3.1. Calcium and Phosphorus
Dietary calcium intake does not meet the recommendations in many parts of the world. Dairy products provide a high level of calcium per serving, which becomes bioavailable under conditions in the gastrointestinal tract [
30,
31]. Crucial in sheep’s milk ice cream is the presence of casein micelles, which are protein colloids containing ~70% of total calcium and ~50% of total inorganic phosphate [
32]. Calcium phosphate is enclosed in casein micelles as small nanoclusters, typically 4–5 nm in diameter. Therefore, casein micelles, which contain several hundred nanoclusters of calcium phosphate and tens of thousands of casein molecules, could be considered a protein-based carrier of calcium phosphate [
33,
34]. To provide adequate transport, some of the calcium from ice cream is complexed with citrate, which is present in milk at a level of 0.20 mg 100 g
−1 [
35]. The proper ratio of calcium to phosphorus in the diet is also essential for adequate phosphate–calcium metabolism. For adults, a beneficial ratio is 1:1 or 1.5:1 [
36,
37], indicating that in the example of the sheep’s milk ice cream studied, the amount of each macroelement is highly favorable because it varies from 1.28:1 to 1.32:1 (
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5).
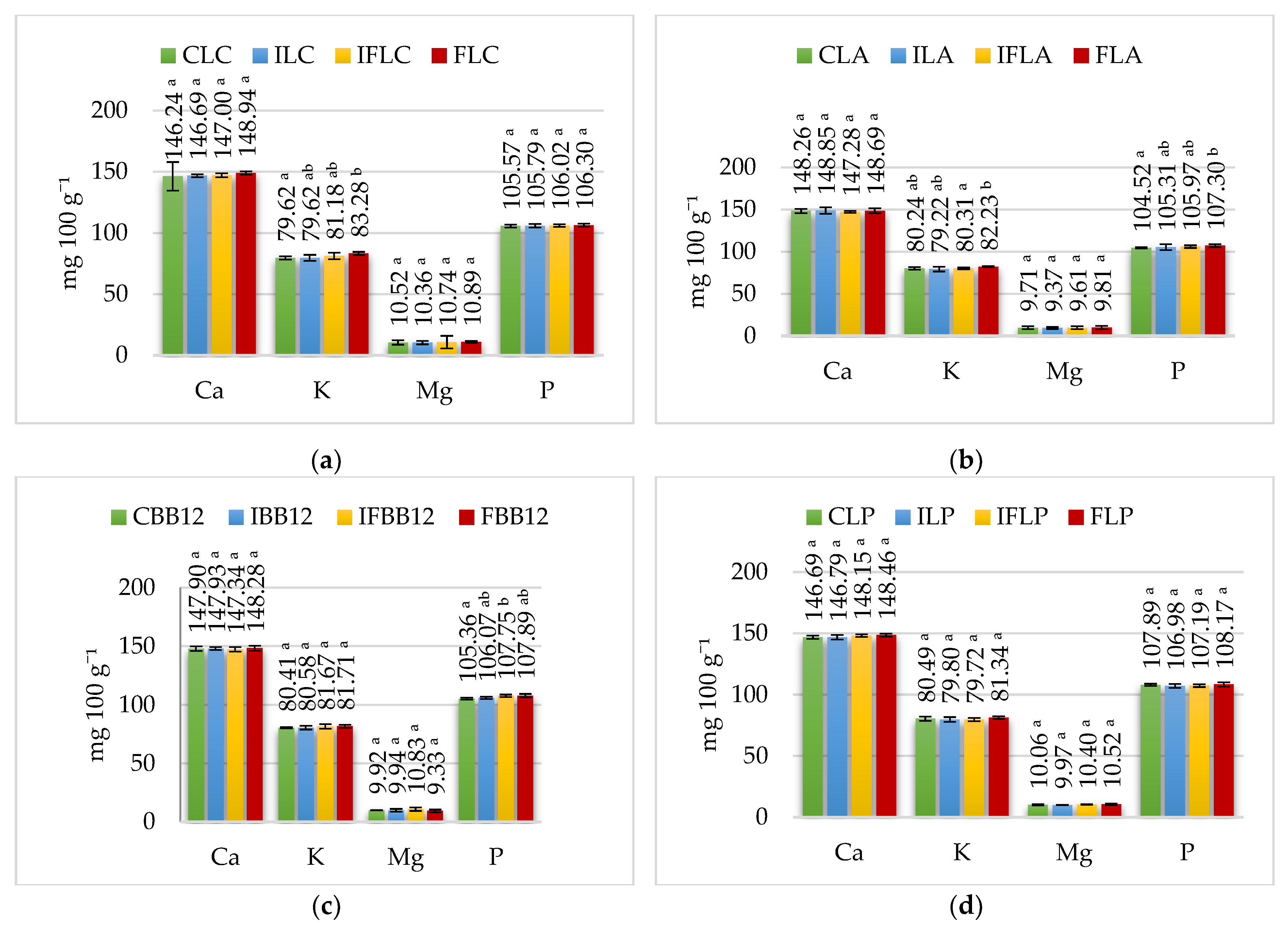
In all control ice cream groups (CLC, CLA, CBB12, CLP, CLR), the calcium content ranged from 199.47 mg 100 g−1 to 202.45 mg 100 g−1 and was comparable to the calcium concentration in sheep milk (202.83 mg 100 g−1). In comparison, the phosphorus content in these groups of ice cream ranged from 153.35 mg 100 g−1 to 157 mg 100 g−1. Adding inulin does not significantly increase the calcium and phosphorus content of ice cream. On the contrary, apple fiber contains 15.96 mg 100 g−1 of calcium and 51.80 mg 100 g−1 of phosphorus, resulting in a proportional increase in calcium and phosphorus in FLC (L. casei), FLA (L. acidophilus), FBB12 (B. animalis), FLP (L. paracasei) and FLR (L. rahamnosus) ice cream.
In the mouth, ice cream samples are mixed with saliva and thus diluted, which contributes to a non-significant decrease in calcium concentration in all samples (
Figure 2). On the contrary, the phosphorus content increased, which was caused by the presence of sodium hydrogen phosphate (Na
2HPO
4) in saliva. In humans, the amount of saliva secreted under resting conditions is 0.5 mL per minute, while after intense secretory stimulation with food, it can increase to 10 mL per minute [
38].
When the ice cream enters the stomach during fasting, it comes into contact with a small amount (~50 mL) of gastric juice, which for an adult will have a pH between 1 and 2 [
39]. However, considering the portion size of the ice cream (~100 mL) with a pH of 4.3–5.3, compared to the amount of gastric juice, the pH of the stomach will quickly rise to a value of about 4. Subsequently, the stomach ice cream is slightly diluted and acidified, resulting in the dissolution of some of the calcium phosphate present in the casein micelles [
40,
41]. Subsequently, gastric juice is secreted into the stomach, gradually lowering the pH [
42].
Therefore, when the pH value is lower than 4 in the stomach, it should be sufficient to dissolve all calcium and phosphate from casein micelles [
34]. Possible enzymatic coagulation of the micelles could impede this process and delay the release of calcium and phosphate from the ice cream. This delayed release may finally benefit ice cream’s calcium absorption due to the gastric phase’s reduced calcium concentration. However, dilution with gastric juice and slow release from the gastric coagulum would significantly reduce the concentration of calcium phosphate in the food content leaving the stomach [
43]. In the gastric phase, the calcium concentration in the analyzed ice cream ranged from 146.24 mg 100 g
−1 to 151.21 mg 100 g
−1 (
Figure 3), representing 72.98% to 73.32% of the calcium content of the ice cream before digestion. However, in the gastric phase, a significantly higher (
p ≤ 0.05) calcium content was determined only in samples fermented by
L. rhamnosus: the IFLR ice cream with inulin and fiber and FLR ice cream with fiber compared to control CLR ice cream and ILR ice cream with inulin. In other ice cream groups, added fiber had no effect on calcium content. In contrast, a significant effect of added apple fiber on the phosphorus content of the gastric phase was found in FLA (fermented by
L. acidophilus) and FBB12 ice cream (fermented by
B. animalis) compared to their control counterparts. Also notable was the tendency in the remaining ice cream with apple fiber to maintain higher phosphorus concentrations than those in samples without fiber.
The rapidity of gastric emptying depends on many factors, including volume flow restrictions, caloric density, pH and rheological properties [
43]. Phosphorus absorption occurs in the duodenum (35%), jejunum (25%) and ileum (40%) [
44]. The naturally occurring phosphorus in food is absorbed from the gastrointestinal tract at 40–60% [
37]. The phosphorus content in the tested samples in the intestinal phase is shown in
Figure 4. The results of the bioavailability of macronutrients are shown in
Table 7,
Table 8,
Table 9,
Table 10 and
Table 11.
The bioavailability of phosphorus in the ice cream ranged from 47.82% in FLC ice cream with fiber fermented by L. casei to 50.94% in the FBB12 group with fiber addition fermented by B. animalis. Adding apple fiber did not significantly reduce the bioavailability of phosphorus in ice cream fermented by L. casei, L. paracasei and L. rhamnosus, nor did it significantly increase bioavailability in ice cream with L. acidophilus (FLA) and B. animalis (FBB12), compared to control counterparts. The highest bioavailability of phosphorus (>50%) was found in sheep’s milk ice cream fermented by B. animalis. The remaining sheep’s milk ice creams had lower phosphorus bioavailability by 1–2.5%.
The bioavailability of calcium in the tested ice cream ranged from 40.63% in FBB12 (with fiber, fermented by
B. animalis) to 54.40% in control CLP samples fermented by
L. paracasei (
Table 7,
Table 8,
Table 9,
Table 10 and
Table 11). The highest bioavailability of calcium, depending on the type of bacteria used in each group, was always shown for the control samples. However, adding 4% inulin already reduced calcium bioavailability by about 3–5%, and a mixture of 2.5% inulin with 1.5% apple fiber reduced calcium bioavailability by 4–8% compared to controls. Adding 4% apple fiber significantly reduced the bioavailability of calcium from sheep’s milk ice cream by up to 6–12%. FLR ice cream with fiber fermented by
L. rhamnosus showed a significant reduction in the bioavailability of >12% compared to control CLR ice cream. Our study and the two-factor analysis of variance (ANOVA) show that the bioavailability of calcium from sheep’s milk ice cream is significantly influenced by the two factors analyzed, which are the fiber (
p = 0.0000) and the type of bacteria (
p = 0.0001), as well as interactions between these factors (
p = 0.0006). Also, a study by Bosscher et al. [
45] showed that dietary fiber inhibits mineral bioavailability more in casein than in whey-based formulations.
Most calcium (about 65%) is absorbed at pH 6.5–7.5. It should be noted that calcium is not absorbed from the stomach [
46]. In order to cross the intestinal barrier, calcium must be in soluble form, generally ionized (Ca
2+) or bound to a soluble organic molecule. The auxiliary organs supporting intestinal digestion and absorption are the pancreas, liver and gallbladder [
38]. Calcium transport involves both active and passive transport mechanisms. Active transport occurs mainly in the duodenum and upper jejunum [
47]. In the ileum, the primary absorption mechanism is passive, since food moves slowly through this area of the gastrointestinal tract. The small intestine is responsible for more than 90% of total calcium uptake in humans, while about 3–6% of calcium is absorbed in the large intestine, depending on calcium loading [
48,
49]. A study by Szalast-Pietrzak et al. [
50] on food products showed the highest percentage of calcium bioavailability from natural yogurt, at 37.73%.
Our study of the effect of applied probiotic strains on calcium bioavailability also supports the results of Sharifi-Rad et al. [
51], where one possible mechanism by which calcium availability is increased is higher calcium absorption and fermentation in the intestine by probiotics. Gilman and Cashman [
52] previously reported that in human intestinal Caco-2 cells in culture,
Lactobacillus salivarius could increase Ca
2+ uptake, although exposure of Caco-2 cells to probiotics has no effect on Ca
2+ transport.
3.2. Magnesium
Sheep milk provides 16–18 mg 100 g
−1 of magnesium, which occurs in the form of soluble compounds (about 73–75% of total Mg) and colloidal compounds (phosphates, citrates). Only about 15% of magnesium is presented in an ionized form. [
3]. The results determining the magnesium content in the analyzed ice cream groups are shown in
Table 2,
Table 3,
Table 4,
Table 5 and
Table 6. In the control ice cream, the content of this macroelement was determined in the range from 15.96 mg 100 g
−1 in control ice cream with
L. casei (CLC) to 17.52 mg 100 g
−1 in control ice cream with
L. paracasei (CLP). Adding apple fiber significantly increased the magnesium content of FLC (
L. casei), FLA (
L. acidophilus), FBB12 (
B. animalis), FLP (
L. paracasei) and FLR (
L. rhamnosus) ice cream by about 0.5–1.4 mg 100 g
−1 due to the presence of magnesium in apple fiber (
Table 1). In contrast, the addition of inulin did not change the magnesium content in the ice cream before digestion compared to its control counterparts. The concentration of magnesium in ice cream in the oral, stomach and intestine stages, depending on the addition of fiber and probiotic strain, is shown in
Figure 2,
Figure 3 and
Figure 4.
The bioavailability of magnesium from sheep’s milk products is poorly studied. In our study, the bioavailability of magnesium in sheep’s milk ice cream ranged from 55.64% in the control ice cream fermented by
L. casei (CLC) to 44.42% in the group with fiber fermented by
L. acidophilus (FLA). The bioavailability of magnesium was highest in the control samples, where it always exceeded 50%. The highest bioavailability was determined in the control CLC ice cream fermented by
L. casei. The addition of fiber had an essential role in modifying the bioavailability of magnesium. Hussain et al. [
53] indicated that apple fiber contains 40% cellulose and 19% water-soluble hemicellulose. Also, as a polysaccharide, inulin is a low-molecular-weight polymer with poor water solubility, contributing to faster intestinal transit. However, it should be mentioned that adding inulin only reduced magnesium’s bioavailability by about 5–6% compared to control counterparts. In contrast, adding apple fiber reduced the bioavailability of magnesium in the ice cream by 8.03% in FLA (fermented by
L. acidophilus), 7.48% in FLC (fermented by
L. casei) and 7.25% in the FLR (fermented by
L. rhamnosus) group. Ice cream with fiber addition, FBB12 fermented by
B. animalis and FLP fermented by
L. paracasei, showed 5–6% lower magnesium bioavailability than controls.
Bielik et al. [
22] consider that the gut microflora influences mineral metabolism by directly influencing mineral absorption in the digestive tract during digestion and producing several enzymes that help release minerals from food. These include bacterial phytases, which catalyze the hydrolysis of phytic acid found in many plant tissues, releasing proper forms of minerals such as calcium, magnesium and phosphorus [
54,
55]. Aljevich et al. [
56] observed a higher bioavailability of magnesium and other minerals from cheese when combined with probiotics. Cultures of
Lactobacillus spp. consumed with Dutch-type cheese increased Mg (~18%) and Ca (~2.5%) availability in vitro [
28]. Similarly, fermented goat milk containing Lactobacillus plantarum increased Mg bioavailability compared to commercial fermented goat milk [
21].
This implies that the bacterial strain conducting the fermentations of the ice cream mixes also significantly affects magnesium bioavailability (p = 0.0001), as confirmed by a two-factor analysis of variance (ANOVA). The calculations show that the bioavailability of magnesium from sheep’s milk ice cream is significantly influenced by both factors analyzed, including the fiber (p = 0.0011) and the interactions between these factors (p = 0.0005).
Comparable values for magnesium absorption from various diets to those in our study have been published [
49]. In an equilibrium study conducted on healthy young men, the apparent magnesium absorption from a mixed Western diet containing 18 g of fiber daily was 46.3%. Knudsen et al. [
57] found an average magnesium absorption of 46%. In another study, magnesium absorption from milk was measured in adolescents aged 9–14 years using the stable isotope multiscale technique. The absorption of magnesium from milk was 42.8% among girls and 45.3% among boys and did not differ significantly between the sexes [
58].
3.3. Potassium
Due to the high potassium content in apple fiber (
Table 1), its addition to ice cream mixes significantly increased the amount of potassium in sheep’s milk ice cream (
Table 2,
Table 3,
Table 4,
Table 5 and
Table 6). The concentration of potassium in ice cream in the mouth, stomach and intestine depending on the addition of fiber and the probiotic strain used for fermentation is shown in
Figure 2,
Figure 3 and
Figure 4.
Potassium is intrinsically soluble and rapidly diffuses into the lumen of the upper gastrointestinal tract. The small intestine is the primary location for potassium absorption, with approximately 90% of dietary potassium being absorbed by passive diffusion [
59,
60]. However, relatively little is known about the bioavailability of potassium, and most work has focused on assessing urinary potassium loss after potassium salt supplementation [
61,
62,
63]. Only potato has been studied for potassium bioavailability, and this food consists mainly of easily digestible starch [
59].
In our study, the bioavailability of potassium was higher in control ice cream than in ice cream with fiber (
Table 7,
Table 8,
Table 9,
Table 10 and
Table 11). In the control ice cream (CLC, CLA, CBB12, CLP, CLR), the bioavailability of potassium was about 60%. In ice cream with inulin, it was lower by 3–4%, and in ice cream with apple fiber, it was lower by 6–9%. A two-factor analysis of variance (ANOVA) shows that the bioavailability of potassium in sheep’s milk ice cream is significantly affected by the addition of fiber (
p = 0.0000), while the type of bacteria (
p = 0.6731) and interactions between these factors (
p = 0.7456) were not significant.
The bioavailability of potassium in whole fruits and vegetables can be as high as 50% to 60%, and there is a lack of evidence linking higher consumption of fruits and vegetables to higher serum potassium concentrations [
64]. The bioavailability of potassium from food additives can be as high as 100% [
64,
65]. Picard [
65] and MacDonald-Clarke et al. [
66] also found that the bioavailability of potassium from fruits and vegetables is 50–60%, compared to 90% from animal protein and 95% from additives. Only a few studies show how well the various forms of potassium contained in dietary supplements are absorbed. A dose–response study showed that people absorb about 94% of the potassium gluconate in supplements, and the absorption rate is similar to that of potassium from potatoes [
66].