The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.1.1. Research Type
2.1.2. Research Subjects
2.1.3. Intervention Measures
2.1.4. Outcome Indicators
2.2. Exclusion Criteria
2.3. Retrieval Strategies
- (1)
- Population (P): patients with T2DM.
- (2)
- Intervention (I): probiotic supplementation.
- (3)
- Comparison (C): placebo.
- (4)
- Outcome (O): BMI, fasting glucose, fasting insulin, HbA1c, HOMA-insulin resistance, TG, TC, HDL, LDL.
- (5)
- Study design (S): randomized clinical trials (RCTs).
2.4. Literature Screening and Data Extraction
2.5. Risk of Bias Assessment
2.6. Statistical Methods
3. Results
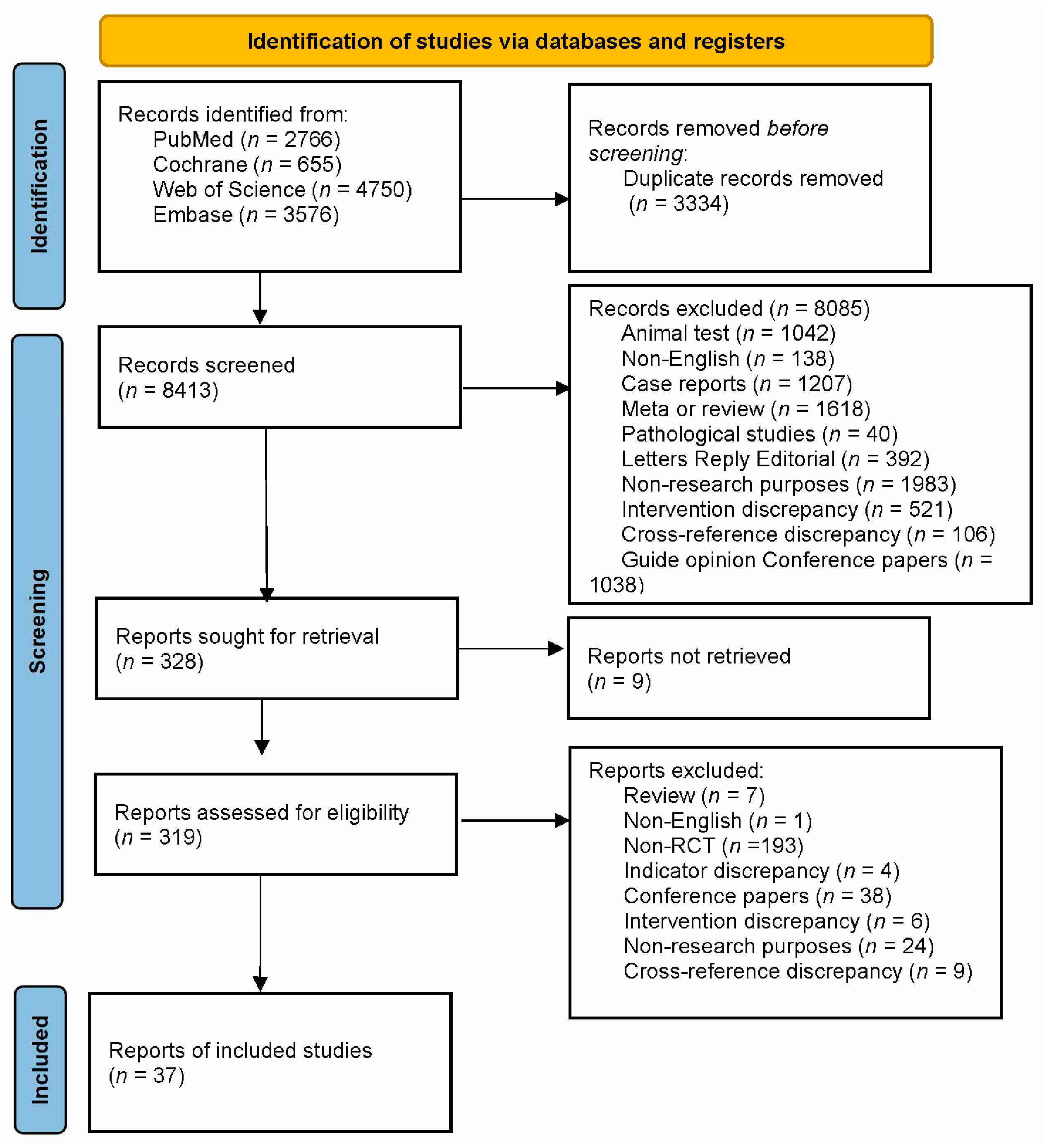
3.1. Literature Search Results
3.2. Basic Characteristics of the Included Studies
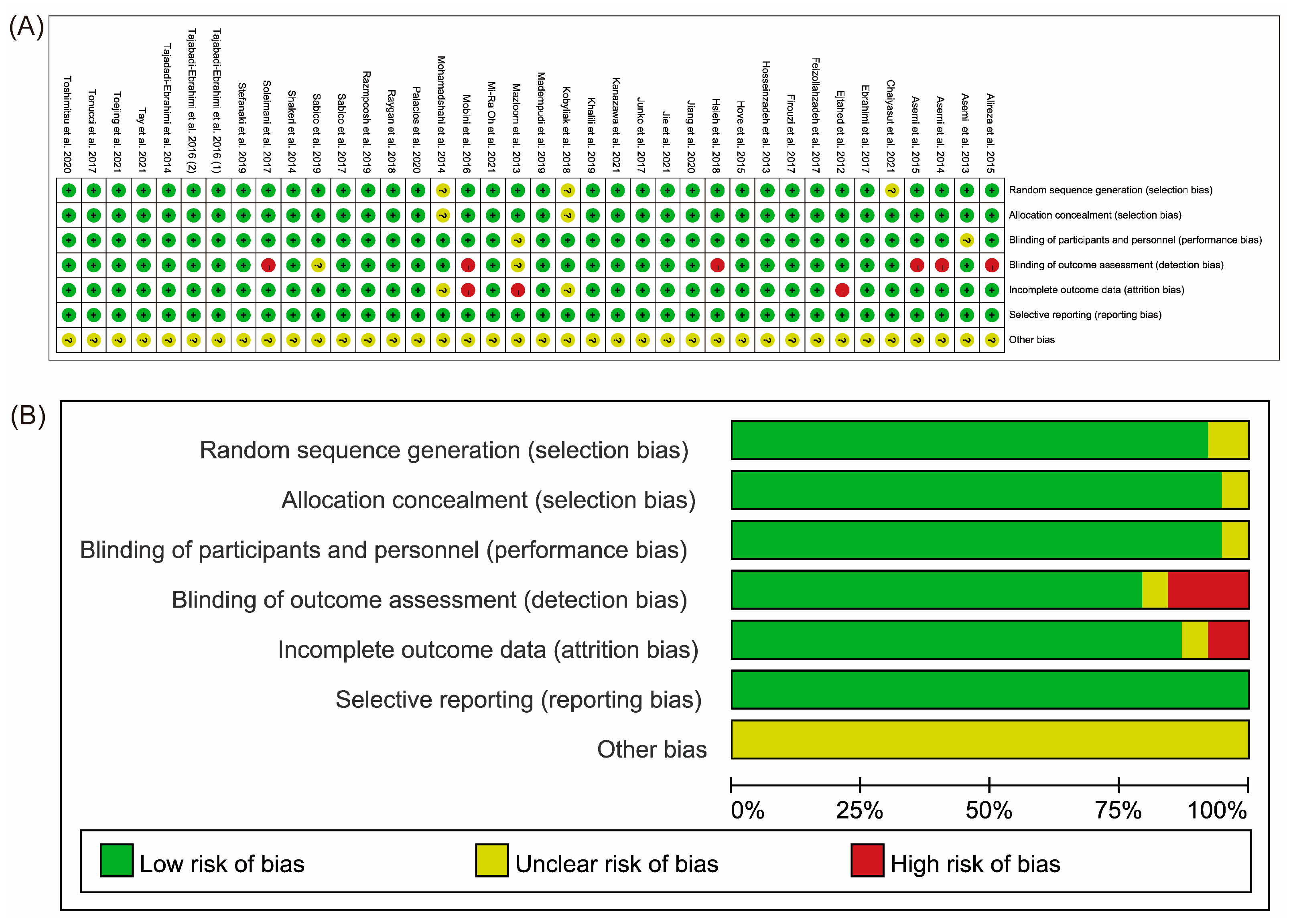
3.3. Quality Assessment of the Included Studies
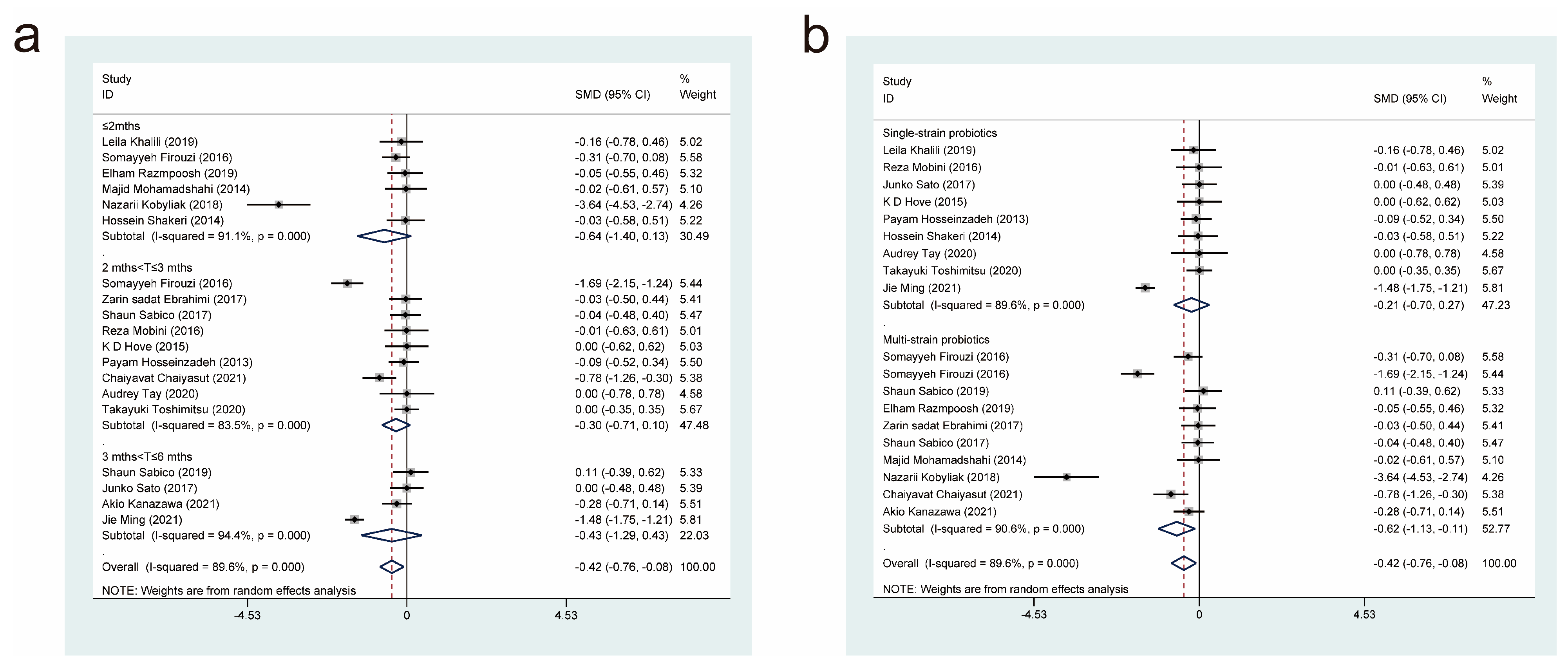
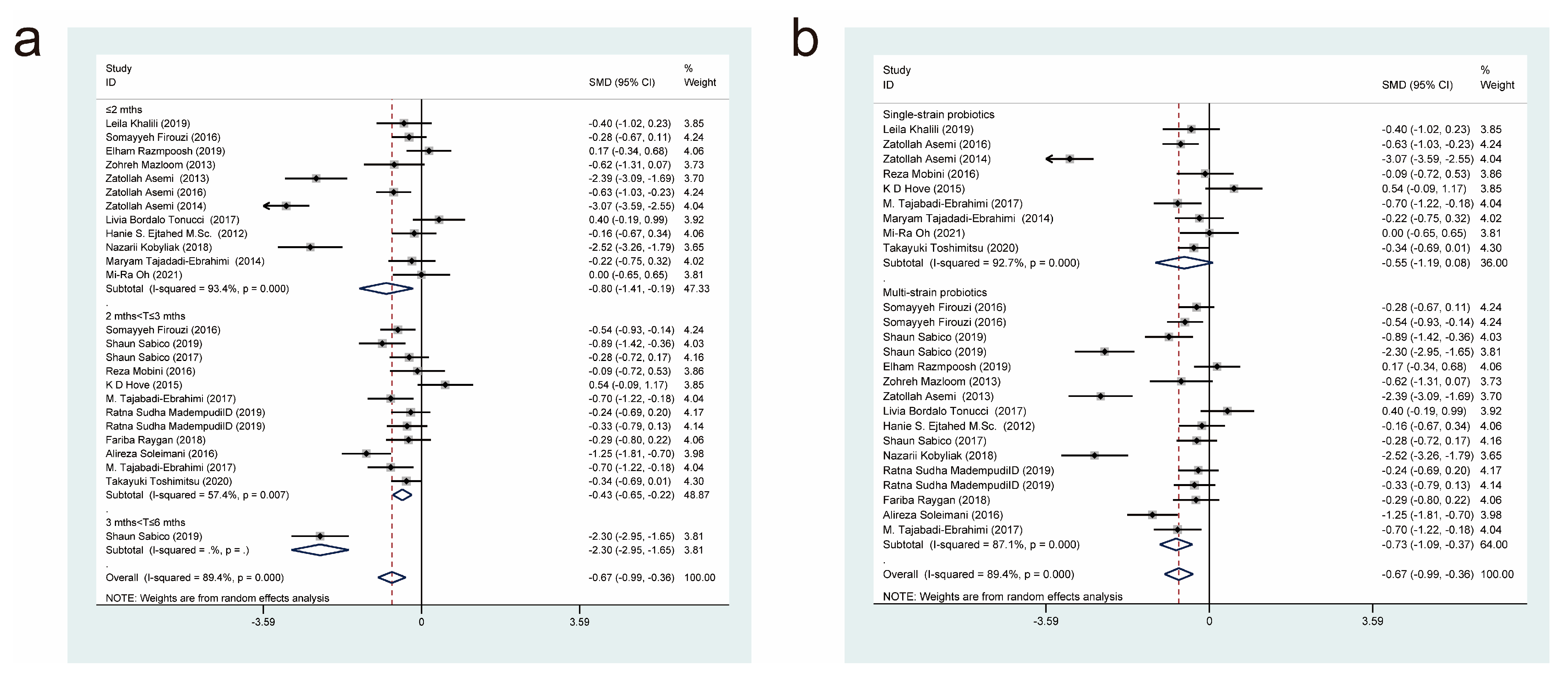
3.4. Meta-Analysis Results
3.4.1. Effects of Probiotic Therapy on Body Mass Index
3.4.2. Effects of Probiotic Therapy on Fasting Blood Glucose Concentration
3.4.3. Effects of Probiotic Therapy on Fasting Insulin Concentration
3.4.4. Effects of Probiotic Therapy on Glycated Haemoglobin Concentration
3.4.5. Effects of Probiotic Therapy on Homeostatic Model Assessment for Insulin Resistance Score
3.4.6. Effects of Probiotic Therapy on Triglyceride Concentration
3.4.7. Effects of Probiotic Therapy on Total Cholesterol Concentration
3.4.8. Effects of Probiotic Therapy on Low-Density Lipoprotein Concentration
3.4.9. Effects of Probiotic Therapy on High-Density Lipoprotein Concentration
3.5. Sensitivity Analysis and Publication Bias Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Wang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Meta-analysis of randomized controlled trials of the effects of probiotics on type 2 diabetes in adults. Clin. Nutr. 2022, 41, 365–373. [Google Scholar] [CrossRef]
- Nie, Q.X.; Hu, J.L.; Gao, H.; Fan, L.L.; Chen, H.H.; Nie, S.P. Polysaccharide from Plantago asiatica L. attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocoll. 2019, 86, 34–42. [Google Scholar] [CrossRef]
- Aschner, P. Insulin Therapy in Type 2 Diabetes. Am. J. Ther. 2020, 27, E79–E90. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Fainberg, U.; Barrett, T. Liraglutide in Children and Teens with Type 2 Diabetes REPLY. N. Engl. J. Med. 2019, 381, 1787. [Google Scholar] [CrossRef]
- Komaroff, A.L. The Microbiome and Risk for Obesity and Diabetes. J. Am. Med. Assoc. 2017, 317, 355–356. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zhang, F.; Ding, X.Y.; Wu, G.J.; Lam, Y.Y.; Wang, X.J.; Fu, H.Q.; Xue, X.H.; Lu, C.H.; Ma, J.L.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Kramer, M.; Gummesson, A.; Perkins, R.; Bergstrom, G.; Backhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390. [Google Scholar] [CrossRef]
- Chen, Z.L.; Radjabzadeh, D.; Chen, L.M.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.Y.; et al. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity a Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Que, Y.Y.; Cao, M.; He, J.Q.; Zhang, Q.; Chen, Q.Y.; Yan, C.S.; Lin, A.Q.; Yang, L.X.; Wu, Z.Z.; Zhu, D.; et al. Gut Bacterial Characteristics of Patients with Type 2 Diabetes Mellitus and the Application Potential. Front. Immunol. 2021, 12, 3218. [Google Scholar] [CrossRef]
- Ke, X.X.; Walker, A.; Haange, S.B.; Lagkouvardos, I.; Liu, Y.W.; Schmitt-Kopplin, P.; Von Bergen, M.; Jehmlich, N.; He, X.; Clavel, T.; et al. Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Mol. Metab. 2019, 22, 96–109. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ansari, M.G.A.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: A double-blind randomized clinical trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Jalili, R.B. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. Daru-J. Pharm. Sci. 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Tao, Y.W.; Gu, Y.L.; Mao, X.Q.; Zhang, L.; Pei, Y.F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar] [CrossRef]
- Hu, Y.M.; Zhou, F.; Yuan, Y.; Xu, Y.C. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med. Clin. 2017, 148, 362–370. [Google Scholar] [CrossRef]
- Kocsis, T.; Molnar, B.; Nemeth, D.; Hegyi, P.; Szakacs, Z.; Balint, A.; Garami, A.; Soos, A.; Marta, K.; Solymar, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef]
- Khalili, L.; Alipour, B.; Asghari Jafar-Abadi, M.; Faraji, I.; Hassanalilou, T.; Mesgari Abbasi, M.; Vaghef-Mehrabany, E.; Alizadeh Sani, M. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran. Biomed. J. 2019, 23, 68–77. [Google Scholar] [CrossRef]
- Firouzi, S.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A.; Barakatun-Nisak, M.Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: A randomized controlled trial. Eur. J. Nutr. 2017, 56, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab. Syndr.-Clin. Res. Rev. 2019, 13, 175–182. [Google Scholar] [CrossRef]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar]
- Ebrahimi, Z.S.; Nasli-Esfahani, E.; Nadjarzade, A.; Mozaffari-Khosravi, H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: A randomized, double-blind, clinical trial. J. Diabetes Metab. Disord. 2017, 16, 23. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes (Publication with Expression of Concern. See, J.A.N.; 2023). Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.A.; Shakeri, H.; Esmaillzadeh, A. Effects of synbiotic food consumption on metabolic status of diabetic patients: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef]
- Tonucci, L.B.; dos Santos, K.M.O.; de Oliveira, L.L.; Ribeiro, S.M.R.; Martino, H.S.D. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naive T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Gheshlaghi, Z.B.; Vahedjabbari, M. Effect of Probiotic Fermented Milk (Kefir) on Glycemic Control and Lipid Profile In Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Mobini, R.; Tremaroli, V.; Stahlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Perkins, R.; Perkins, R.; Baackhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Feizollahzadeh, S.; Ghiasvand, R.; Rezaei, A.; Khanahmad, H.; Sadeghi, A.; Hariri, M. Effect of Probiotic Soy Milk on Serum Levels of Adiponectin, Inflammatory Mediators, Lipid Profile, and Fasting Blood Glucose among Patients with Type II Diabetes Mellitus. Probiotics Antimicrob. Proteins 2017, 9, 41–47. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 12115. [Google Scholar] [CrossRef]
- Hove, K.D.; Brons, C.; Faerch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: A randomised double-blind placebo-controlled study. Eur. J. Endocrinol. 2015, 172, 11–20. [Google Scholar] [CrossRef]
- Tajabadi-Ebrahimi, M.; Sharifi, N.; Farrokhian, A.; Raygan, F.; Karamali, F.; Razzaghi, R.; Taheri, S.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effect of Synbiotic Administration on Markers of Insulin Metabolism and Lipid Profiles in Overweight Type 2 Diabetic Patients with—Coronary Heart Disease. Exp. Clin. Endocrinol. Diabetes 2017, 125, 21–27. [Google Scholar] [CrossRef]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Shahbazian, H.; Kaydani, G.A.; Mohammadi, F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014, 4, 83–88. [Google Scholar]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr.-Clin. Res. Rev. 2018, 12, 617–624. [Google Scholar] [CrossRef]
- Tajadadi-Ebrahimi, M.; Bahmani, F.; Shakeri, H.; Hadaegh, H.; Hijijafari, M.; Abedi, F.; Asemi, Z. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: A double-blind, randomized, controlled clinical trial. Ann. Nutr. Metab. 2014, 65, 34–41. [Google Scholar] [CrossRef]
- Hosseinzadeh, P.; Javanbakht, M.H.; Mostafavi, S.A.; Djalali, M.; Derakhshanian, H.; Hajianfar, H.; Bahonar, A.; Djazayery, A. Brewer’s Yeast Improves Glycemic Indices in Type 2 Diabetes Mellitus. Int J. Prev. Med. 2013, 4, 1131–1138. [Google Scholar]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: A double blind, randomized, placebo controlled study. PLoS ONE 2019, 14, e0225168. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Kesika, P.; Khongtan, S.; Khampithum, N.; Thangaleela, S.; Peerajan, S.; Bumrungpert, A.; Chaiyasut, K.; Sirilun, S.; et al. Synbiotic Supplementation Improves Obesity Index and Metabolic Biomarkers in Thai Obese Adults: A Randomized Clinical Trial. Foods 2021, 10, 1580. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xu, D.; Wang, Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J. Clin. Lab. Anal. 2021, 35, e23650. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.R.; Jang, H.Y.; Lee, S.Y.; Jung, S.J.; Chae, S.W.; Lee, S.O.; Park, B.H. Lactobacillus plantarum HAC01 Supplementation Improves Glycemic Control in Prediabetic Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2337. [Google Scholar] [CrossRef]
- Tay, A.; Pringle, H.; Penning, E.; Plank, L.D.; Murphy, R. PROFAST: A Randomized Trial Assessing the Effects of Intermittent Fasting and Lacticaseibacillus rhamnosus Probiotic among People with Prediabetes. Nutrients 2020, 12, 3530. [Google Scholar] [CrossRef] [PubMed]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Lam, Y.Y.; Manuel, R.; Briskey, D.; Hendy, C.; Kim, J.N.; Ishoey, T.; et al. Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study. Nutrients 2020, 12, 2041. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Gotou, A.; Sashihara, T.; Hachimura, S.; Shioya, N.; Suzuki, S.; Asami, Y. Effects of 12-Week Ingestion of Yogurt Containing Lactobacillus plantarum OLL2712 on Glucose Metabolism and Chronic Inflammation in Prediabetic Adults: A Randomized Placebo-Controlled Trial. Nutrients 2020, 12, 374. [Google Scholar] [CrossRef]
- Stefanaki, C.; Michos, A.; Mastorakos, G.; Mantzou, A.; Landis, G.; Zosi, P.; Bacopoulou, F. Probiotics in Adolescent Prediabetes: A Pilot RCT on Glycemic Control and Intestinal Bacteriome. J. Clin. Med. 2019, 8, 1743. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Tsai, W.H.; Jheng, Y.P.; Su, S.L.; Wang, S.Y.; Lin, C.C.; Chen, Y.H.; Chang, W.W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Borzabadi, S.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51. [Google Scholar] [CrossRef]
- Soleimani, A.; Zarrati Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Ming, J.; Yu, X.; Xu, X.; Wang, L.; Ding, C.; Wang, Z.; Xie, X.; Li, S.; Yang, W.; Luo, S.; et al. Effectiveness and safety of Bifidobacterium and berberine in human hyperglycemia and their regulatory effect on the gut microbiota: A multi-center, double-blind, randomized, parallel-controlled study. Genome Med. 2021, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Real, J.M.F.; Guarner, F.; Gueimonde, M.; Rodriguez, J.M.; de Pipaon, M.S.; Sanz, Y. Gut microbes and health. Gastroenterol. Y Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Lau, W.L.; Tran, T.; Rhee, C.M.; Kalantar-Zadeh, K.; Vaziri, N.D. Diabetes and the Gut Microbiome. Semin. Nephrol. 2021, 41, 104–113. [Google Scholar] [CrossRef]
- Zhai, L.X.; Wu, J.Y.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef] [PubMed]
- Wieers, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; de Foy, J.M.P.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Duque, A.; Saad, S.M.I.; Sivieri, K. Gut microbiome approaches to treat obesity in humans. Appl. Microbiol. Biotechnol. 2019, 103, 1081–1094. [Google Scholar] [CrossRef]
- Salazar, J.; Angarita, L.; Morillo, V.; Navarro, C.; Martinez, M.S.; Chacin, M.; Torres, W.; Rajotia, A.; Rojas, M.; Cano, C.; et al. Microbiota and Diabetes Mellitus: Role of Lipid Mediators. Nutrients 2020, 12, 3039. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Dilidaxi, D.; Wu, Y.C.; Sailike, J.; Sun, X.; Nabi, X.H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Singer-Englar, T.; Barlow, G.; Mathur, R. Obesity, diabetes, and the gut microbiome: An updated review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Allayee, H.; Hazen, S.L. Contribution of Gut Bacteria to Lipid Levels Another Metabolic Role for Microbes? Circ. Res. 2015, 117, 750–754. [Google Scholar] [CrossRef]
- Kumar, R.; Grover, S.; Batish, V.K. Bile Salt Hydrolase (Bsh) Activity Screening of Lactobacilli: In Vitro Selection of Indigenous Lactobacillus Strains with Potential Bile Salt Hydrolysing and Cholesterol-Lowering Ability. Probiotics Antimicrob. Proteins 2012, 4, 162–172. [Google Scholar] [CrossRef]
- Del Bas, J.M.; Ricketts, M.L.; Vaque, M.; Sala, E.; Quesada, H.; Ardevol, A.; Salvado, M.J.; Blay, M.; Arola, L.; Moore, D.D.; et al. Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol. Nutr. Food Res. 2009, 53, 805–814. [Google Scholar] [CrossRef]
- Pols, T.W.H.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The Bile Acid Membrane Receptor TGR5: A Valuable Metabolic Target. Dig. Dis. 2011, 29, 37–44. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Taranto, M.P.; Murga, M.L.F.; Lorca, G.; de Valdez, G.F. Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri. J. Appl. Microbiol. 2003, 95, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Cipriani, S.; Renga, B.; Bruno, A.; D’Amore, C.; Distrutti, E.; Fiorucci, S. VSL#3 Resets Insulin Signaling and Protects against NASH and Atherosclerosis in a Model of Genetic Dyslipidemia and Intestinal Inflammation. PLoS ONE 2012, 7, e45425. [Google Scholar]
- Zhang, M.; Wang, C.L.; Wang, C.H.; Zhao, H.Y.; Zhao, C.Q.; Chen, Y.P.; Wang, Y.H.; McClain, C.; Feng, W.K. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J. Nutr. Biochem. 2015, 26, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Guerrero-Bonmatty, R.; Gil-Fernandez, G.; Rodriguez-Velasco, F.J.; Espadaler-Mazo, J. A Combination of Lactoplantibacillus plantarum Strains CECT7527, CECT7528, and CECT7529 Plus Monacolin K Reduces Blood Cholesterol: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1206. [Google Scholar] [CrossRef]
- Robert, S.; Steidler, L. Recombinant Lactococcus lactis can make the difference in antigen-specific immune tolerance induction, the Type 1 Diabetes case. Microb. Cell Factories 2014, 13, S11. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Hanninen, A.; Toivonen, R.; Poysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]

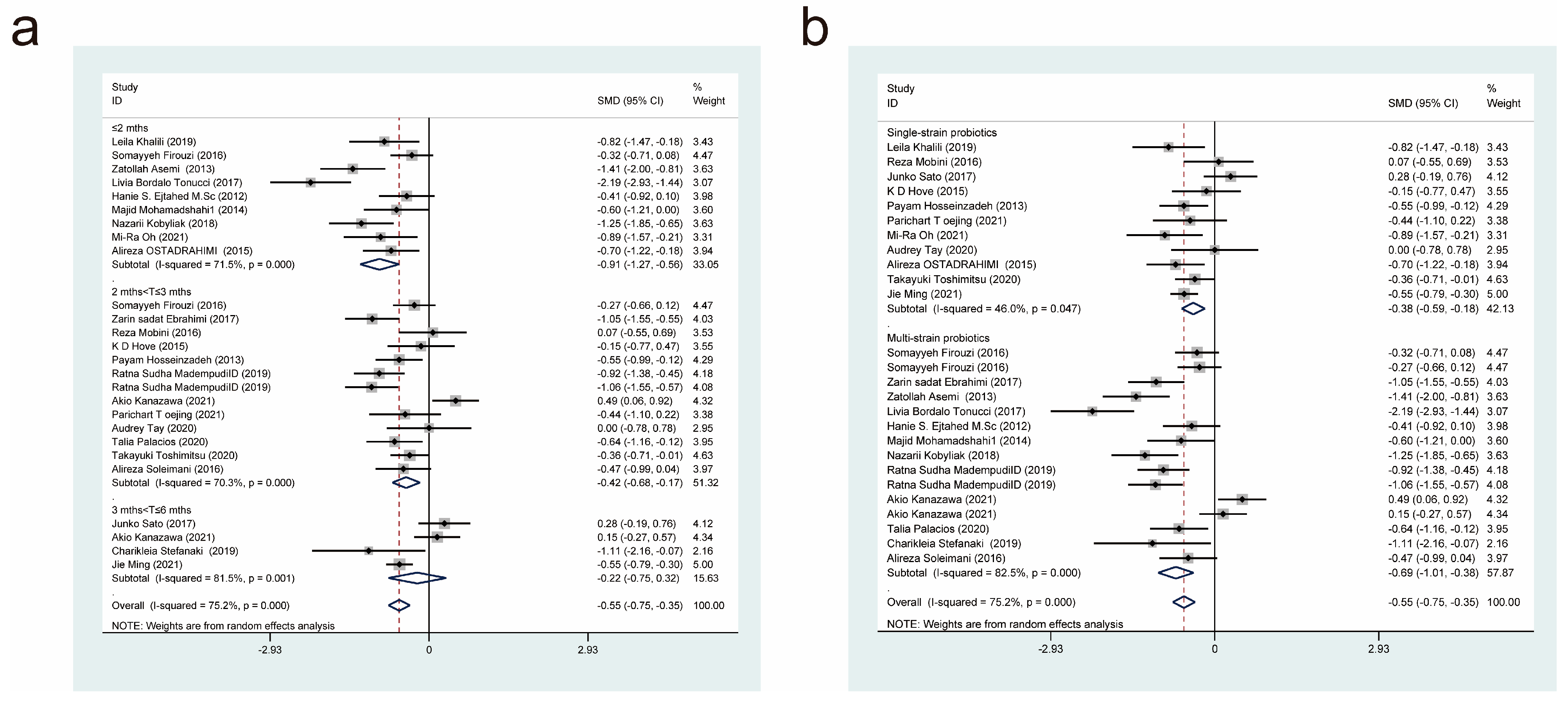

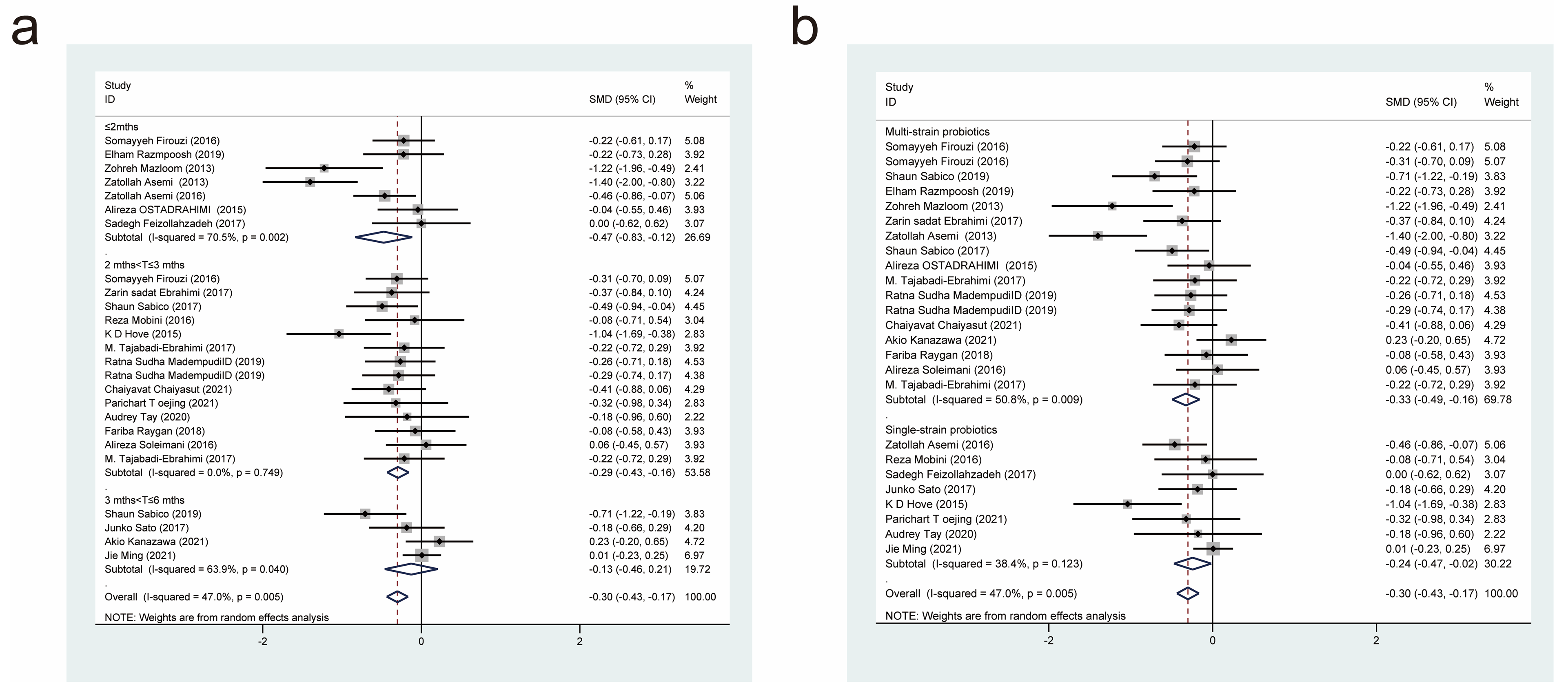
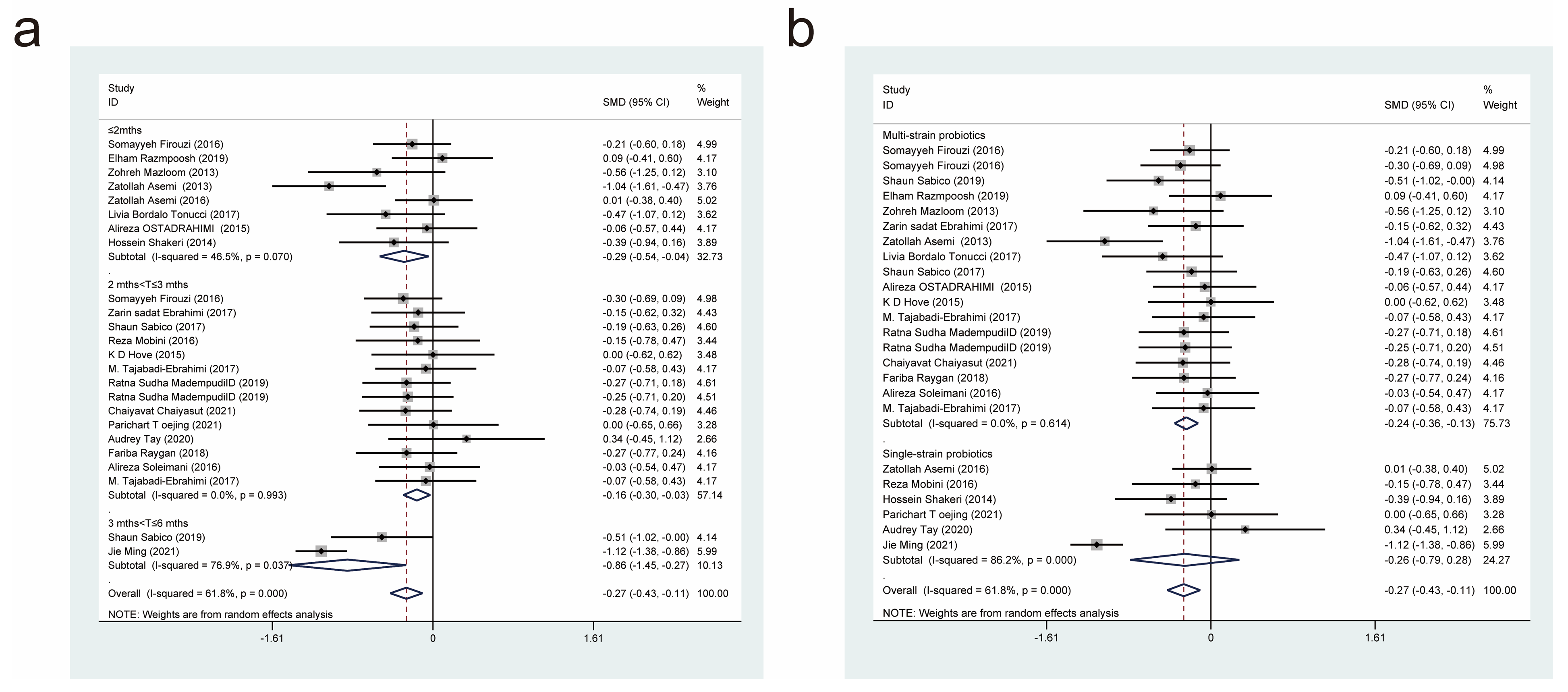

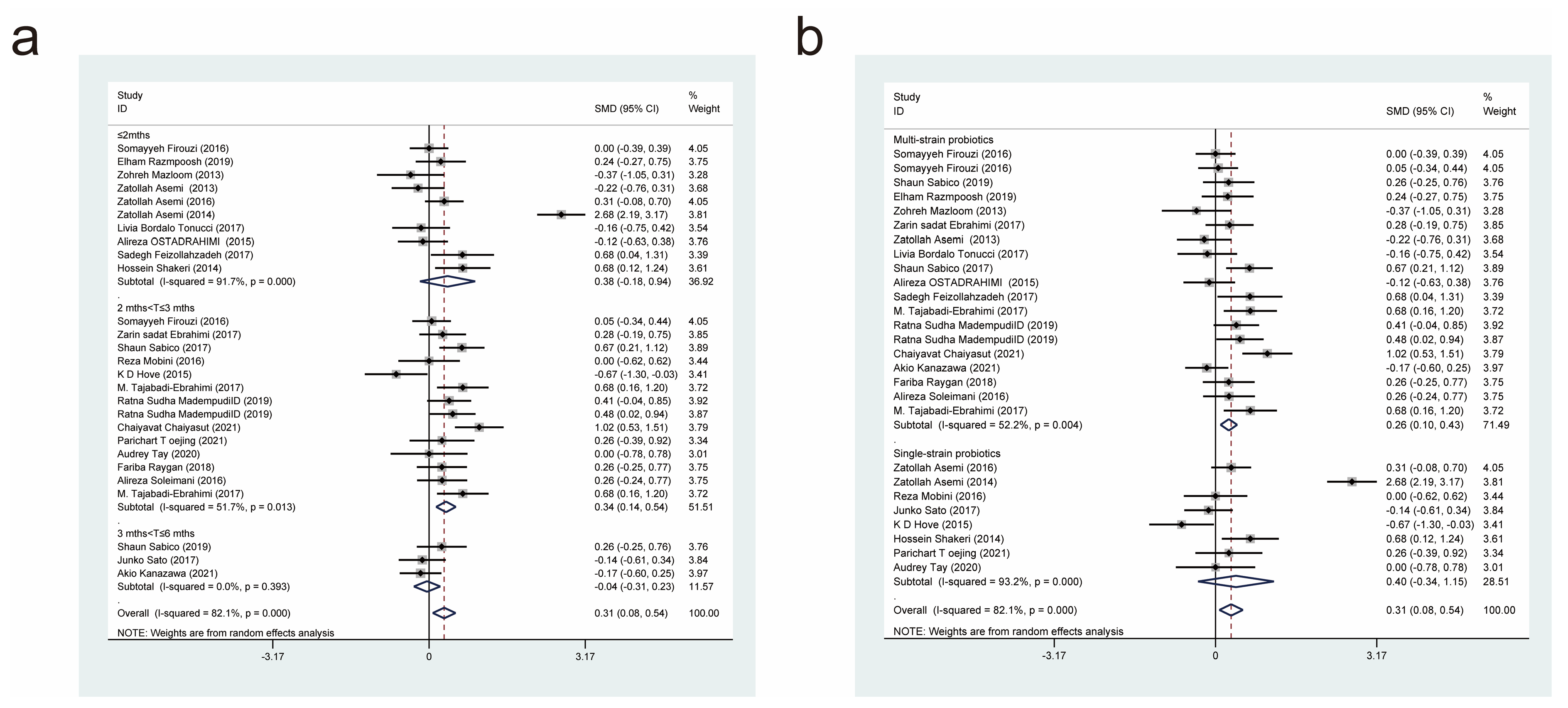
| Author/Year | Country | Intervention/Control (Size) | Age (Year) | Female/Male | Probiotic/Control | Dose (CFU/d) | Form | Duration | Measure Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Khalili et al., 2019 [17] | Iran | Probiotics (20) | 43.95 ± 8.14 | 13/7 | Lacidophilus casei | 2 × 108 | Capsules | 8 weeks | FBS/HbA1c/Insulin/HOMA-IR/ BMI |
| Control (20) | 45.00 ± 5.37 | 13/7 | Maltodextrin | ||||||
| Firouzi et al., 2017 [18] | Malaysia | Probiotics (68) | 52.90 ± 9.20 | NR | L. acidophilus, L. casei, L. lactis, Bifdobacterium, Actinobacteria, B. bifdum, B.longum, and B. infantis | 6 × 1010 | Powder | 12 weeks | FBG/HbA1c/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (68) | 54.20 ± 8.30 | NR | Placebo | ||||||
| Sabico et al., 2019 [11] | Saudi Arabia | Probiotics (39) | 48.00 ± 8.30 | 20/19 | B. bifdum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L.salivarius W24, L. lactis W19, and L. lactis W58 | 5 × 109 | Sachets | 12 weeks | Glucose/Insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (39) | 46.60 ± 5.90 | 18/21 | Maize starch and maltodextrins | ||||||
| Razmpoosh et al., 2019 [19] | Iran | Probiotic (30) | 58.6 ± 6.5 | 13/17 | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophilus | 4.9 × 1010 | Capsules | 6 weeks | FBG/HbA1c/insulin/HOMA-IR/ TC/TG/BMI |
| Control (30) | 61.3 ± 5.2 | 14/16 | Fructo-oligosaccharide, and Magnesium stearate | ||||||
| Mazloom et al., 2013 [20] | Iran | Probiotic (16) | 55.4 | NR | L. acidophilus, L. bulgaricus, L. bifidum, and L. casei. | NR | Capsules | 6 weeks | FBG/insulin/HOMA-IR/ TC/TG |
| Control (18) | 54.2 | NR | Magnesium stearate | ||||||
| Ebrahimi et al., 2017 [21] | Iran | Probiotic (35) | 58.71 ± 8.20 | 12/23 | Lactobacillus family, Bifidobacterium family, Streptococus thermophilus | NR | Capsules | 9 weeks | FBG/TC/TG/HDL-C/LDL-C |
| Control (35) | 58.71 ± 8.20 | 16/19 | Row starch | ||||||
| Asemi et al., 2013 [22] | Iran | Probiotic (27) | 50.51 ± 9.82 | NR | L. acidophilus, L. case, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. longum, Streptococcus thermophilus | 3.92 × 1010 | Capsules | 8 weeks | FBG/HbA1c/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (27) | 52.59 ± 7.14 | NR | Placebo | ||||||
| Asemi et al., 2016 [23] | Iran | Probiotic (51) | NR | NR | Lactobacillus sporogenes | 2.7 × 108 | Package | 6 weeks | FBG/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (51) | NR | NR | Isomalt, sorbitol and stevia | ||||||
| Asemi et al., 2014 [24] | Iran | Probiotic (62) | NR | NR | Lactobacillus sporogenes | 2.7 × 108 | Synbiotic food | 6 weeks | FBG/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (62) | NR | NR | Control food | ||||||
| Tonucci et al., 2017 [25] | Brazil | Probiotic (23) | 51.83 ± 6.64 | 11/12 | L. acidophilus La-5 B. lactis BB-12 | 109 | Fermented goat milk | 6 weeks | FBG/HbA1c/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C |
| Control (22) | 50.95 ± 7.20 | 8/14 | Conventional fermented milk | ||||||
| Ejtahed et al., 2012 [26] | Iran | Probiotic (30) | 50.87 ± 7.68 | 19/11 | B. lactis Bb12 and L. acidophilus La5 | 4 × 109 | Yogurt | 6 weeks | FBG/HbA1c/insulin |
| Control (30) | 51.00 ±7.32 | 18/12 | Conventional yogurts | ||||||
| Sabico et al., 2017 [27] | Saudi Arabia | Probiotic (39) | 48.0 ± 8.3 | 20/19 | B. bifdum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, L. lactis W19, and L. lactis W58 | 2 × 109 | Powder | 12 weeks | FBG/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C |
| Control (39) | 46.6 ± 5.9 | 18/21 | Maize starch and maltodextrins | ||||||
| Alireza et al., 2015 [28] | Iran | Probiotic (30) | NR | 12/18 | Fermented milk (kefir) containing L. casei, L. acidophilus, and Bifidobacteria | 4.6 × 1010 | Fermented milk | 8 weeks | FBG/HbA1c/TC/TG/HDL-C/ LDL-C |
| Control (30) | NR | 14/16 | Fermented milk (dough) | ||||||
| Mobini et al., 2016 [29] | Sweden | Probiotics (14) | 64.00 ± 6.00 | 3/11 | L. reuteri DSM 17938 | 108 or 1010 | Powder | 12 weeks | FBG/HbA1c/insulin/TC/ TG/HDL-C/LDL-C |
| Control (15) | 65.00 ± 5.00 | 4/11 | Placebo | ||||||
| Feizollahzadeh et al., 2017 [30] | Iran | Probiotics (20) | 56.9 ± 1.81 | 11/9 | Soy milk containing L. planetarum | 107 | Soy milk | 8 weeks | FBS/TG/HDL-C/LDL-C |
| Control (20) | 53.60 ± 1.60 | 10/10 | Conventional soy milk | ||||||
| Junko et al., 2017 [31] | Japan | Probiotic (34) | 64.0 ± 9.2 | 5/29 | Lactobacillus casei strain Shirota-fermented milk | 4 × 1010 | Fermented milk | 16 weeks | FBG/HbA1c/TC/TG/HDL-C/ BMI |
| Control (34) | 65.0 ± 8.3 | 14/20 | Did not receive a probiotic | ||||||
| Hove et al., 2015 [32] | Denmark | Probiotic (23) | 58.5 ± 7.7 | NR | L. helveticus Cardi04 | NR | Yogurt | 12 weeks | FBG/HbA1c/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (18) | 60.6 ± 5.2 | NR | Artificially acidified milk | ||||||
| Tajabadi-Ebrahimi et al., 2017 [33] | Iran | Probiotic (30) | 64.20 ± 12.00 | NR | L. acidophilus, L. casei, L. lactis, ifdobacterium, Actinobacteria, B. bifdum, B.longum, and B. infantis | 2 × 109 | Capsule | 12 weeks | FBG/HbA1c/insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C |
| Control (30) | 64.00 ± 11.70 | NR | Starch | ||||||
| Mohamadshahi et al., 2014 [34] | Iran | Probiotic (22) | 53.00 ±5.9 | NR | L. acidophilus (La5) and B. lactic (Bb12) | 1.1 × 109 | Yogurt | 8 weeks | FBG/HbA1c |
| Control (22) | 49.00 ± 7.08 | NR | Conventional yogurts | ||||||
| Kobyliak et al., 2018 [35] | Ukraine | Probiotic (31) | 52.23± 1.74 | NR | LactobacillusþLactococcusþ Bifidobacteriumþ Propionibacteriumþ Acetobacter | 1012 | “Symbiter” | 8 weeks | FBS/HbA1c/Insulin |
| Control (22) | 57.18± 2.06 | NR | Placebos | ||||||
| Tajadadi-Ebrahimi et al., 2014 [36] | Iran | Probiotic (27) | 52.0 ± 7.2 | NR | L. sporogenes | 1 × 108 | Bread | 8 weeks | FBG/insulin/HOMA-IR |
| Control (27) | 53.4 ± 7.5 | NR | Control bread | ||||||
| Hosseinzadeh et al., 2013 [37] | Iran | Probiotic (42) | 46.8 ± 6.21 | 32/10 | Brewer’s yeast | 1.8 g | Tablets | 12 weeks | FBS/HbA1c/HOMA-IR |
| Control (42) | 45.7 ± 6.11 | 31/11 | Cellulose microcrystalline compounds, magnesium stearate, caramel, malt, and stearic acid. | ||||||
| Shakeri et al., 2014 [38] | Iran | Probiotic (26) | 52.3± 8.2 | NR | L. sporogenes | 1.2 × 1010 | Breads | 8 weeks | FBG/BMI/TC/TG/HDL-C/ LDL-C |
| Control (26) | 53.1± 7.5 | NR | Control bread | ||||||
| Madempudi et al., 2019 [39] | India | Probiotic (37) | 53.60 | 7/30 | L. salivarius UBLS22, L. casei UBLC42, L. plantarum UBLP40, L. acidophilus UBLA34, B. breve UBBr01, and B. coagulans Unique IS2 | 3 × 1010 | Capsules | 12 weeks | FBS/HbA1c/Insulin/HOMA-IR/TC/TG/HDL-C/ LDL-C |
| Control (37) | 50.50 | 9/28 | Maltodextrin | ||||||
| Chaiyasut et al., 2021 [40] | Thailand | Probiotic (36) | 54.78 ± 1.92 | NR | Lactobacillus paracasei, Bifidobacterium longum, Bifidobac-terium breve | 1011 | Sachet | FBG/BMI/TC/TG/HDL-C/ LDL-C | |
| Control (36) | 58.94 ± 1.32 | NR | Corn starch | ||||||
| Jiang et al., 2020 [41] | China | Probiotic (42) | 55.96 ± 8.45 | 27/15 | Bifidobacterium bifidum, Lactobacillus acidophilus, Streptococcus thermophilus | 9.7 × 109 | Sachet | 12 weeks | FBS/HbA1c/Insulin/HOMA-IR |
| Control (34) | 56.12 ± 8.23 | 22/12 | Starch | ||||||
| Kanazawa et al., 2021 [42] | Japan | Probiotic (44) | 61.1 ± 11.0 | 13/31 | Lacticaseibacillus paracasei YIT 9029 (strain Shirota:LcS) organisms, Bifidobacterium breve YIT 12272 | 6 × 108 | Powder | 24 weeks | FBS/HbA1c/TC/TG/HDL-C/ LDL-C |
| Control (42) | 55.9 ± 10.7 | 8/34 | Placebo | ||||||
| Toejing et al., 2021 [43] | Thailand | Probiotic (18) | 63.50 ± 5.94 | 12/6 | L. paracasei | 5 × 1010 | Powder | 12 weeks | FBS/HbA1c/TC/TG/HDL-C/ LDL-C |
| Control (18) | 61.78 ± 7.73 | 16/2 | Corn starch | ||||||
| Mi-Ra Oh et al., 2021 [44] | Korea | Probiotic (20) | 56.40 ± 11.57 | 14/6 | L. plantarum HAC01. | 4 × 109 | Capsules | 8 weeks | FBS/HbA1c/Insulin/HOMA-IR |
| Control (20) | 53.55 ± 10.18 | 17/3 | Microcrystalline cellulose | ||||||
| Tay et al., 2021 [45] | New Zealand | Probiotic (15) | 52.9 ± 8.7 | 9/6 | L. rhamnosus HN001 | 6 × 109 | Capsules | 12 weeks | FBS/HbA1c/TC/TG/HDL-C/ LDL-C |
| Control (11) | 54.1 ± 6.4 | 9/2 | Microcrystalline cellulose and dextrose anhydrate | ||||||
| Palacios et al., 2020 [46] | Australia | Probiotic (30) | 61.4± 8.9 | 13/17 | Lactobacillus plantarum Lp-115, Lactobacillus bulgaricus Lb-64, Lactobacillus gasseri Lg-36, Bifidobacterium breve Bb-03, Bifidobacterium animalis sbsp. lactis Bi-07, Bifidobacterium bifidum Bb-06, Streptococcus thermophilus St-21, Saccharomyces boulardii DBVPG 6763 | 5 × 1010 | Capsules | 12 weeks | FBS/HbA1c/Insulin/HOMA-IR |
| Control (30) | 56.1 ± 12.3 | 19/11 | 0 mg microcrystalline cellulose, 5 mg silica, and 10 mg magnesium stearate | ||||||
| Toshimitsu et al., 2020 [47] | Japan | Probiotic (62) | 50.6 ± 6.9 | 20/42 | OLL2712 | 5 × 109 | Yogurt | 12 weeks | FBS/HbA1c/Insulin/HOMA-IR |
| Control (64) | 51.2 ± 7.6 | 20/44 | Placebo | ||||||
| Stefanaki et al., 2019 [48] | Greece | Probiotic (7) | 15 | 4/3 | Streptococcus thermophilus (DSM24731), Bifidobacteria breve (DSM24732), Bifidobacteria longum (DSM2473), Bifidobacteria infantis (DSM24737), Lactobacillus acidophilus (DSM24735), Lactobacillus plantarum (DSM24730), Lactobacillus paracasei (DSM24733), Lactobacillus delbreuckii subspecies bulgaricus (DSM24734) | 4.5 × 1011 | Powder | 24 weeks | FBS/TC/TG/HDL-C/LDL-C |
| Control (10) | 13.50 | 5/5 | Placebo | ||||||
| Hsieh et al., 2018 [49] | China Taiwan | Probiotic (22) | NR | NR | L. reuteri | 4 × 109 | Capsules | 24 weeks | FBS/HbA1c/Insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C |
| Control (22) | NR | NR | Placebo | ||||||
| Raygan et al., 2018 [50] | Iran | Probiotic (30) | 60.7 ± 9.4 | NR | Bifidobacterium bifidum, Lactobacillus casei, Lactobacillus acidophilus | 6 × 109 | Capsules | 12 weeks | FBS/Insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (30) | 61.8 ± 9.8 | NR | Placebo | ||||||
| Soleimani et al., 2017 [51] | Iran | Probiotic (30) | NR | 10/20 | L. acidophilus, L. casei, and B. bifidum | 2 × 109 | Capsules | 12 weeks | FBS/HbA1c/Insulin/HOMA-IR/ TC/TG/HDL-C/LDL-C/BMI |
| Control (30) | NR | 10/20 | Placebo | ||||||
| Jie et al., 2021 [52] | china | Probiotic (100) | 54.16 ± 9.10 | 41/59 | Bifidobacterium | 2 × 108 | Capsules | 16 weeks | FBS/HbA1c/TC/TG/HDL-C/ LDL-C/BMI |
| Control (99) | 52.73 ± 9.35 | 55/54 | Lactose and magnesium stearate |
| Outcome Indicators | Items | Effect Size | Standard Error | 95% CI | t Value | p |
|---|---|---|---|---|---|---|
| BMI | slope | −1.06756 | 0.6448588 | (−2.37, 0.35) | −1.56 | 0.137 |
| bias | 2.333851 | 2.712093 | (−3.39, 8.06) | 0.86 | 0.401 | |
| HDL | slope | 0.6000644 | 0.7204764 | (−0.88, 2.08) | 0.83 | 0.413 |
| bias | −1.106745 | 2.812447 | (−6.90, 4.69) | −0.39 | 0.697 | |
| HOMA-IR | slope | 0.62113839 | 0.4490837 | (−0.31, 1.55) | 1.38 | 0.179 |
| bias | −0.58518 | 1.844773 | (−9.39, −1.78) | −3.03 | 0.006 | |
| LDL | slope | −0.8541399 | 0.3113323 | (−1.50, −0.21) | −2.74 | 0.012 |
| bias | 2.536676 | 1.277253 | (−0.11, 5.18) | 1.99 | 0.059 | |
| TC | slope | −1.236445 | 0.2768925 | (−1.81, −0.66) | −4.47 | 0.000 |
| bias | 3.821232 | 1.143507 | (1.45, 6.19) | 3.34 | 0.003 | |
| TG | slope | 0.2946404 | 0.2313827 | (−0.18, 0.77) | 1.27 | 0.216 |
| bias | −2.406254 | 0.9667479 | (−4.41, −0.41) | −2.49 | 0.020 | |
| FBG | slope | 0.3008114 | 0.4888035 | (−0.69, 1.29) | 0.62 | 0.542 |
| bias | −3.62217 | 1.900053 | (−7.47, 0.22) | −1.91 | 0.064 | |
| Fasting insulin | slope | 0. 5509178 | 0.7954488 | (−1.09, 2.20) | 0.69 | 0.496 |
| bias | −4.468728 | 3.06054 | (−10.78, 1.86) | −1.46 | 0.158 | |
| glycosylated haemoglobin | slope | −0.0440752 | 0.3279199 | (−0.72, 0.63) | −0.13 | 0.894 |
| bias | −1.885872 | 1.327238 | (−4.63, 0.85) | −1.42 | 0.168 |
| Outcome Indicators | Phase | Effect Size | 95% CI | Z Value | p | No. of Studies |
|---|---|---|---|---|---|---|
| HOMA-IR | Before | −0.879 | (−1.169, −0.589) | −5.944 | 0 | 26 |
| After | −0.879 | (−1.169, −0.589) | −5.944 | 0 | 26 | |
| TC | Before | −0.267 | (−0.429, −0.105) | −3.235 | 0.001 | 24 |
| After | −0.430 | (−0.583, −0. 277) | −5.494 | 0.000 | 32 | |
| TG | Before | −0.301 | (−0. 435, −0.167) | −4.413 | 0.000 | 25 |
| After | −0.301 | (−0. 435, −0.167) | −4.413 | 0.000 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, R.; Wang, L.; Tian, P.; Jin, X.; Zhao, J.; Zhang, H.; Wang, G.; Zhu, M. The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3240. https://doi.org/10.3390/nu15143240
Xiao R, Wang L, Tian P, Jin X, Zhao J, Zhang H, Wang G, Zhu M. The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(14):3240. https://doi.org/10.3390/nu15143240
Chicago/Turabian StyleXiao, Rui, Linlin Wang, Peijun Tian, Xing Jin, Jianxin Zhao, Hao Zhang, Gang Wang, and Minmin Zhu. 2023. "The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis" Nutrients 15, no. 14: 3240. https://doi.org/10.3390/nu15143240
APA StyleXiao, R., Wang, L., Tian, P., Jin, X., Zhao, J., Zhang, H., Wang, G., & Zhu, M. (2023). The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients, 15(14), 3240. https://doi.org/10.3390/nu15143240





