitochondrial protein maturation mechanism. J. Biol. Chem. 2020, 295, 3029–3039. [Google Scholar] [CrossRef]
- Gutzke, G.; Fischer, B.; Mendel, R.R.; Schwarz, G. Thiocarboxylation of molybdopterin synthase provides evidence for the mechanism of dithiolene formation in metal-binding pterins. J. Biol. Chem. 2001, 276, 36268–36274. [Google Scholar] [CrossRef] [Green Version]
- Matthies, A.; Rajagopalan, K.; Mendel, R.R.; Leimkühler, S. Evidence for the physiological role of a rhodanese-like protein for the biosynthesis of the molybdenum cofactor in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 5946–5951. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.; Dosche, C.; Löhmannsröben, H.-G.; Leimkühler, S. Dual role of the molybdenum cofactor biosynthesis protein MOCS3 in tRNA thiolation and molybdenum cofactor biosynthesis in humans. J. Biol. Chem. 2012, 287, 17297–17307. [Google Scholar] [CrossRef] [Green Version]
- Llamas, A.; Mendel, R.R.; Schwarz, G. Synthesis of adenylated molybdopterin: An essential step for molybdenum insertion. J. Biol. Chem. 2004, 279, 55241–55246. [Google Scholar] [CrossRef] [Green Version]
- Belaidi, A.A.; Schwarz, G. Metal insertion into the molybdenum cofactor: Product–substrate channelling demonstrates the functional origin of domain fusion in gephyrin. Biochem. J. 2013, 450, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Llamas, A.; Otte, T.; Multhaup, G.; Mendel, R.R.; Schwarz, G. The mechanism of nucleotide-assisted molybdenum insertion into molybdopterin: A novel route toward metal cofactor assembly. J. Biol. Chem. 2006, 281, 18343–18350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Peretz, H.; Naamati, M.S.; Levartovsky, D.; Lagziel, A.; Shani, E.; Horn, I.; Shalev, H.; Landau, D. Identification and characterization of the first mutation (Arg776Cys) in the C-terminal domain of the Human Molybdenum Cofactor Sulfurase (HMCS) associated with type II classical xanthinuria. Mol. Genet. Metab. 2007, 91, 23–29. [Google Scholar] [CrossRef]
- Cordas, C.M.; Moura, J.J. Molybdenum and tungsten enzymes redox properties—A brief overview. Coord. Chem. Rev. 2019, 394, 53–64. [Google Scholar] [CrossRef]
- Hille, R.; Nishino, T.; Bittner, F. Molybdenum enzymes in higher organisms. Coord. Chem. Rev. 2011, 255, 1179–1205. [Google Scholar] [CrossRef] [Green Version]
- Kubitza, C.; Bittner, F.; Ginsel, C.; Havemeyer, A.; Clement, B.; Scheidig, A.J. Crystal structure of human mARC1 reveals its exceptional position among eukaryotic molybdenum enzymes. Proc. Natl. Acad. Sci. USA 2018, 115, 11958–11963. [Google Scholar] [CrossRef] [Green Version]
- Hille, R. Molybdenum and tungsten in biology. Trends Biochem. Sci. 2002, 27, 360–367. [Google Scholar] [CrossRef]
- Ferreira, P.; Cerqueira, N.M.S.A.; Fernandes, P.A.; Romão, M.J.O.; Ramos, M.J.o. Catalytic mechanism of human aldehyde oxidase. ACS Catal. 2020, 10, 9276–9286. [Google Scholar] [CrossRef]
- Schwarz, G. Molybdenum cofactor and human disease. Curr. Opin. Chem. Biol. 2016, 31, 179–187. [Google Scholar] [CrossRef]
- Claerhout, H.; Witters, P.; Régal, L.; Jansen, K.; Van Hoestenberghe, M.R.; Breckpot, J.; Vermeersch, P. Isolated sulfite oxidase deficiency. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2018, 41, 101–108. [Google Scholar] [CrossRef]
- Pais, V.M., Jr.; Lowe, G.; Lallas, C.D.; Preminger, G.M.; Assimos, D.G. Xanthine urolithiasis. Urology 2006, 67, 1084.e9–1084.e11. [Google Scholar] [CrossRef]
- Mraz, M.; Hurba, O.; Bartl, J.; Dolezel, Z.; Marinaki, A.; Fairbanks, L.; Stiburkova, B. Modern diagnostic approach to hereditary xanthinuria. Urolithiasis 2015, 43, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lü, J.-M.; Yao, Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: An overview. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giulia Battelli, M.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in drug metabolism: Beyond a role as a detoxifying enzyme. Curr. Med. Chem. 2016, 23, 4027–4036. [Google Scholar] [CrossRef] [Green Version]
- Garattini, E.; Terao, M. Aldehyde oxidase and its importance in novel drug discovery: Present and future challenges. Expert Opin. Drug Discov. 2013, 8, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.M.; Fernandes, H.S.; Maia, L.B.; Sousa, S.F.; Moura, J.J.; Cerqueira, N.M. The complete catalytic mechanism of xanthine oxidase: A computational study. Inorg. Chem. Front. 2021, 8, 405–416. [Google Scholar] [CrossRef]
- Okamoto, K.; Kusano, T.; Nishino, T. Chemical nature and reaction mechanisms of the molybdenum cofactor of xanthine oxidoreductase. Curr. Pharm. Des. 2013, 19, 2606–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributions and controls. Appl. Geochem. 2017, 84, 387–432. [Google Scholar] [CrossRef] [Green Version]
- Novotny, J.A.; Peterson, C.A. Molybdenum. Adv. Nutr. 2018, 9, 272–273. [Google Scholar] [CrossRef] [Green Version]
- Hattori, H.; Ashida, A.; ITÔ, C.; Yoshida, M. Determination of molybdenum in foods and human milk, and an estimate of average molybdenum intake in the Japanese population. J. Nutr. Sci. Vitaminol. 2004, 50, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Hunt, C.D.; Meacham, S.L. Aluminum, boron, calcium, copper, iron, magnesium, manganese, molybdenum, phosphorus, potassium, sodium, and zinc: Concentrations in common western foods and estimated daily intakes by infants; toddlers; and male and female adolescents, adults, and seniors in the United States. J. Am. Diet. Assoc. 2001, 109, 1058–1060. [Google Scholar] [CrossRef]
- Szira, F.; Monostori, I.; Galiba, G.; Rakszegi, M.; Bálint, A.F. Micronutrient contents and nutritional values of commercial wheat flours and flours of field-grown wheat varieties—A survey in Hungary. Cereal Res. Commun. 2014, 42, 293–302. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific Opinion on Dietary Reference Values for molybdenum. EFSA J. 2013, 11, 3333. [Google Scholar] [CrossRef]
- Institute of Medicine, Panel on Micronutrients. Molybdenum. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 3 July 2023). [CrossRef]
- Lener, J.; Bíbr, B. Effects of molybdenum on the organism (a review). J. Hyg. Epidemiol. Microbiol. Immunol. 1984, 28, 405–419. [Google Scholar] [PubMed]
- Mařík, T.; Kselíková, M.; Bíbr, B.; Lener, J. Specific molybdenum binding to spectrin subunits. Cell Biochem. Funct. 1984, 2, 21–22. [Google Scholar] [CrossRef]
- Kruse, T. Moco Carrier and Binding Proteins. Molecules 2022, 27, 6571. [Google Scholar] [CrossRef] [PubMed]
- Giussani, A. A recycling systemic model for the biokinetics of molybdenum radionuclides. Sci. Total Environ. 2008, 404, 44–55. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Iida, S.; Ueoka-Nakanishi, H.; Niimi, T.; Tomioka, R.; Maeshima, M. Exploring dynamics of molybdate in living animal cells by a genetically encoded FRET nanosensor. PLoS ONE 2013, 8, e58175. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Lee, S.K.; Yang, J.Y.; In, S.W.; Kim, K.W.; Chung, K.H.; Chung, M.G.; Choung, S.Y. Organ distribution of heavy metals in autopsy material from normal Korean. J. Health Sci. 2002, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Hattori, H.; Ôta, S.; Yoshihara, K.; Kodama, N.; Yoshitake, Y.; Nishimuta, M. Molybdenum balance in healthy young Japanese women. J. Trace Elem. Med. Biol. 2006, 20, 245–252. [Google Scholar] [CrossRef]
- Zeisler, R.; Greenberg, R.; Stone, S. Radiochemical and instrumental neutron activation analysis procedures for the determination of low level trace elements in human livers. J. Radioanal. Nucl. Chem. 1988, 124, 47–63. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. The 22nd Australian Total Diet Survey: A Total Survey of Dietary Exposure of the Australian Population to the Trace Elements Iodine, Selenium, Molybdenum, Chromium, and Nickel; FSANZ: Canberra, Australia; Wellington, New Zealand, 2008; ISBN 978-0-642-34561-5. Available online: https://www.foodstandards.gov.au/publications/documents/atds.pdf (accessed on 23 June 2023).
- Holzinger, S.; Anke, M.; Röhrig, B.; Gonzalez, D. Molybdenum intake of adults in Germany and Mexico. Analyst 1998, 123, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Turconi, G.; Minoia, C.; Ronchi, A.; Roggi, C. Dietary exposure estimates of twenty-one trace elements from a Total Diet Study carried out in Pavia, Northern Italy. Br. J. Nutr. 2008, 101, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
- Basket, N.M. Chemical Analysis, Exposure Estimation and Health-Related Assessment of Nutrients and Toxic Compounds in Swedish Food Baskets; Swedish National Food Agency: Uppsala, Sweden, 2012.
- Kapil, U.; Verma, D.; Goel, M.; Saxena, N.; Gnanasekaran, N.; Goindi, G.; Nayar, D. Dietary intake of trace elements and minerals among adults in underprivileged communities of rural Rajasthan, India. Asia Pac. J. Clin. Nutr. 1998, 7, 29–32. [Google Scholar] [PubMed]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Keyes, W.R.; Peiffer, G.L.; Chiang, G. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men during depletion and repletion. Am. J. Clin. Nutr. 1995, 61, 1102–1109. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Keyes, W.R.; Peiffer, G.L. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men at five intakes of dietary molybdenum. Am. J. Clin. Nutr. 1995, 62, 790–796. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; Australian Government Department of Health and Aged Care, Ed.; National Health and Medical Research Council: Canberra, Australia, 2006.
- Ministry of Health, Labour and Welfare (Health Service Bureau). Dietary Reference Intakes for Japanese 2015; Health Service Bureau, Ministry of Health, Labour and Welfare, Ed.; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2015.
- Department of Health. Dietary reference values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1991, 41, 1–210. [Google Scholar]
- D-A-CH (Deutsche Gesellschaft für Ernährung, Ö.G.f.E., Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung). Referenzwerte für die Nährstoffzufuhr; D-A-CH: Frankfurt, Germany, 2013. [Google Scholar]
- Novotny, J.A.; Turnlund, J.R. Molybdenum Intake Influences Molybdenum Kinetics in Men1. J. Nutr. 2007, 137, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Werner, E.; Giussani, A.; Heinrichs, U.; Roth, P.; Greim, H. Biokinetic studies in humans with stable isotopes as tracers. Part 2: Uptake of molybdenum from aqueous solutions and labelled foodstuffs. Isot. Environ. Health Stud. 1998, 34, 297–301. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Weaver, C.M.; Kim, S.K.; Keyes, W.R.; Gizaw, Y.; Thompson, K.H.; Peiffer, G.L. Molybdenum absorption and utilization in humans from soy and kale intrinsically labeled with stable isotopes of molybdenum. Am. J. Clin. Nutr. 1999, 69, 1217–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnlund, J.R.; Keyes, W.R. Plasma molybdenum reflects dietary molybdenum intake. J. Nutr. Biochem. 2004, 15, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.N.; Schneider, A.J.; Steel, D.; Rogers, L.S. Amino acid intolerance during prolonged total parenteral nutrition reversed by molybdate therapy. Am. J. Clin. Nutr. 1981, 34, 2551–2559. [Google Scholar] [CrossRef]
- Spiegel, R.; Schwahn, B.C.; Squires, L.; Confer, N. Molybdenum cofactor deficiency: A natural history. J. Inherit. Metab. Dis. 2022, 45, 456–469. [Google Scholar] [CrossRef]
- Alkan, M.; Kip, G.; Şahin, Ş.; Atabek, D. Choice of anesthesia in molybdenum cofactor deficiency: A case report. J. Res. Med. Sci. 2014, 19, 1103–1105. [Google Scholar] [PubMed]
- Kang, C. Fosdenopterin: First Approval. Drugs 2021, 81, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Koval’Skii, V.V.; Iarovaia, G.A.; Shmavonian, D.M. Modification of human and animal purine metabolism in conditions of various molybdenum bio-geochemical areas. Zhurnal Obs. Biol. 1961, 22, 179–191. [Google Scholar]
- Seldén, A.I.; Berg, N.P.; Söderbergh, A.; Bergström, B.E. Occupational molybdenum exposure and a gouty electrician. Occup. Med. 2005, 55, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Walravens, P.A.; Moure-Eraso, R.; Solomons, C.C.; Chappell, W.R.; Bentley, G. Biochemical Abnormalities in Workers Exposed to Molybdenum Dust. Arch. Environ. Health Int. J. 1979, 34, 302–308. [Google Scholar] [CrossRef]
- Neary, E.R. The use of molybdenized ferrous sulfate in the treatment of true iron deficiency anemia of pregnancy. Am. J. Med. Sci. 1946, 212, 76–82. [Google Scholar] [CrossRef]
- Fungwe, T.V.; Buddingh, F.; Demick, D.S.; Lox, C.D.; Yang, M.T.; Yang, S.P. The role of dietary molybdenum on estrous activity, fertility, reproduction and molybdenum and copper enzyme activities of female rats. Nutr. Res. 1990, 10, 515–524. [Google Scholar] [CrossRef]
- Vasto, S.; Di Gaudio, F.; Raso, M.; Sabatino, L.; Caldarella, R.; De Pasquale, C.; Di Rosa, L.; Baldassano, S. Impact on Glucose Homeostasis: Is Food Biofortified with Molybdenum a Workable Solution? A Two-Arm Study. Nutrients 2022, 14, 1351. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, L.; Brolli, B.; Negro, A.; Zoino, J.L.; Schicchi, A.; Petrolini, V.M.; Lonati, D.; Ronchi, A.; Locatelli, C.A. Accidental ingestion of sodium molybdate at the workplace followed by short-term biomonitoring. Med. Lav. 2022, 113, e2022015. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple Micronutrient Needs in Pregnancy in Industrialized Countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, E.; Nandakumaran, M.; Al-Shammari, M.; Al-Falah, F.; Al-Harouny, A. Assessment of maternal–fetal status of some essential trace elements in pregnant women in late gestation: Relationship with birth weight and placental weight. J. Matern.-Fetal Neonatal Med. 2004, 16, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Srinivas, S.R.; Fei, Y.J.; Gopal, E.; Umapathy, N.S.; Wang, H.; Conway, S.J.; Ganapathy, V.; Prasad, P.D. Functional characteristics of NaS2, a placenta-specific Na+-coupled transporter for sulfate and oxyanions of the micronutrients selenium and chromium. Placenta 2006, 27, 550–559. [Google Scholar] [CrossRef]
- Wang, X.; Oberleas, D.; Yang, M.T.; Yang, S.P. Molybdenum requirement of female rats. J. Nutr. 1992, 122, 1036–1041. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakagawa, M.; Hosomi, R.; Nishiyama, T.; Fukunaga, K. Low molybdenum state induced by tungsten as a model of molybdenum deficiency in rats. Biol. Trace Elem. Res. 2015, 165, 75–80. [Google Scholar] [CrossRef]
- Cao, G.H.; Yan, S.M.; Yuan, Z.K.; Wu, L.; Liu, Y.F. A study of the relationship between trace element Mo and gastric cancer. World J. Gastroenterol. 1998, 4, 55–56. [Google Scholar] [CrossRef]
- Fang, W.; Wu, P.; Hu, R. Environmental Se–Mo–B Deficiency and its Possible Effects in Jiantou Keshan Disease Area in Shaanxi Province, China. Environ. Geochem. Health 2002, 24, 349–358. [Google Scholar] [CrossRef]
- Ahmed, F.; Khosravi-Boroujeni, H.; Khan, M.R.; Roy, A.K.; Raqib, R. Prevalence and Predictors of Vitamin D Deficiency and Insufficiency among Pregnant Rural Women in Bangladesh. Nutrients 2021, 13, 449. [Google Scholar] [CrossRef]
- Filipowicz, D.; Szczepanek-Parulska, E.; Kłobus, M.; Szymanowski, K.; Chillon, T.S.; Asaad, S.; Sun, Q.; Mikulska-Sauermann, A.A.; Karaźniewicz-Łada, M.; Główka, F.K.; et al. Selenium Status and Supplementation Effects in Pregnancy—A Study on Mother–Child Pairs from a Single-Center Cohort. Nutrients 2022, 14, 3082. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Fischer, P.R.; Thacher, T.D.; Topazian, M.D.; Bourassa, M.W.; Combs, G.F. Thiamin deficiency in low- and middle-income countries: Disorders, prevalences, previous interventions and current recommendations. Nutr. Health 2019, 25, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.; Perkins, A.V. Selenoproteins in the Human Placenta: How Essential Is Selenium to a Healthy Start to Life? Nutrients 2022, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.; Vanderlelie, J.J.; Perkins, A.V. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta 2013, 34, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Hubalewska-Dydejczyk, A.; Duntas, L.; Gilis-Januszewska, A. Pregnancy, thyroid, and the potential use of selenium. Hormones 2020, 19, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.-M.; Zhang, Y.-L.; Liu, F.-J.; Zhou, T.-Z.; Yang, Z.-J.; Gao, S.-Y.; Wang, S.-D.; Chen, X.-L.; Zhai, X.-W.; Ma, X.-G.; et al. The effect of molybdenum on the in vitro development of mouse preimplantation embryos. Syst. Biol. Reprod. Med. 2013, 59, 69–73. [Google Scholar] [CrossRef]
- Ahmed, A.A. Beneficial effects of combined administration of sodium molybdate with atorvastatin in hyperlipidemic hamsters. Drug Discov. 2009, 3, 62–70. [Google Scholar]
- Lee, S.; Nam, K.-H.; Seong, J.K.; Ryu, D.-Y. Molybdate Attenuates Lipid Accumulation in the Livers of Mice Fed a Diet Deficient in Methionine and Choline. Biol. Pharm. Bull. 2018, 41, 1203–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, K.; Bailey, J.; MacRory, C.; Friis, C.; Vogels, C.M.; Broderick, T.; Westcott, S.A. A newly synthesised molybdenum/ascorbic acid complex alleviates some effects of cardiomyopathy in streptozocin-induced diabetic rats. Drugs R. D. 2006, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ozcelikay, A.T.; Becker, D.J.; Ongemba, L.N.; Pottier, A.M.; Henquin, J.C.; Brichard, S.M. Improvement of glucose and lipid metabolism in diabetic rats treated with molybdate. Am. J. Physiol.-Endocrinol. Metab. 1996, 270, E344–E352. [Google Scholar] [CrossRef]
- Panneerselvam, R.S.; Govindaswamy, S. Effect of sodium molybdate on carbohydrate metabolizing enzymes in alloxan-induced diabetic rats. J. Nutr. Biochem. 2002, 13, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Reul, B.A.; Becker, D.J.; Ongemba, L.N.; Bailey, C.J.; Henquin, J.C.; Brichard, S.M. Improvement of glucose homeostasis and hepatic insulin resistance in ob/ob mice given oral molybdate. J. Endocrinol. 1997, 155, 55–64. [Google Scholar] [CrossRef]
- Barberà, A.; Gomis, R.R.; Prats, N.; Rodríguez-Gil, J.E.; Domingo, M.; Gomis, R.; Guinovart, J.J. Tungstate is an effective antidiabetic agent in streptozotocin-induced diabetic rats: A long-term study. Diabetologia 2001, 44, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.K.; Mehdi, M.Z. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabet. Med. 2005, 22, 2–13. [Google Scholar] [CrossRef]
- Singh, K.B.; Maret, W. The interactions of metal cations and oxyanions with protein tyrosine phosphatase 1B. BioMetals 2017, 30, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, E.; Birla Singh, K.; Massarotti, A.; Hogstrand, C.; Maret, W. The metal face of protein tyrosine phosphatase 1B. Coord. Chem. Rev. 2016, 327–328, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, M.; Dominguez, L.J.; Resnick, L.M. Insulin-mimetic action of vanadate: Role of intracellular magnesium. Hypertension 2001, 38, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Huang, Y.; Luo, C.; Peng, X.; Jiao, Y.; Zhou, L.; Yin, J.; Liu, L. Inverse Association of Plasma Molybdenum with Metabolic Syndrome in a Chinese Adult Population: A Case-Control Study. Nutrients 2021, 13, 4544. [Google Scholar] [CrossRef]
- Ajibola, R.; Ogundahunsi, O.A.; Soyinka, O.O.; Ogunyemi, E.O.; Odewabi, A.O. Serum Chromium, Molybdenum, Zinc and Magnesium Levels in Diabetes Mellitus Patients in Sagamu, South West Nigeria. Asian J. Med. Sci. 2014, 6, 15–19. [Google Scholar] [CrossRef]
- Flores, C.R.; Puga, M.P.; Wrobel, K.; Garay Sevilla, M.E.; Wrobel, K. Trace elements status in diabetes mellitus type 2: Possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res. Clin. Pract. 2011, 91, 333–341. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Bai, Y.; Cheng, Z. Sex-specific and dose-response relationships of urinary cobalt and molybdenum levels with glucose levels and insulin resistance in U.S. adults. J. Environ. Sci. 2023, 124, 42–49. [Google Scholar] [CrossRef]
- Menke, A.; Guallar, E.; Cowie, C.C. Metals in Urine and Diabetes in U.S. Adults. Diabetes 2015, 65, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Mukherjee, B.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Batterman, S.; Harlow, S.D.; Park, S.K. Urinary metal mixtures and longitudinal changes in glucose homeostasis: The Study of Women’s Health Across the Nation (SWAN). Environ. Int. 2020, 145, 106109. [Google Scholar] [CrossRef]
- Wang, X.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Mukherjee, B.; Harlow, S.D.; Park, S.K. Urinary metals and incident diabetes in midlife women: Study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res. Care 2020, 8, e001233. [Google Scholar] [CrossRef]
- Toro-Román, V.; Robles-Gil, M.C.; Muñoz, D.; Bartolomé, I.; Siquier-Coll, J.; Maynar-Mariño, M. Extracellular and Intracellular Concentrations of Molybdenum and Zinc in Soccer Players: Sex Differences. Biology 2022, 11, 1710. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Weisskopf, M.; Williams, P.L.; Parsons, P.J.; Palmer, C.D.; Buck Louis, G.M.; James-Todd, T. A Prospective Study of Early Pregnancy Essential Metal(loid)s and Glucose Levels Late in the Second Trimester. J. Clin. Endocrinol. Metab. 2019, 104, 4295–4303. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Weisskopf, M.G.; Williams, P.L.; Claus Henn, B.; Parsons, P.J.; Palmer, C.D.; Buck Louis, G.M.; James-Todd, T. Evaluating associations between early pregnancy trace elements mixture and 2nd trimester gestational glucose levels: A comparison of three statistical approaches. Int. J. Hyg. Environ. Health 2020, 224, 113446. [Google Scholar] [CrossRef]
- McAlpine, J.M.; McKeating, D.R.; Vincze, L.; Vanderlelie, J.J.; Perkins, A.V. Essential Mineral Intake During Pregnancy and Its Association With Maternal Health and Birth Outcomes in South East Queensland, Australia. Nutr. Metab. Insights 2019, 12, 1178638819879444. [Google Scholar] [CrossRef]
- Al-Saleh, E.; Nandakumaran, M.; Al-Shammari, M.; Al-Harouny, A. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in patients with gestational diabetes. J. Matern. Fetal Neonatal Med. 2004, 16, 15–21. [Google Scholar] [CrossRef]
- Al-Saleh, E.; Nandakumaran, M.; Al-Rashdan, I.; Al-Harmi, J.; Al-Shammari, M. Maternal-foetal status of copper, iron, molybdenum, selenium and zinc in obese gestational diabetic pregnancies. Acta Diabetol. 2007, 44, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, E.; Nandakumaran, M.; Al-Shammari, M.; Makhseed, M.; Sadan, T.; Harouny, A. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in insulin-dependent diabetic pregnancies. Arch. Gynecol. Obstet. 2005, 271, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Bartho, L.A.; Perkins, A.V.; Holland, O.J. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin. Exp. Pharmacol. Physiol. 2020, 47, 176–184. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; He, J.; Shen, X.; Zhao, K.; Wang, Y. The Effects of Oral Administration of Molybdenum Fertilizers on Immune Function of Nanjiang Brown Goat Grazing on Natural Pastures Contaminated by Mixed Heavy Metal. Biol. Trace Elem. Res. 2022, 200, 2750–2757. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Liu, F.-J.; Chen, X.-L.; Zhang, Z.-Q.; Shu, R.-Z.; Yu, X.-L.; Zhai, X.-W.; Jin, L.-J.; Ma, X.-G.; Qi, Q.; et al. Dual effects of molybdenum on mouse oocyte quality and ovarian oxidative stress. Syst. Biol. Reprod. Med. 2013, 59, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.-W.; Zhang, Y.-L.; Qi, Q.; Bai, Y.; Chen, X.-L.; Jin, L.-J.; Ma, X.-G.; Shu, R.-Z.; Yang, Z.-J.; Liu, F.-J. Effects of molybdenum on sperm quality and testis oxidative stress. Syst. Biol. Reprod. Med. 2013, 59, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: Nitrite reduction by molybdoenzymes. Redox Biol. 2018, 19, 274–289. [Google Scholar] [CrossRef]
- McKeating, D.R.; Clifton, V.L.; Hurst, C.P.; Fisher, J.J.; Bennett, W.W.; Perkins, A.V. Elemental Metabolomics for Prediction of Term Gestational Outcomes Utilising 18-Week Maternal Plasma and Urine Samples. Biol. Trace Elem. Res. 2021, 199, 26–40. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Ingle, M.E.; Domino, S.E.; Treadwell, M.C.; Dolinoy, D.C.; Burant, C.; Meeker, J.D.; Padmanabhan, V. First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother-Infant Pairs study. J. Dev. Orig. Health Dis. 2019, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, W.; Zhang, X.; Zhu, Q.; Tang, J.; He, H.; Chen, G.; Qin, J. Associations between molybdenum exposure and ultrasound measures of fetal growth parameters: A pilot study. Chemosphere 2021, 269, 128709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Meeker, J.D.; Aung, M.T.; Yu, Y.; Mukherjee, B.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Urinary trace metals in association with fetal ultrasound measures during pregnancy. Environ. Epidemiol. 2020, 4, e075. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Meeker, J.D.; Carroll, R.; Zhao, S.; Mourgas, M.J.; Richards, M.J.; Aung, M.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Urinary trace metals individually and in mixtures in association with preterm birth. Environ. Int. 2018, 121, 582–590. [Google Scholar] [CrossRef]
- McKeating, D.R.; Fisher, J.J.; MacDonald, T.; Walker, S.; Tong, S.; Bennett, W.W.; Kaitu’u-Lino, T.J.; Perkins, A.V. Circulating trace elements for the prediction of preeclampsia and small for gestational age babies. Metabolomics 2021, 17, 90. [Google Scholar] [CrossRef]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2010, 45, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Fu, J.; Yuan, Z.; Gu, H. Impact of prenatal exposure to metallic elements on neural tube defects: Insights from human investigations. Ecotoxicol. Environ. Saf. 2023, 255, 114815. [Google Scholar] [CrossRef]
- Tian, T.; Yin, S.; Jin, L.; Liu, J.; Wang, C.; Wei, J.; Liu, M.; Li, Z.; Wang, L.; Yin, C.; et al. Single and mixed effects of metallic elements in maternal serum during pregnancy on risk for fetal neural tube defects: A Bayesian kernel regression approach. Environ. Pollut. 2021, 285, 117203. [Google Scholar] [CrossRef]
- Yan, L.; Wang, B.; Li, Z.; Liu, Y.; Huo, W.; Wang, J.; Li, Z.; Ren, A. Association of essential trace metals in maternal hair with the risk of neural tube defects in offspring. Birth Defects Res. 2017, 109, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jin, L.; Yu, J.; Su, Z.; Sun, Y.; Liu, Y.; Xie, Q.; Li, Z.; Wang, L.; Ren, A. Essential trace elements in umbilical cord tissue and risk for neural tube defects. Reprod. Toxicol. 2020, 98, 149–156. [Google Scholar] [CrossRef]
- Ovayolu, A.; Ovayolu, G.; Karaman, E.; Yuce, T.; Ozek, M.A.; Turksoy, V.A. Amniotic fluid levels of selected trace elements and heavy metals in pregnancies complicated with neural tube defects. Congenit. Anom. 2020, 60, 136–141. [Google Scholar] [CrossRef]
- Tian, T.; Liu, J.; Lu, X.; Qiu, X.; Wei, J.; Wang, C.; Liu, M.; Yin, S.; Jin, L.; Wang, L.; et al. Selenium protects against the likelihood of fetal neural tube defects partly via the arginine metabolic pathway. Clin. Nutr. 2022, 41, 838–846. [Google Scholar] [CrossRef]
- Yin, S.; Wang, C.; Wei, J.; Wang, D.; Jin, L.; Liu, J.; Wang, L.; Li, Z.; Ren, A.; Yin, C. Essential trace elements in placental tissue and risk for fetal neural tube defects. Environ. Int. 2020, 139, 105688. [Google Scholar] [CrossRef]
- Kim, S.S.; Meeker, J.D.; Keil, A.P.; Aung, M.T.; Bommarito, P.A.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers. Environ. Res. 2019, 179, 108854. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Li, W.-D.; Xu, X.-D.; Cui, F.-P.; Chen, P.-P.; Deng, Y.-L.; Miao, Y.; Luo, Q.; Zeng, J.-Y.; et al. Individual and mixtures of metal exposures in associations with biomarkers of oxidative stress and global DNA methylation among pregnant women. Chemosphere 2022, 293, 133662. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Massa, V.; George, T.M.; Qureshy, S.; Bulfamante, G.P.; Finnell, R.H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019, 111, 1455–1467. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharm. Rev. 2011, 63, 218–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the Role of Nitric Oxide and Its Clinical Applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 56. [Google Scholar] [CrossRef]
- Zullino, S.; Buzzella, F.; Simoncini, T. Nitric oxide and the biology of pregnancy. Vasc. Pharmacol. 2018, 110, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kundu, T.K.; Velayutham, M.; Zweier, J.L. Aldehyde Oxidase Functions as a Superoxide Generating NADH Oxidase: An Important Redox Regulated Pathway of Cellular Oxygen Radical Formation. Biochemistry 2012, 51, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Sultana, Z.; Aitken, R.J.; Morris, J.; Park, F.; Andrew, B.; Riley, S.C.; Smith, R. Evidence that fetal death is associated with placental aging. Am. J. Obstet. Gynecol. 2017, 217, 441.e1–441.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Many, A.; Westerhausen-Larson, A.; Kanbour-Shakir, A.; Roberts, J.M. Xanthine oxidase/dehydrogenase is present in human placenta. Placenta 1996, 17, 361–365. [Google Scholar] [CrossRef]
- Many, A.; Hubel, C.A.; Fisher, S.J.; Roberts, J.M.; Zhou, Y. Invasive Cytotrophoblasts Manifest Evidence of Oxidative Stress in Preeclampsia. Am. J. Pathol. 2000, 156, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, Y.; Fan, K.; Jin, L. Serum uric acid in early pregnancy and risk of gestational diabetes mellitus: A cohort study of 85,609 pregnant women. Diabetes Metab. 2022, 48, 101293. [Google Scholar] [CrossRef]
- Woo, W.H.; Yang, H.; Wong, K.P.; Halliwell, B. Sulphite oxidase gene expression in human brain and in other human and rat tissues. Biochem. Biophys. Res. Commun. 2003, 305, 619–623. [Google Scholar] [CrossRef]
- Klein, J.M.; Schwarz, G. Cofactor-dependent maturation of mammalian sulfite oxidase links two mitochondrial import pathways. J. Cell Sci. 2012, 125, 4876–4885. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Krizowski, S.; Fischer-Schrader, K.; Niks, D.; Tejero, J.; Sparacino-Watkins, C.; Wang, L.; Ragireddy, V.; Frizzell, S.; Kelley, E.E. Sulfite Oxidase Catalyzes Single-Electron Transfer at Molybdenum Domain to Reduce Nitrite to Nitric Oxide. Antioxid. Redox Signal. 2015, 23, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hänsch, R.; Lang, C.; Riebeseel, E.; Lindigkeit, R.; Gessler, A.; Rennenberg, H.; Mendel, R.R. Plant Sulfite Oxidase as Novel Producer of H2O2: Combination of enzyme catalysis with a subsequent non-enzymatic reaction step. J. Biol. Chem. 2006, 281, 6884–6888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, R.S.; Hänsch, R.; Mendel, R.R.; Hille, R. Oxidative half-reaction of arabidopsis thaliana sulfite oxidase: Generation of superoxide by a peroxisomal enzyme. J. Biol. Chem. 2009, 284, 35479–35484. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.F.; Chi, C.S.; Tsai, C.R.; Chen, H.C.; Lee, I.C. Prenatal brain disruption in isolated sulfite oxidase deficiency. Orphanet J. Rare Dis. 2017, 12, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, E.P.; Köfeler, H.; Hendriks, D.F.; Nordling, Å.; Gogvadze, V.; Mkrtchian, S.; Näslund, E.; Ingelman-Sundberg, M. Expression and Function of mARC: Roles in Lipogenesis and Metabolic Activation of Ximelagatran. PLoS ONE 2015, 10, e0138487. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.M.; Busch, J.D.; Potting, C.; Baker, M.J.; Langer, T.; Schwarz, G. The Mitochondrial Amidoxime-reducing Component (mARC1) Is a Novel Signal-anchored Protein of the Outer Mitochondrial Membrane. J. Biol. Chem. 2012, 287, 42795–42803. [Google Scholar] [CrossRef] [Green Version]
- Ott, G.; Havemeyer, A.; Clement, B. The mammalian molybdenum enzymes of mARC. JBIC J. Biol. Inorg. Chem. 2015, 20, 265–275. [Google Scholar] [CrossRef]
- Krompholz, N.; Krischkowski, C.; Reichmann, D.; Garbe-Schönberg, D.; Mendel, R.-R.; Bittner, F.; Clement, B.; Havemeyer, A. The Mitochondrial Amidoxime Reducing Component (mARC) Is Involved in Detoxification of N-Hydroxylated Base Analogues. Chem. Res. Toxicol. 2012, 25, 2443–2450. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.E.; Tejero, J.; Sun, B.; Gauthier, M.C.; Thomas, J.; Ragireddy, V.; Merchant, B.A.; Wang, J.; Azarov, I.; Basu, P.; et al. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J. Biol. Chem. 2014, 289, 10345–10358. [Google Scholar] [CrossRef] [Green Version]
- Lewis, L.C.; Chen, L.; Hameed, L.S.; Kitchen, R.R.; Maroteau, C.; Nagarajan, S.R.; Norlin, J.; Daly, C.E.; Szczerbinska, I.; Hjuler, S.T.; et al. Hepatocyte mARC1 promotes fatty liver disease. JHEP Rep. 2023, 5, 100693. [Google Scholar] [CrossRef]
- Emdin, C.A.; Haas, M.E.; Khera, A.V.; Aragam, K.; Chaffin, M.; Klarin, D.; Hindy, G.; Jiang, L.; Wei, W.Q.; Feng, Q.; et al. A missense variant in Mitochondrial Amidoxime Reducing Component 1 gene and protection against liver disease. PLoS Genet. 2020, 16, e1008629. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Seaman, C.J.; Paragas, E.M.; Xi, H.; Herpoldt, K.L.; King, N.P.; Jones, J.P.; Isoherranen, N. Aldehyde Oxidase Contributes to All-Trans-Retinoic Acid Biosynthesis in Human Liver. Drug. Metab. Dispos. 2021, 49, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucl. Nucl. Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, W.G.; Martins-Santos, M.E.S.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef]
- Pleskacova, A.; Bartakova, V.; Chalasova, K.; Pacal, L.; Kankova, K.; Tomandl, J. Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus-The Effect on Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2018, 19, 3696. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Fukushima, K.; Takao, T.; Seki, H.; Takeda, S.; Wake, N. Oxidative stress produced by xanthine oxidase induces apoptosis in human extravillous trophoblast cells. J. Reprod. Dev. 2013, 59, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Grings, M.; Moura, A.P.; Parmeggiani, B.; Pletsch, J.T.; Cardoso, G.M.F.; August, P.M.; Matté, C.; Wyse, A.T.S.; Wajner, M.; Leipnitz, G. Bezafibrate prevents mitochondrial dysfunction, antioxidant system disturbance, glial reactivity and neuronal damage induced by sulfite administration in striatum of rats: Implications for a possible therapeutic strategy for sulfite oxidase deficiency. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2135–2148. [Google Scholar] [CrossRef]
- Taylor, C.E. A novel treatment for “morning sickness”: Nausea of pregnancy could be induced by excess sulfite which molybdenum can help alleviate. Med. Hypotheses 2016, 95, 31–33. [Google Scholar] [CrossRef]
- Havemeyer, A.; Bittner, F.; Wollers, S.; Mendel, R.; Kunze, T.; Clement, B. Identification of the Missing Component in the Mitochondrial Benzamidoxime Prodrug-converting System as a Novel Molybdenum Enzyme. J. Biol. Chem. 2006, 281, 34796–34802. [Google Scholar] [CrossRef] [Green Version]
- Tejada-Jimenez, M.; Chamizo-Ampudia, A.; Calatrava, V.; Galvan, A.; Fernandez, E.; Llamas, A. From the Eukaryotic Molybdenum Cofactor Biosynthesis to the Moonlighting Enzyme mARC. Molecules 2018, 23, 3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Authors | Method of Detection | Population Number | Population Details | Matrix | Country | Results |
|---|---|---|---|---|---|---|
| Ajibola et al., 2014 [128] | AAS | 148 subjects (98 diabetes, 50 control) | Diabetes: 65.3% female, control: no details provided; diabetes: 55.92 ± 12.82 years, control: 42.06 ± 7.31 years | Plasma (fasting) | Nigeria | Significantly increased Mo in diabetics in comparison to control. |
| Flores et al., 2011 [129] | ICP-MS | 88 subjects (52 slight-to-moderate diabetes complications, 24 severe diabetes complications, 12 control | Either sex, no further details provided; 52 ± 8 years | 24 h urine, fasting serum | Mexico | Urinary Mo was significantly decreased in severe diabetics in comparison to moderate diabetics; Serum Mo was increased in diabetic patients in comparison to control, and significantly increased in severe vs. moderate diabetic subjects. |
| Li et al., 2021 [127] | ICP-MS | 5356 subjects (2678 ‘metabolic syndrome’ diagnosed, 2678 control) | MetS: 55.2% male, 54.5 (10.9) years; control: 55.2% male, 54.8 (10.8) years | Plasma (fasting) | China | Plasma Mo inversely correlated with risk of Metabolic Syndrome, in a dose-response manner. |
| Menke et al., 2015 [131] | ICP-MS | 9447 subjects (1364 diabetes, 8083 control) | Diabetes: 51.7% ± 1.75 female, 58.6 ± 0.54 years, control: 50.5 ± 0.69 female, 45.6 ± 0.30 years | Spot urine | US | Higher quartiles of Mo were associated with greater HOMA of insulin resistance; Mo was positively associated with diabetes prevalence. |
| Wang et al., 2020 [133] | ICP-MS | 1237 subjects, random selection | Female only; diabetes: 50.0 years, control: 49.5 years | Spontaneously voided urine (first morning) | US | No association found between urinary Mo and diabetes. |
| Wang et al., 2020 [132] | ICP-MS | 1262 subjects, random selection | Female only; 49.7 (2.8) years at baseline | Spontaneously voided urine (first morning) | US | Urinary Mo concentration was significantly inversely associated with HOMA of insulin resistance at baseline. |
| Yang et al., 2023 [130] | ICP-MS | 1423 subjects, random selection | 56.5% male, 46.9 ± 17.2 years | Spot urine | US | High urinary Mo levels were associated with elevated FPG and HbA1c levels. |
| Authors | Method of Detection | Population Number | Population Details | Matrix | Country | Results |
|---|---|---|---|---|---|---|
| Al-Saleh et al., 2004 [138] | AAS | 30 subjects (15 gestational diabetics, 15 control) | Late third trimester (gestational diabetics: 39.0 ± 0.3 weeks, control: 40.0 ± 0.4 weeks) | Blood/Serum | Kuwait | No significant difference found in maternal Mo serum concentrations between control and GDM group. Significantly increased Mo concentrations in umbilical vein and artery of GDM group in comparison to control. |
| Al-Saleh et al., 2005 [140] | AAS | 31 subjects (14 insulin-dependent diabetics, 17 control) | Late third trimester (insulin-dependent diabetics: 38 ± 0.31 weeks, control: 40 ± 0.4 weeks) | Serum | Kuwait | No significant difference in maternal Mo serum concentrations between control and diabetic group. Significantly increased Mo concentrations in umbilical vein and artery of diabetic group in comparison to control. |
| Al-Saleh et al., 2007 [139] | AAS | 21 subjects (10 obese gestational diabetics, 11 control obese) | Late third trimester (obese gestational diabetics: 38.0 ± 0.40 weeks, obese control: 40.0 ± 0.50) | Serum | Kuwait | Increased Mo serum concentration in maternal vein and umbilical artery of obese gestational diabetics in comparison to obese controls. |
| McAlpine et al., 2019 [137] | ICP-MS | 127 subjects (89 used supplements, 38 did not) | Late second and early third trimester, 180–210 days’ gestation (25–30 weeks) | Serum (Fasting) | Australia | No evidence of significant association between Mo in serum or dietary levels and the incidence of GDM. |
| Zheng et al., 2019 [135] | ICP-MS | 1857 healthy non-obese subjects | Late first trimester (median: 12 weeks’ gestation) | Plasma | US | Every 50% increase in molybdenum concentration was associated with 1.2 mg/dL lower mean glucose level (95% CI: −2.3, −0.1 mg/dL) |
| Zheng et al., 2020 [136] | ICP-MS | 1720 healthy non-obese subjects | Late first trimester (median: 12 weeks’ gestation) | Plasma | US | Inverse association between Mo plasma levels and glucose levels, in three different statistical models. Strong associations were not observed in BKMR modelling in comparison to adaptive LASSO and GAM. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foteva, V.; Fisher, J.J.; Qiao, Y.; Smith, R. Does the Micronutrient Molybdenum Have a Role in Gestational Complications and Placental Health? Nutrients 2023, 15, 3348. https://doi.org/10.3390/nu15153348
Foteva V, Fisher JJ, Qiao Y, Smith R. Does the Micronutrient Molybdenum Have a Role in Gestational Complications and Placental Health? Nutrients. 2023; 15(15):3348. https://doi.org/10.3390/nu15153348
Chicago/Turabian StyleFoteva, Vladimira, Joshua J. Fisher, Yixue Qiao, and Roger Smith. 2023. "Does the Micronutrient Molybdenum Have a Role in Gestational Complications and Placental Health?" Nutrients 15, no. 15: 3348. https://doi.org/10.3390/nu15153348
APA StyleFoteva, V., Fisher, J. J., Qiao, Y., & Smith, R. (2023). Does the Micronutrient Molybdenum Have a Role in Gestational Complications and Placental Health? Nutrients, 15(15), 3348. https://doi.org/10.3390/nu15153348






