The Genetic Architecture of Vitamin D Deficiency among an Elderly Lebanese Middle Eastern Population: An Exome-Wide Association Study
Abstract
1. Introduction
2. Methods
2.1. Participants’ Characteristics
2.2. Quantification of 25(OH)D and Related Covariates
2.3. Whole-Exome Sequencing and Bioinformatics Analyses
2.4. Exome-Wide Association Analysis
2.5. Meta-Analysis
2.6. Validation of Replicated Loci Previously Associated with 25(OH)D
2.7. Analysis of Polygenic Risk Scores
2.8. Functional Insights and Annotation of Variants
3. Results
3.1. Study Description
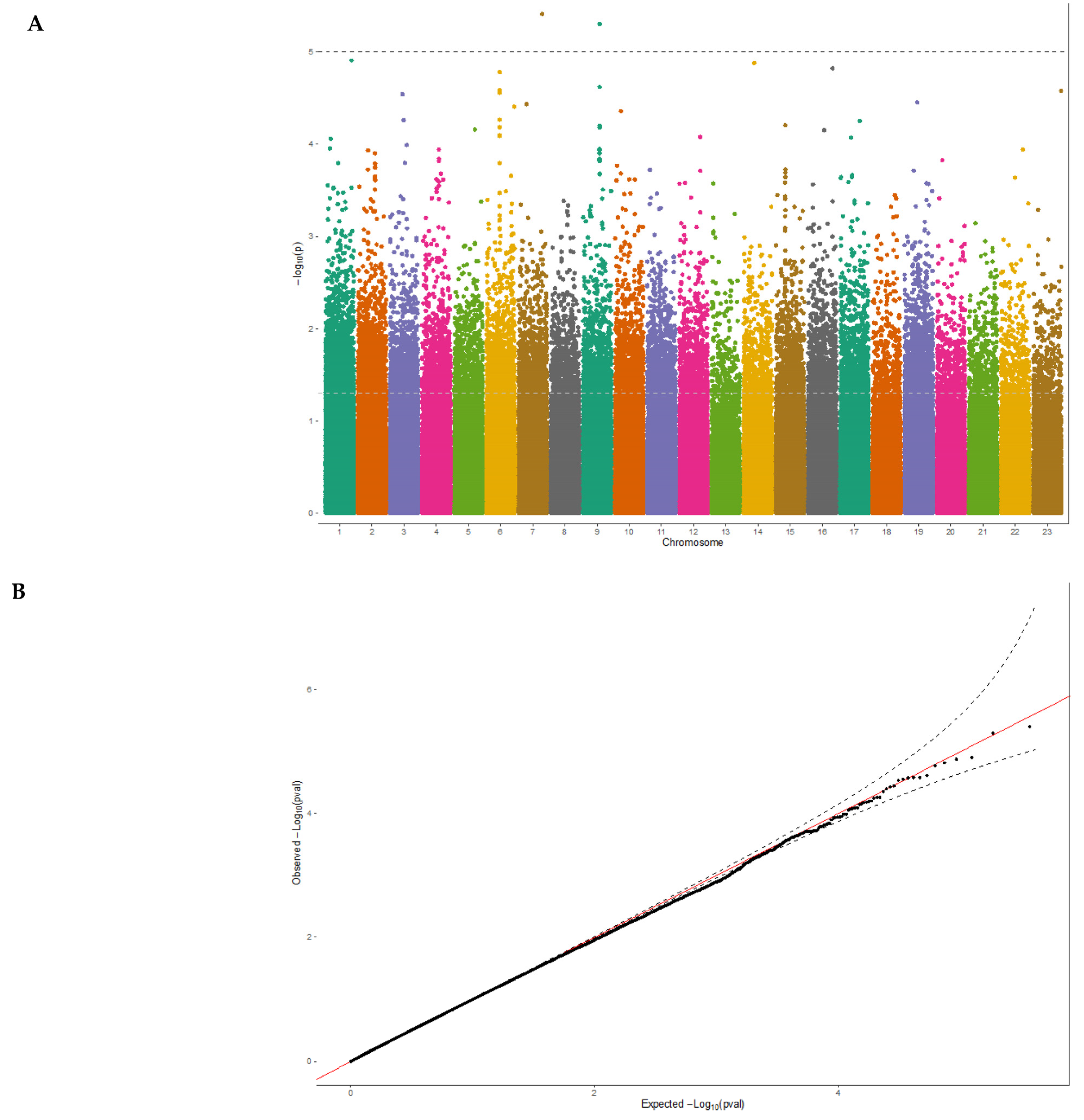
3.2. Exome-Wide Association Study on 25(OH)D
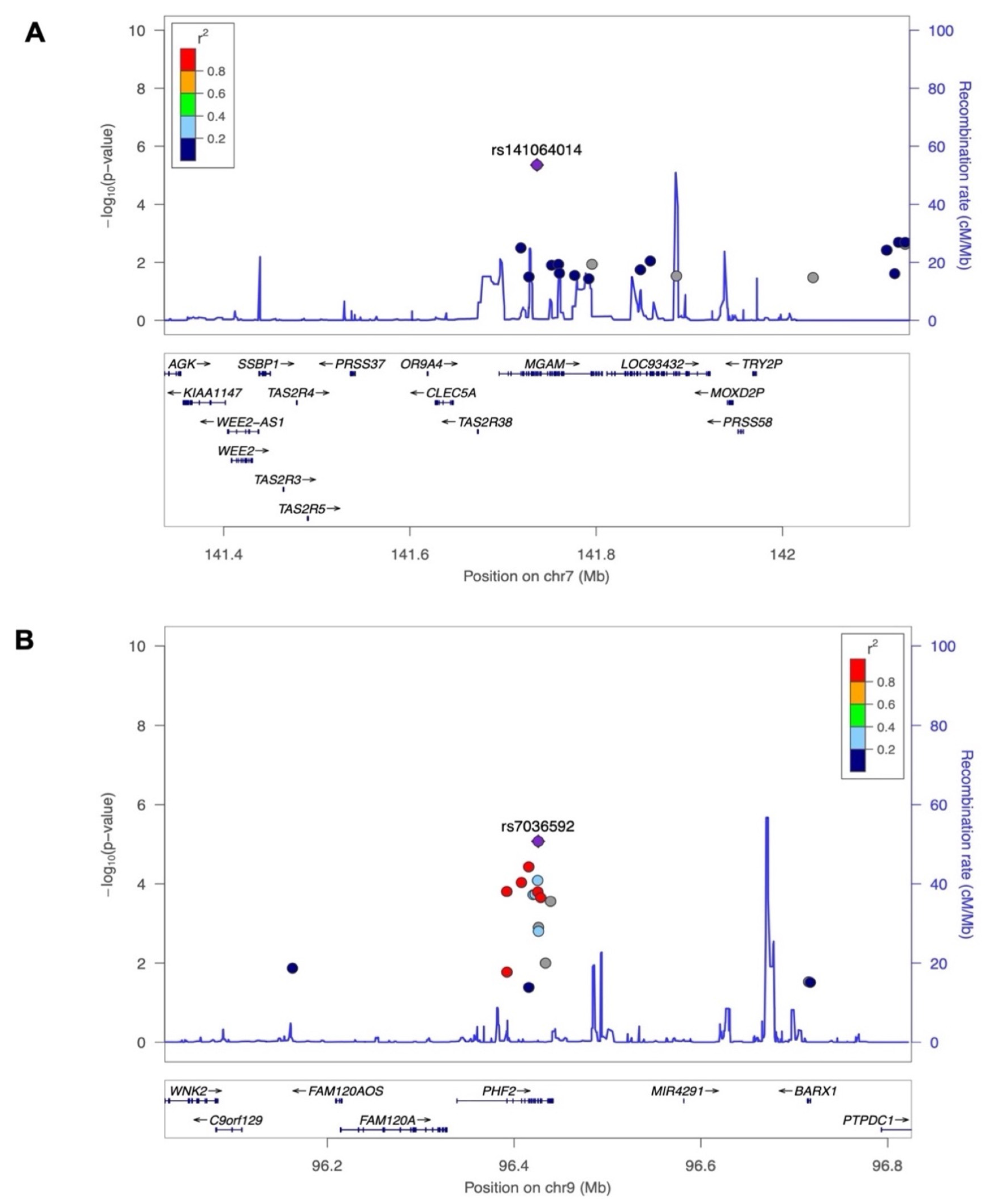
3.3. Evaluation of Common Loci Replication
3.4. Meta-Analysis of Vitamin D GWAS
3.5. Analysis of Functional Variant Expression and Frequency
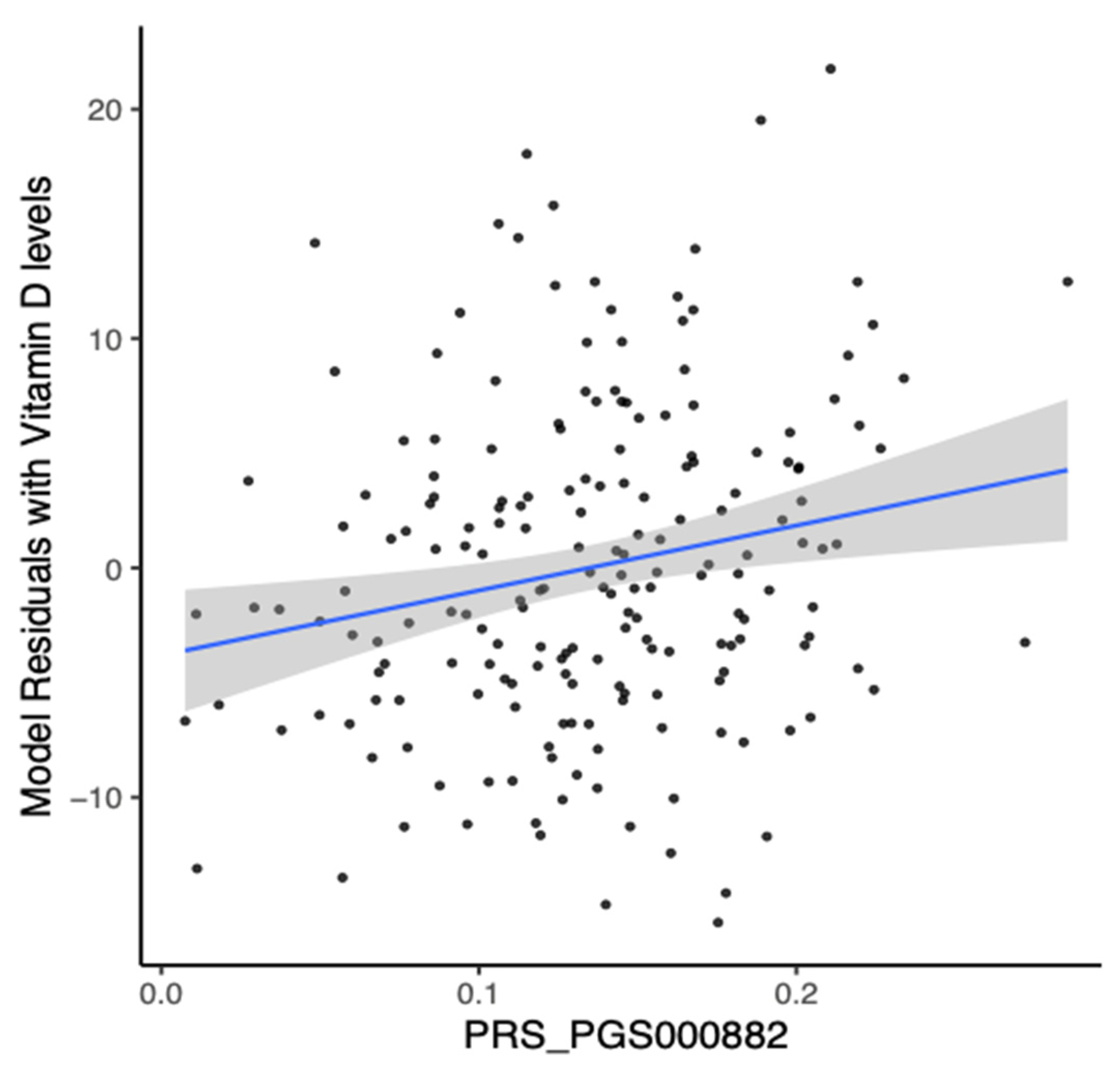
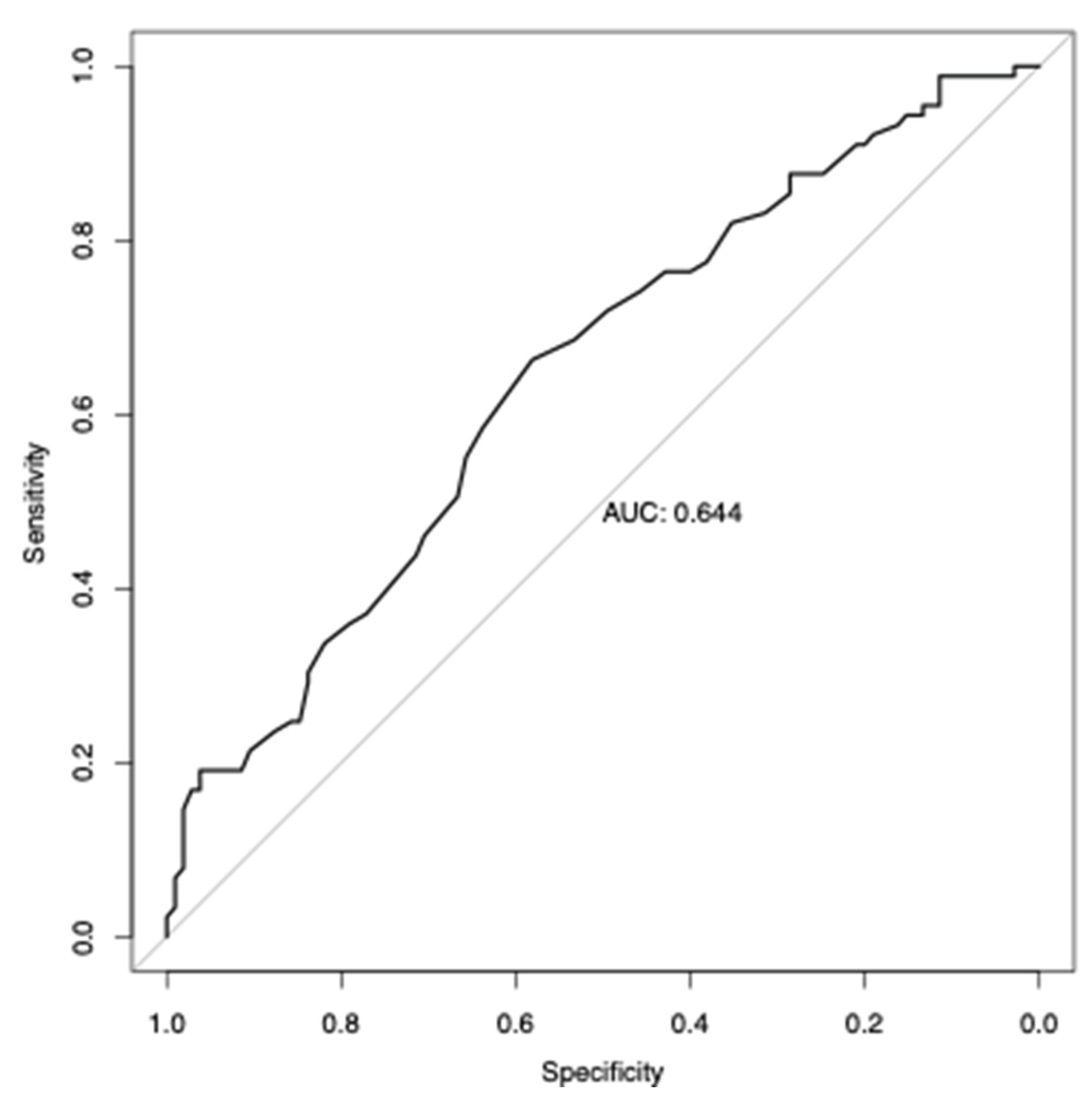
3.6. Analysis of Polygenic Risk Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz-Garcia, A.; Pallares-Carratala, V.; Turegano-Yedro, M.; Torres, F.; Sapena, V.; Martin-Gorgojo, A.; Martin-Moreno, J.M. Vitamin D Supplementation and Its Impact on Mortality and Cardiovascular Outcomes: Systematic Review and Meta-Analysis of 80 Randomized Clinical Trials. Nutrients 2023, 15, 1810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, W. Meta-analysis of randomized controlled trials on vitamin D supplement and cancer incidence and mortality. Biosci. Rep. 2019, 39, BSR20190369. [Google Scholar] [CrossRef] [PubMed]
- Rahme, M.; Sharara, S.L.; Baddoura, R.; Habib, R.H.; Halaby, G.; Arabi, A.; Singh, R.J.; Kassem, M.; Mahfoud, Z.; Hoteit, M.; et al. Impact of Calcium and Two Doses of Vitamin D on Bone Metabolism in the Elderly: A Randomized Controlled Trial. J. Bone Miner. Res. 2017, 32, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Ritz, C.; Kiely, M.; Odin, C. Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses. Nutrients 2017, 9, 469. [Google Scholar] [CrossRef]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A systematic review and meta-analysis of randomized controlled trials. Medicina 2019, 55, 368. [Google Scholar]
- Mitchell, B.L.; Zhu, G.; Medland, S.E.; Renteria, M.E.; Eyles, D.W.; Grasby, K.L.; McGrath, J.J.; Martin, N.G. Half the Genetic Variance in Vitamin D Concentration is Shared with Skin Colour and Sun Exposure Genes. Behav. Genet. 2019, 49, 386–398. [Google Scholar] [CrossRef]
- Ammar, M.; Heni, S.; Tira, M.S.; Khalij, Y.; Hamdouni, H.; Amor, D.; Ksibi, S.; Omezzine, A.; Bouslama, A. Variability in response to vitamin D supplementation according to vitamin D metabolism related gene polymorphisms in healthy adults. Eur. J. Clin. Nutr. 2023, 77, 189–194. [Google Scholar] [CrossRef]
- Datta, P.; Philipsen, P.A.; Olsen, P.; Petersen, B.; Andersen, J.D.; Morling, N.; Wulf, H.C. Pigment genes not skin pigmentation affect UVB-induced vitamin D. Photochem. Photobiol. Sci. 2019, 18, 448–458. [Google Scholar] [CrossRef]
- Wjst, M.; Altmuller, J.; Braig, C.; Bahnweg, M.; Andre, E. A genome-wide linkage scan for 25-OH-D(3) and 1,25-(OH)2-D3 serum levels in asthma families. J. Steroid Biochem. Mol. Biol. 2007, 103, 799–802. [Google Scholar] [CrossRef]
- Manousaki, D.; Mitchell, R.; Dudding, T.; Haworth, S.; Harroud, A.; Forgetta, V.; Shah, R.L.; Luan, J.; Langenberg, C.; Timpson, N.J.; et al. Genome-wide Association Study for Vitamin D Levels Reveals 69 Independent Loci. Am. J. Hum. Genet. 2020, 106, 327–337. [Google Scholar] [CrossRef]
- Sinnott-Armstrong, N.; Tanigawa, Y.; Amar, D.; Mars, N.; Benner, C.; Aguirre, M.; Venkataraman, G.R.; Wainberg, M.; Ollila, H.M.; Kiiskinen, T.; et al. Author Correction: Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021, 53, 1622. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.-H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Rahme, M.; Chamoun, N.; Fuleihan, G.E.-H. Vitamin D in the Middle East and North Africa. Bone Rep. 2018, 8, 135–146. [Google Scholar] [CrossRef]
- Salman, S.; Khouzami, M.; Harb, M.; Saleh, B.; Boushnak, M.O.; Moussa, M.K.; Mohsen, Z.H. Prevalence and Predictors of Vitamin D Inadequacy: A Sample of 2,547 Patients in a Mediterranean Country. Cureus 2021, 13, e14881. [Google Scholar] [CrossRef]
- McMahon, A.; Lewis, E.; Buniello, A.; Cerezo, M.; Hall, P.; Sollis, E.; Parkinson, H.; Hindorff, L.A.; Harris, L.W.; MacArthur, J.A. Sequencing-based genome-wide association studies reporting standards. Cell Genom. 2021, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- McCaw, Z.R.; Lane, J.M.; Saxena, R.; Redline, S.; Lin, X. Operating characteristics of the rank-based inverse normal transformation for quantitative trait analysis in genome-wide association studies. Biometrics 2020, 76, 1262–1272. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.; Sirotkin, K. dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res. 1999, 9, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Zhou, W.; Nielsen, J.B.; Fritsche, L.G.; Dey, R.; Gabrielsen, M.E.; Wolford, B.N.; LeFaive, J.; VandeHaar, P.; Gagliano, S.A.; Gifford, A.; et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018, 50, 1335–1341. [Google Scholar] [CrossRef]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [PubMed]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Lambert, S.A.; Gil, L.; Jupp, S.; Ritchie, S.C.; Xu, Y.; Buniello, A.; McMahon, A.; Abraham, G.; Chapman, M.; Parkinson, H.; et al. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat. Genet. 2021, 53, 420–425. [Google Scholar] [CrossRef]
- Hunt, S.E.; Moore, B.; Amode, R.M.; Armean, I.M.; Lemos, D.; Mushtaq, A.; Parton, A.; Schuilenburg, H.; Szpak, M.; Thormann, A.; et al. Annotating and prioritizing genomic variants using the Ensembl Variant Effect Predictor—A tutorial. Hum. Mutat. 2022, 43, 986–997. [Google Scholar] [CrossRef]
- Lachmann, A.; Torre, D.; Keenan, A.B.; Jagodnik, K.M.; Lee, H.J.; Wang, L.; Silverstein, M.C.; Ma’ayan, A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Chen, Y.; Bao, S.; Hou, X.; Hu, J.; Mu, O.Y.N.; Song, Y.; Shan, L. Identification of subgroups along the glycolysis-cholesterol synthesis axis and the development of an associated prognostic risk model. Hum. Genom. 2021, 15, 53. [Google Scholar] [CrossRef]
- Chiruvella, V.; Cheema, A.; Arshad, H.M.S.; Chan, J.T.; Yap, J.E.L. Sucrase-Isomaltase Deficiency Causing Persistent Bloating and Diarrhea in an Adult Female. Cureus 2021, 13, e14349. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Ohtake, F.; Igarashi, K.; Kanno, J.; Matsumoto, T.; Takada, I.; Kato, S.; Imai, Y. Epigenetic regulation of adipogenesis by PHF2 histone demethylase. Diabetes 2013, 62, 1426–1434. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Y.-D.; He, F.; Tong, C.-J.; Liu, K.; Liu, H.; Zhu, S.-Z.; Luo, J.-Z.; Yuan, B. HIF-1alpha-mediated augmentation of miRNA-18b-5p facilitates proliferation and metastasis in osteosarcoma through attenuation PHF2. Sci. Rep. 2022, 12, 10398. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Barbáchano, A.; Singh, P.K.; Campbell, M.J.; Muñoz, A.; Larriba, M.J. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle 2012, 11, 1081–1089. [Google Scholar] [CrossRef]
- Sawatsubashi, S.; Nishimura, K.; Mori, J.; Kouzmenko, A.; Kato, S. The Function of the Vitamin D Receptor and a Possible Role of Enhancer RNA in Epigenomic Regulation of Target Genes: Implications for Bone Metabolism. J. Bone Metab. 2019, 26, 3–12. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W.; Lee, K.H.; Yoon, H.; Shin, D.H.; Ju, U.I.; Seok, S.H.; Lim, S.H.; Lee, Z.H.; Kim, H.H.; et al. Plant homeodomain finger protein 2 promotes bone formation by demethylating and activating Runx2 for osteoblast differentiation. Cell Res. 2014, 24, 1231–1249. [Google Scholar] [CrossRef]
- Tripathi, R.; Aggarwal, T.; Lindberg, F.A.; Klemm, A.H.; Fredriksson, R. SLC38A10 Regulate Glutamate Homeostasis and Modulate the AKT/TSC2/mTOR Pathway in Mouse Primary Cortex Cells. Front Cell Dev. Biol. 2022, 10, 854397. [Google Scholar] [CrossRef]
- Patel, R.A.; Musharoff, S.A.; Spence, J.P.; Pimentel, H.; Tcheandjieu, C.; Mostafavi, H.; Sinnott-Armstrong, N.; Clarke, S.L.; Smith, C.J.; Program, V.A.M.V.; et al. Genetic interactions drive heterogeneity in causal variant effect sizes for gene expression and complex traits. Am. J. Hum. Genet. 2022, 109, 1286–1297. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Male | Female | Total |
|---|---|---|---|
| Age (years) | 72.7 (±5.50) | 69.77 (±3.51) | 71.13 (±4.82) |
| BMI (kg/m2) | 28.69 (±3.35) | 31.60 (±5.10) | 30.24 (±4.60) |
| Serum 25(OH)D (ng/mL) | 19.36 (±6.25) | 20.83 (±7.93) | 20.12 (±7.22) |
| Sample size | 90 (46.4%) | 104 (53.6%) | 194 |
| SNP | Gene | HGVS ID | CHR | Position | A1 | A2 | Beta | SE (Beta) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| rs141064014 | MGAM | NC_000007.13:g.141736273G>A | 7 | 141736273 | G | A | −2.38 | 0.52 | 4.4 × 10−6 |
| rs7036592 | PHF2 | NC_000009.11:g.96425777C>T | 9 | 96425777 | C | T | −0.54 | 0.12 | 8.4 × 10−6 |
| Populations | Frequency for rs141064014 in MGAM | Frequency for rs7036592 in PHF2 | Frequency for rs2725405 in SLC38A10 |
|---|---|---|---|
| Lebanese elderly population | 0.0103 | 0.2408 | 0.4845 |
| European population of ALFA | 0.00794 | 0.39533 | 0.5729 |
| Controls of gnomAD populations | |||
| European | 0.00634 | 0.3829 | 0.5468 |
| East Asian | 0.001 | 0.2136 | 0.3467 |
| African | 0.001 | 0.2783 | 0.9234 |
| All populations | 0.00553 | 0.3216 | 0.5084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendi, N.N.; Chakhtoura, M.; Al-Sarraj, Y.; Basha, D.S.; Albagha, O.; Fuleihan, G.E.-H.; Nemer, G. The Genetic Architecture of Vitamin D Deficiency among an Elderly Lebanese Middle Eastern Population: An Exome-Wide Association Study. Nutrients 2023, 15, 3216. https://doi.org/10.3390/nu15143216
Hendi NN, Chakhtoura M, Al-Sarraj Y, Basha DS, Albagha O, Fuleihan GE-H, Nemer G. The Genetic Architecture of Vitamin D Deficiency among an Elderly Lebanese Middle Eastern Population: An Exome-Wide Association Study. Nutrients. 2023; 15(14):3216. https://doi.org/10.3390/nu15143216
Chicago/Turabian StyleHendi, Nagham Nafiz, Marlene Chakhtoura, Yasser Al-Sarraj, Dania Saleh Basha, Omar Albagha, Ghada El-Hajj Fuleihan, and Georges Nemer. 2023. "The Genetic Architecture of Vitamin D Deficiency among an Elderly Lebanese Middle Eastern Population: An Exome-Wide Association Study" Nutrients 15, no. 14: 3216. https://doi.org/10.3390/nu15143216
APA StyleHendi, N. N., Chakhtoura, M., Al-Sarraj, Y., Basha, D. S., Albagha, O., Fuleihan, G. E.-H., & Nemer, G. (2023). The Genetic Architecture of Vitamin D Deficiency among an Elderly Lebanese Middle Eastern Population: An Exome-Wide Association Study. Nutrients, 15(14), 3216. https://doi.org/10.3390/nu15143216






