Polyunsaturated Fatty Acid Intake during Complementary Feeding and Neurodevelopmental Outcome in Very Low Birth Weight Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subgroup
2.2. Standardized Feeding Concept and Dietary Intake Analysis
2.3. Neurodevelopmental Assessment
2.4. Outcome Parameters
2.5. Statistical Analysis
3. Results
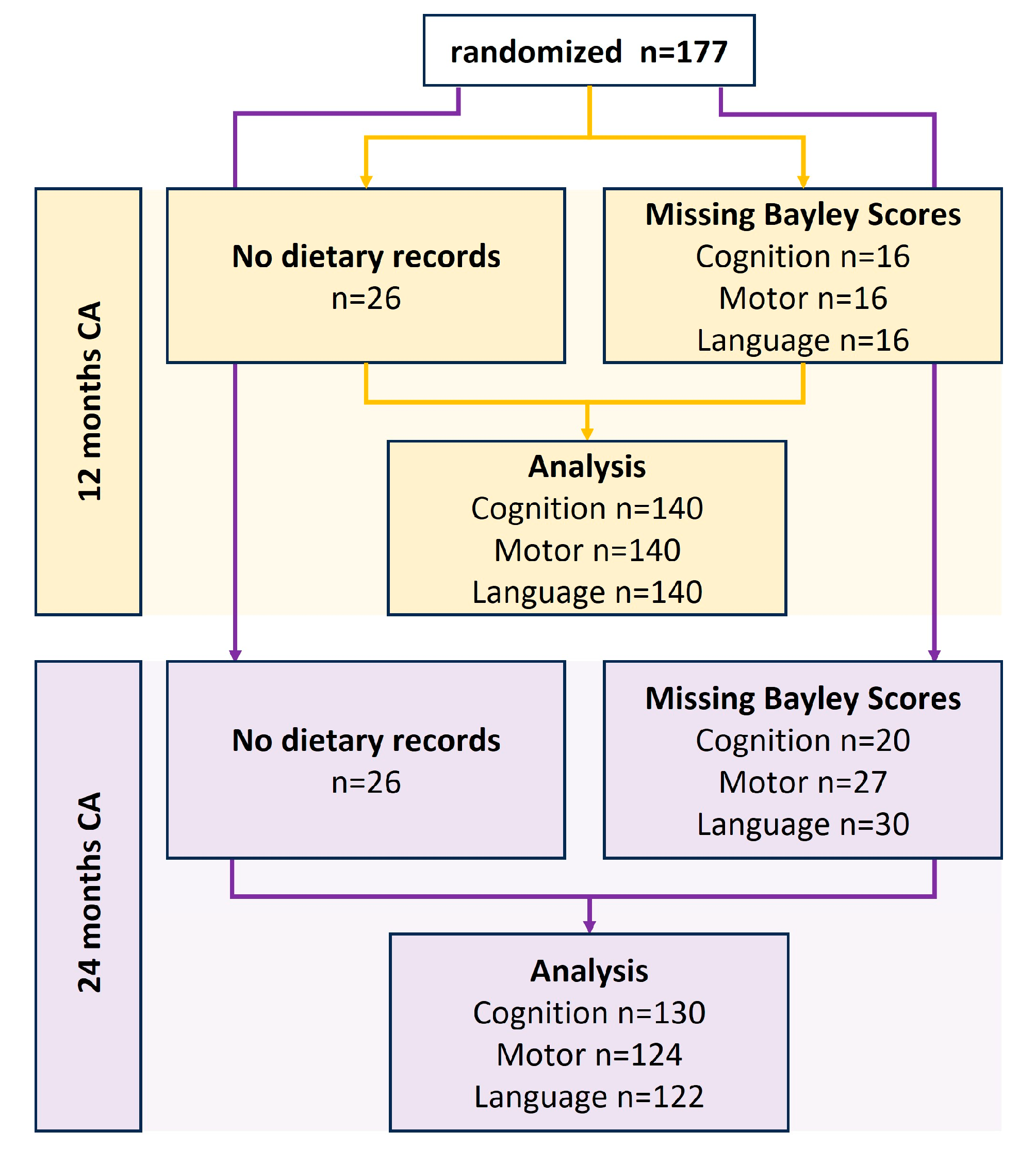
3.1. Screening and Participants
3.2. Baseline Characteristics and Neonatal Morbidity
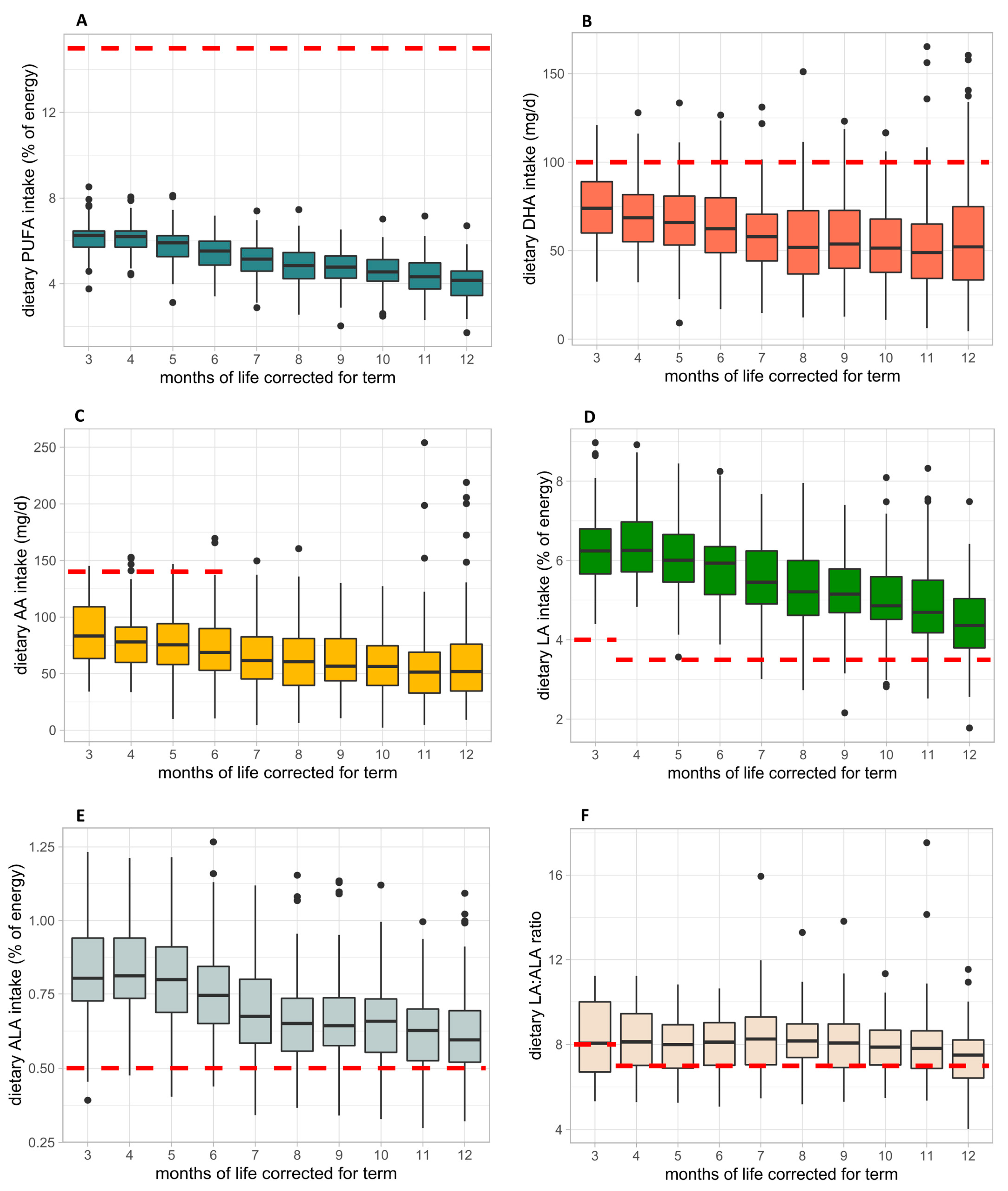
3.3. Dietary Total PUFA, DHA and Intake during the First Year of Life
3.4. Fatty Acid Intake from 3 to 12 Months CA and Comparison with Current Dietary Intake Recommendations
3.5. Bayley-III: Cognition, Motor, Language
3.6. Total PUFA Intake and Neurological Development
3.7. DHA Intake and Neurological Development
3.8. AA Intake and Neurological Development
4. Discussion
4.1. Dietary Intake of Total PUFAs and Neurodevelopmental Outcome
4.2. Dietary Intakes of DHA and AA and Neurodevelopmental Outcome
4.3. Dietary Intakes of LA, ALA and LA/ALA Ratio
4.4. How to Improve Dietary PUFA Intake to Meet a Guideline Diet?
4.5. Study Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klevebro, S.; Juul, S.E.; Wood, T.R. A More Comprehensive Approach to the Neuroprotective Potential of Long-Chain Polyunsaturated Fatty Acids in Preterm Infants Is Needed—Should We Consider Maternal Diet and the n-6:n-3 Fatty Acid Ratio? Front. Pediatr. 2020, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.J.; Klevebro, S.; Wood, T.R. Maternal and Neonatal Polyunsaturated Fatty Acid Intake and Risk of Neurodevelopmental Impairment in Premature Infants. Int. J. Mol. Sci. 2022, 23, 700. [Google Scholar] [CrossRef]
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011, 7, 17–26. [Google Scholar] [CrossRef]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Matern. Health Neonatol. Perinatol. 2017, 3, 22. [Google Scholar] [CrossRef]
- Gow, R.V.; Hibbeln, J.R. Omega-3 Fatty Acid and Nutrient Deficits in Adverse Neurodevelopment and Childhood Behaviors. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatr. 2009, 169, 149–164. [Google Scholar] [CrossRef]
- Libuda, L.; Mesch, C.M.; Stimming, M.; Demmelmair, H.; Koletzko, B.; Warschburger, P.; Blanke, K.; Reischl, E.; Kalhoff, H.; Kersting, M. Fatty acid supply with complementary foods and LC-PUFA status in healthy infants: Results of a randomised controlled trial. Eur. J. Nutr. 2015, 55, 1633–1644. [Google Scholar] [CrossRef]
- Kalhoff, H.; Mesch, C.M.; Stimming, M.; Israel, A.; Spitzer, C.; Beganovic, L.; Perez, R.E.; Koletzko, B.; Warschburger, P.; Kersting, M.; et al. Effects of LC-PUFA supply via complementary food on infant development—A food based intervention (RCT) embedded in a total diet concept. Eur. J. Clin. Nutr. 2019, 74, 682–690. [Google Scholar] [CrossRef]
- Haiden, N.; Thanhaeuser, M.; Eibensteiner, F.; Huber-Dangl, M.; Gsoellpointner, M.; Ristl, R.; Kroyer, B.; Brandstetter, S.; Kornsteiner-Krenn, M.; Binder, C.; et al. Randomized Controlled Trial of Two Timepoints for Introduction of Standardized Complementary Food in Preterm Infants. Nutrients 2022, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Toeller, M.; Buyken, A.; Heitkamp, G.; Milne, R.; Klischan, A.; Gries, F. EURODIAB IDDM Complications Study Group Repeatability of three-day dietary records in the EURODIAB IDDM Complications Study. Eur. J. Clin. Nutr. 1997, 51, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kim, M.K.; Hwang, S.H.; Ahn, Y.; Shim, J.E.; Kim, D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010, 4, 142–148. [Google Scholar] [CrossRef]
- Dewey, K.G.; Beaton, G.; Fjeld, C.; Lönnerdal, B.; Reeds, P. Protein requirements of infants and children. Eur. J. Clin. Nutr. 1996, 50 (Suppl. 1), S119–S147; discussion S147–S150. [Google Scholar] [PubMed]
- Catalá, A. Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects. J. Lipids 2013, 2013, 710290. [Google Scholar] [CrossRef] [PubMed]
- Gsoellpointner, M.; Eibensteiner, F.; Thanhaeuser, M.; Ristl, R.; Jilma, B.; Berger, A.; Haiden, N. Effects of early introduction of solid foods on nutrient intake in preterm infants during their 1st year of life: A secondary outcome analysis of a prospective, randomized intervention study. Front. Nutr. 2023, 10, 1124544. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.A.; Grieve, A.J. Test Review: Bayley, N. Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 2007, 25, 180–190. [Google Scholar] [CrossRef]
- Joint, F. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation, 10–14 November 2008, Geneva; FAO: Rome, Italy, 2010. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current Information and Asian Perspectives on Long-Chain Polyunsaturated Fatty Acids in Pregnancy, Lactation, and Infancy: Systematic Review and Practice Recommendations from an Early Nutrition Academy Workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung; Österreichische Gesellschaft für Ernährung; Schweizerische Gesellschaft für Ernährungsforschung; Schweizerische Vereinigung für Ernährung. Referenzwerte für die Nährstoffzufuhr, 2nd ed.; 7th updated ed.; Bonn, Germany, 2021. [Google Scholar]
- Fleith, M.; Clandinin, M.T. Dietary PUFA for Preterm and Term Infants: Review of Clinical Studies. Crit. Rev. Food Sci. Nutr. 2005, 45, 205–229. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Collins, C.T.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Smithers, L.G.; et al. Neurodevelopmental Outcomes of Preterm Infants Fed High-Dose Docosahexaenoic Acid. JAMA 2009, 301, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Drossard, C.; Dube, K.; Kannenberg, F.; Kunz, C.; Kalhoff, H.; Kersting, M. Dietary intake and plasma concentrations of PUFA and LC-PUFA in breastfed and formula fed infants under real-life conditions. Eur. J. Nutr. 2009, 49, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Julvez, J.; Guxens, M.; Carsin, A.-E.; Forns, J.; Mendez, M.; Turner, M.C.; Sunyer, J. A cohort study on full breastfeeding and child neuropsychological development: The role of maternal social, psychological, and nutritional factors. Dev. Med. Child Neurol. 2013, 56, 148–156. [Google Scholar] [CrossRef]
- Guxens, M.; Mendez, M.A.; Moltó-Puigmartí, C.; Julvez, J.; García-Esteban, R.; Forns, J.; Ferrer, M.; Vrijheid, M.; López-Sabater, M.C.; Sunyer, J. Breastfeeding, Long-Chain Polyunsaturated Fatty Acids in Colostrum, and Infant Mental Development. Pediatrics 2011, 128, e880–e889. [Google Scholar] [CrossRef]
- Bernard, J.; Armand, M.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.A.; Heude, B. The EDEN Mother-Child Cohort Study Group The association between linoleic acid levels in colostrum and child cognition at 2 and 3 y in the EDEN cohort. Pediatr. Res. 2015, 77, 829–835. [Google Scholar] [CrossRef]
- Vidakovic, A.J.; Gishti, O.; Voortman, T.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Jaddoe, V.W.; et al. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: The Generation R Study. Am. J. Clin. Nutr. 2016, 103, 1017–1025. [Google Scholar] [CrossRef]
- Strain, J.; Davidson, P.W.; Bonham, M.P.; Duffy, E.M.; Stokes-Riner, A.; Thurston, S.W.; Wallace, J.M.; Robson, P.J.; Shamlaye, C.F.; Georger, L.A.; et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology 2008, 29, 776–782. [Google Scholar] [CrossRef]
- Schwartz, J.; Dube, K.; Sichert-Hellert, W.; Kannenberg, F.; Kunz, C.; Kalhoff, H.; Kersting, M. Modification of dietary polyunsaturated fatty acids via complementary food enhances n-3 long-chain polyunsaturated fatty acid synthesis in healthy infants: A double blinded randomised controlled trial. Arch. Dis. Child. 2009, 94, 876–882. [Google Scholar] [CrossRef]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Bührer, C.; Genzel-Boroviczény, O.; Jochum, F.; Kauth, T.; Kersting, M.; Koletzko, B.; Mihatsch, W.; Przyrembel, H.; Reinehr, T.; Zimmer, P. Ernährung gesunder Säuglinge. Ernährungskommission der Deutschen Gesellschaft für Kinder- und Jugendmedizin (DGKJ). Monatsschrift Kinderheilkd. 2014, 162, 527–538. [Google Scholar] [CrossRef]
- Komprda, T.; Zelenka, J.; Fajmonová, E.; Fialová, M.; Kladroba, D. Arachidonic Acid and Long-Chain n−3 Polyunsaturated Fatty Acid Contents in Meat of Selected Poultry and Fish Species in Relation to Dietary Fat Sources. J. Agric. Food Chem. 2005, 53, 6804–6812. [Google Scholar] [CrossRef] [PubMed]
- Taber, L.; Chiu, C.-H.; Whelan, J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids 1998, 33, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ng, A.; Mann, N.J.; Sinclair, A.J. Contribution of meat fat to dietary arachidonic acid. Lipids 1998, 33, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Operto, F.F.; Matricardi, S.; Pastorino, G.M.G.; Verrotti, A.; Coppola, G. The Ketogenic Diet for the Treatment of Mood Disorders in Comorbidity with Epilepsy in Children and Adolescents. Front. Pharmacol. 2020, 11, 578396. [Google Scholar] [CrossRef]

| Fatty Acid Family | Number of Double Bonds | Name |
|---|---|---|
| n-3 fatty acids | 18:3 | alpha-Linolenic acid (ALA) |
| 22:6 | Docosahexaenoic acid (DHA) | |
| 20:5 | Eicosapentaenoic acid (EPA) | |
| n-6 fatty acids | 18:2 | Linoleic acid (LA) |
| 20:4 | Arachidonic Acid (AA) |
| Baseline Characteristics (n = 140) | |
|---|---|
| Neonatal outcomes | |
| Birth weight (g) | 928 (719–1125) |
| Height at birth (cm) | 35.0 (32.5–37.0) |
| Head circumference at birth (cm) | 25 (23.0–26.3) |
| Gestational age (days) | 192 (177–200) |
| Gestational age (weeks) | 27/3 |
| Time to full enteral feeds (days) | 21 (14–31) |
| Male sex | 79 (56%) |
| Intraventricular hemorrhage Grade III + IV | 7 (5%) |
| Retinopathy of prematurity (any) | |
| Grade 1 | 11 (8%) |
| Grade 2 | 24 (17%) |
| Grade 3 | 6 (4%) |
| Nutrition at discharge | |
| Breastmilk | 44 (31%) |
| Formula | 44 (31%) |
| Mixed | 52 (37%) |
| Necrotizing enterocolitis | |
| Grade 1 | 1 (0%) |
| Grade 2 | 2 (1%) |
| Periventricular leukomalacia | 2 (1%) |
| Obstetric and parental parameters | |
| Age of mother at birth (years) | 33 (30–37) |
| Multiple birth | 43 (31%) |
| Cesarean delivery | 129 (92%) |
| Preeclampsia | 12 (9%) |
| Highest parental education | |
| Primary education | 43 (31%) |
| Secondary education | 23 (16%) |
| Tertiary education | 58 (41%) |
| Maternal smoking habit | |
| Before pregnancy | 27 (19%) |
| During pregnancy | 4 (3%) |
| After pregnancy | 3 (2%) |
| Always | 19 (14%) |
| Bayley-III | Bayley Assessment (Months CA) | n | ERS | 95% CI | p-Value Unadjusted | p-Value Adjusted |
|---|---|---|---|---|---|---|
| Cognition | 12 | 140 | 7.32 | 3.84 to 10.80 | 0.0005 | 0.003 |
| 24 | 130 | 2.74 | −1.71 to 7.18 | 0.20 | 0.31 | |
| Motor | 12 | 140 | 4.73 | 1.80 to 7.67 | 0.004 | 0.01 |
| 24 | 124 | 3.37 | −0.24 to 6.97 | 0.06 | 0.13 | |
| Language | 12 | 140 | 1.65 | −1.46 to 4.75 | 0.27 | 0.32 |
| 24 | 122 | −0.61 | −5.29 to 4.10 | 0.78 | 0.77 |
| Bayley-III | Bayley Assessment (Months CA) | n | ERS | 95% CI | p-Value Unadjusted | p-Value Adjusted |
|---|---|---|---|---|---|---|
| Cognition | 12 | 140 | 0.16 | 0.00 to 0.32 | 0.04 | 0.13 |
| 24 | 130 | 0.03 | −0.16 to 0.23 | 0.75 | 0.77 | |
| Motor | 12 | 140 | 0.21 | 0.10 to 0.34 | 0.002 | 0.01 |
| 24 | 124 | 0.03 | −0.13 to 0.19 | 0.68 | 0.77 | |
| Language | 12 | 140 | 0.05 | −0.08 to 0.18 | 0.42 | 0.77 |
| 24 | 122 | −0.03 | −0.24 to 0.18 | 0.77 | 0.77 |
| Bayley-III | Bayley Assessment (Months CA) | n | ERS | 95% CI | p-Value Unadjusted | p-Value Adjusted |
|---|---|---|---|---|---|---|
| Cognition | 12 | 140 | 0.11 | −0.01 to 0.24 | 0.07 | 0.21 |
| 24 | 130 | −0.07 | −0.22 to 0.07 | 0.32 | 0.48 | |
| Motor | 12 | 140 | 0.16 | 0.06 to 0.26 | 0.004 | 0.03 |
| 24 | 124 | −0.07 | −0.18 to 0.05 | 0.24 | 0.48 | |
| Language | 12 | 140 | 0.02 | −0.09 to 0.12 | 0.75 | 0.74 |
| 24 | 122 | −0.05 | −0.22 to 0.12 | 0.53 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gsoellpointner, M.; Thanhaeuser, M.; Eibensteiner, F.; Ristl, R.; Jilma, B.; Fuiko, R.; Brandstetter, S.; Berger, A.; Haiden, N. Polyunsaturated Fatty Acid Intake during Complementary Feeding and Neurodevelopmental Outcome in Very Low Birth Weight Infants. Nutrients 2023, 15, 3141. https://doi.org/10.3390/nu15143141
Gsoellpointner M, Thanhaeuser M, Eibensteiner F, Ristl R, Jilma B, Fuiko R, Brandstetter S, Berger A, Haiden N. Polyunsaturated Fatty Acid Intake during Complementary Feeding and Neurodevelopmental Outcome in Very Low Birth Weight Infants. Nutrients. 2023; 15(14):3141. https://doi.org/10.3390/nu15143141
Chicago/Turabian StyleGsoellpointner, Melanie, Margarita Thanhaeuser, Fabian Eibensteiner, Robin Ristl, Bernd Jilma, Renate Fuiko, Sophia Brandstetter, Angelika Berger, and Nadja Haiden. 2023. "Polyunsaturated Fatty Acid Intake during Complementary Feeding and Neurodevelopmental Outcome in Very Low Birth Weight Infants" Nutrients 15, no. 14: 3141. https://doi.org/10.3390/nu15143141
APA StyleGsoellpointner, M., Thanhaeuser, M., Eibensteiner, F., Ristl, R., Jilma, B., Fuiko, R., Brandstetter, S., Berger, A., & Haiden, N. (2023). Polyunsaturated Fatty Acid Intake during Complementary Feeding and Neurodevelopmental Outcome in Very Low Birth Weight Infants. Nutrients, 15(14), 3141. https://doi.org/10.3390/nu15143141







