Personalized Nutritional Strategies to Reduce Knee Osteoarthritis Severity and Ameliorate Sarcopenic Obesity Indices: A Practical Guide in an Orthopedic Setting
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Diagnosis of KOA
3.2. Screening/Diagnosis of SO in Patients with KOA
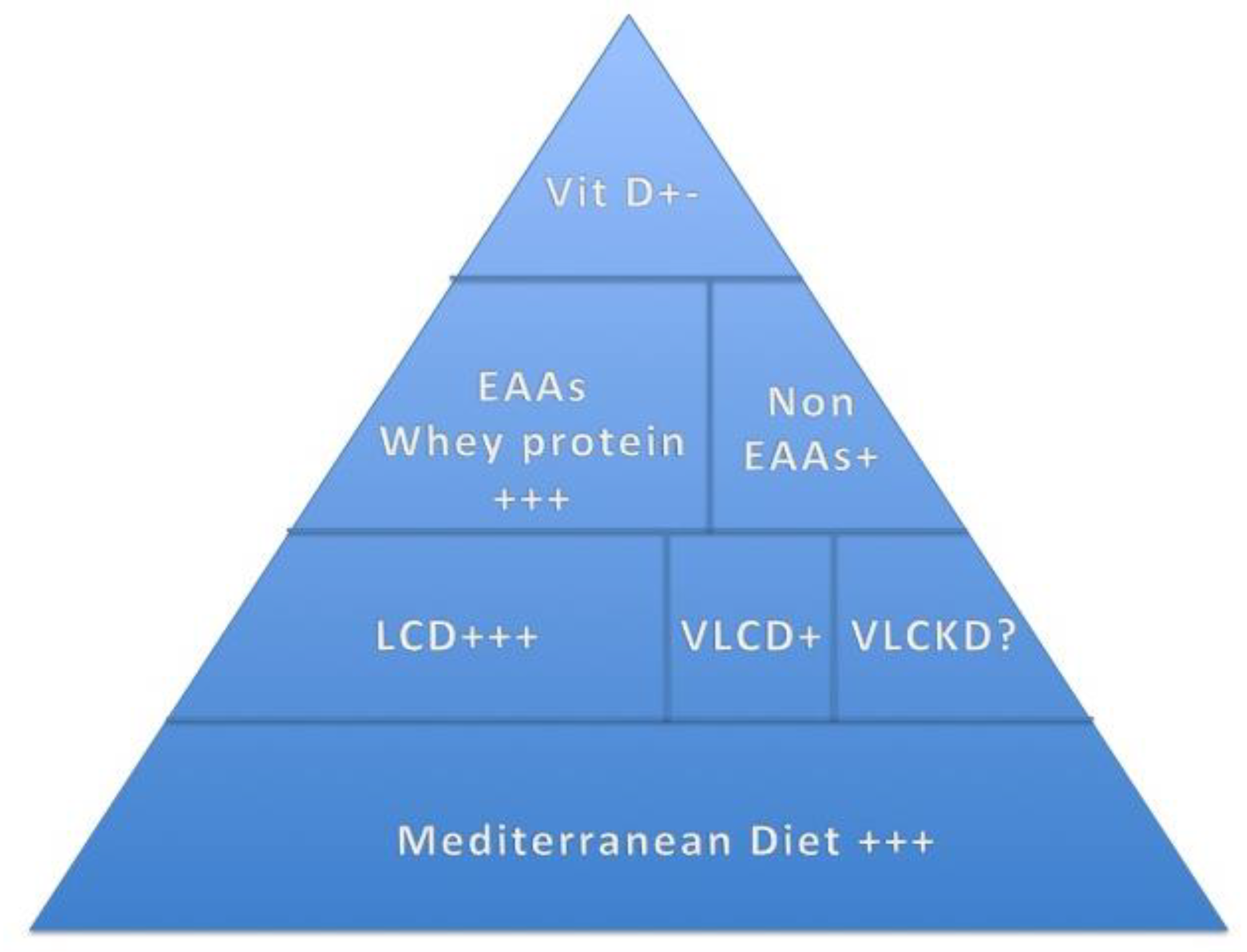
3.3. Nutritional Management in Patients with KOA and SO Diets
- (1)
- Mediterranean Diet
- (2)
- Weight loss
- (3)
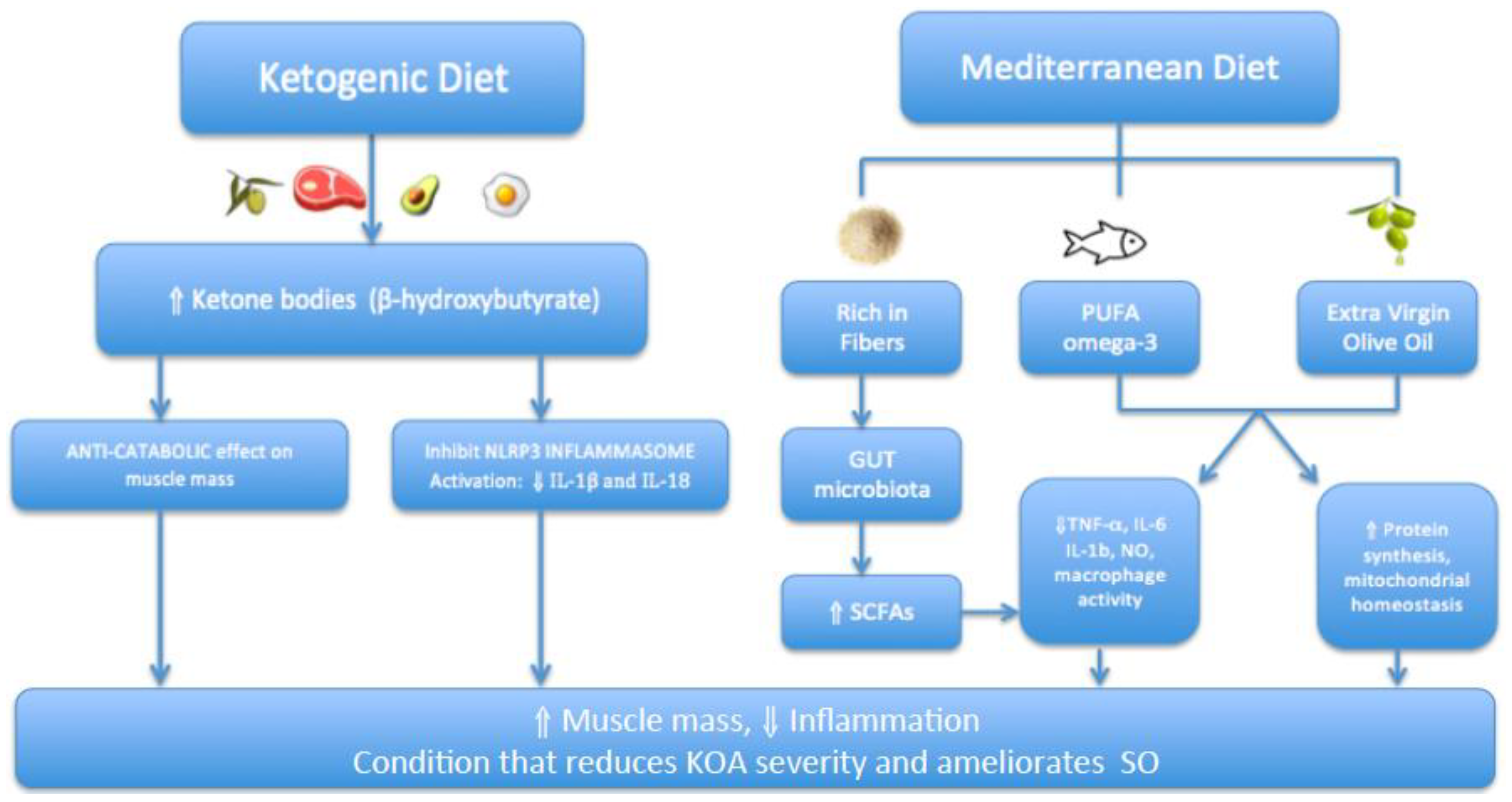
- Very-Low-Calorie Ketogenic Diet (VLCKD)
3.4. Supplementation
- (1)
- Vitamin D
- (2)
- Antioxidant Supplements
- (3)
- Amino Acid Supplementation
- EAA Supplementation, KOA and SO
- Non-EAA Supplementation and KOA
- (4)
- Protein Supplementation
- Whey protein supplements, KOA and SO
| Grade | Nutritional Approach | Suggested Duration | Type of Evidence | |
|---|---|---|---|---|
| Prevention program | 0 |
| Lifelong |
|
| Lifestyle intervention (no surgery) | I and II |
| 18 months |
|
| Intensive lifestyle intervention (surgery) | III and IV |
| 5–7 weeks |
4. Discussion
4.1. Interaction between KOA and SO
4.2. Findings
- (1)
- Prevention Program
- (2)
- Lifestyle Intervention (LI) Program (72 weeks)
- (3)
- Intensive Lifestyle Intervention (ILI) Program (Five Weeks)
4.3. Clinical Implications
4.4. Strengths and Limitations
4.5. New Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Pérez Martín, A. Symptoms. Localizations: Knee, hip, hands, spine, other localizations. Aten. Primaria. 2014, 46, 11–17. [Google Scholar]
- Brandt, K.D.; Dieppe, P.; Radin, E. Etiopathogenesis of Osteoarthritis. Med. Clin. N. Am. 2009, 93, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. Eclinicalmedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Szilagyi, I.A.; Waarsing, J.H.; van Meurs, J.B.J.; Bierma-Zeinstra, S.M.A.; Schiphof, D. A systematic review of the sex differences in risk factors for knee osteoarthritis. Rheumatology 2023, 62, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kean, W. Obesity and knee osteoarthritis. Inflammopharmacology 2012, 20, 53–58. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Wang, Y.; Li, H. Association between sarcopenia and osteoarthritis: A protocol for meta-analysis. PLoS ONE 2022, 17, e0272284. [Google Scholar] [CrossRef]
- Nezameddin, R.; Itani, L.; Kreidieh, D.; El Masri, D.; Tannir, H.; El Ghoch, M. Understanding Sarcopenic Obesity in Terms of Definition and Health Consequences: A Clinical Review. Curr. Diabetes Rev. 2020, 16, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Khadra, D.; Itani, L.; Tannir, H.; Kreidieh, D.; El Masri, D.; El Ghoch, M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J. Diabetes 2019, 10, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Khadra, D.; Itani, L.; Chebaro, Y.; Obeid, M.; Jaber, M.; Ghanem, R.; Ayton, A.; Kreidieh, D.; El Masri, D.; Kimura, A.; et al. Association Between Sarcopenic Obesity and Metabolic Syndrome in Adults: A Systematic Review and Meta-Analysis. Curr. Cardiol. Rev. 2020, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Khazem, S.; Itani, L.; Kreidieh, D.; El Masri, D.; Tannir, H.; Citarella, R.; El Ghoch, M. Reduced Lean Body Mass and Cardiometabolic Diseases in Adult Males with Overweight and Obesity: A Pilot Study. Int. J. Environ. Res. Public Health 2018, 15, 2754. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.; Fielding, R.A.; Felson, D.T.; Niu, J.; Brown, C.; Nevitt, M.; Lewis, C.E.; Torner, J.; Neogi, T.; The MOST Study. Risk of Knee Osteoarthritis With Obesity, Sarcopenic Obesity, and Sarcopenia. Arthritis Rheumatol. 2019, 71, 232–237. [Google Scholar] [CrossRef]
- Narrative Review Checklist. Available online: https://www.elsevier.com/__data/promis_misc/ANDJNarrativeReviewChecklistpdf (accessed on 1 June 2023).
- Jang, S.; Lee, K.; Ju, J. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Godziuk, K.; Woodhouse, L.J.; Prado, C.M.; Forhan, M. Clinical screening and identification of sarcopenic obesity in adults with advanced knee osteoarthritis. Clin. Nutr. ESPEN 2020, 40, 340–348. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Martinez-Lacoba, R.; Pardo-Garcia, I.; Amo-Saus, E.; Escribano-Sotos, F. Mediterranean diet and health outcomes: A systematic meta-review. Eur. J. Public Health 2018, 28, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. From gut microbiota dysfunction to obesity: Could short-chain fatty acids stop this dangerous course? Hormones 2019, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Ni, Y.-F.; Wang, J.; Yan, X.-L.; Tian, F.; Zhao, J.-B.; Wang, Y.-J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010, 11, 33. [Google Scholar] [CrossRef]
- Säemann, M.D.; Böhmig, G.A.; Osterreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The Immune Protective Effect of the Mediterranean Diet against Chronic Low-grade Inflammatory Diseases. Endocr. Metab. Immune Disord.—Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Inflammatory Cytokine Storms. Mo Med. 2020, 117, 539–542. [Google Scholar] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Luchini, C.; Smith, T.O.; Cooper, C.; Guglielmi, G.; Reginster, J.-Y.; Rizzoli, R.; et al. Adherence to a Mediterranean diet is associated with lower prevalence of osteoarthritis: Data from the osteoarthritis initiative. Clin. Nutr. 2017, 36, 1609–1614. [Google Scholar] [CrossRef]
- Veronese, N.; La Tegola, L.; Crepaldi, G.; Maggi, S.; Rogoli, D.; Guglielmi, G. The association between the Mediterranean diet and magnetic resonance parameters for knee osteoarthritis: Data from the Osteoarthritis Initiative. Clin. Rheumatol. 2018, 37, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Koyanagi, A.; Stubbs, B.; Cooper, C.; Guglielmi, G.; Rizzoli, R.; Punzi, L.; Rogoli, D.; Caruso, M.G.; Rotolo, O.; et al. Mediterranean diet and knee osteoarthritis outcomes: A longitudinal cohort study. Clin. Nutr. 2019, 38, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Calvani, R.; Marzetti, E.; Picca, A.; Coelho-Júnior, H.J.; Martone, A.M.; Massaro, C.; Tosato, M.; Landi, F. Low Adherence to Mediterranean Diet Is Associated with Probable Sarcopenia in Community-Dwelling Older Adults: Results from the Longevity Check-Up (Lookup) 7+ Project. Nutrients 2023, 15, 1026. [Google Scholar] [CrossRef]
- Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients 2022, 14, 3567. [Google Scholar] [CrossRef]
- Yarla, N.S.; Polito, A.; Peluso, I. Effects of Olive Oil on TNF-α and IL-6 in Humans: Implication in Obesity and Frailty. Endocr. Metab. Immune Disord.—Drug Targets 2018, 18, 63–74. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.-P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Y.-B.; Tao, X.-H.; Pan, W.-L.; Wu, Y.-X.; Wang, X.-H.; He, Y.-Q.; Xiao, W.-F.; Li, Y.-S. Mitochondrial Quality Control in Sarcopenia: Updated Overview of Mechanisms and Interventions. Aging Dis. 2021, 12, 2016. [Google Scholar] [CrossRef]
- Messier, S.P.; Gutekunst, D.J.; Davis, C.; DeVita, P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005, 52, 2026–2032. [Google Scholar] [CrossRef]
- Christensen, R.; Bartels, E.M.; Astrup, A.; Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2007, 66, 433–439. [Google Scholar] [CrossRef]
- Chu, I.J.H.; Lim, A.Y.T.; Ng, C.L.W. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1597–1607. [Google Scholar] [CrossRef]
- Panunzi, S.; Maltese, S.; De Gaetano, A.; Capristo, E.; Bornstein, S.R.; Mingrone, G. Comparative efficacy of different weight loss treatments on knee osteoarthritis: A network meta-analysis. Obes. Rev. 2021, 22, e13230. [Google Scholar] [CrossRef]
- Tannir, H.; Itani, L.; Kreidieh, D.; El Masri, D.; El Ghoch, M. Can Intentional Weight Loss Ameliorate Sarcopenia in Individuals with Obesity? A Longitudinal Interventional Study. Clin. Pract. 2022, 12, 106–112. [Google Scholar] [CrossRef]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO) European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Ting, J.P. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J. Immunol. 2009, 183, 7623–7629. [Google Scholar] [CrossRef]
- Fioravanti, A.; Tenti, S.; McAllister, M.; Chemaly, M.; Eakin, A.; McLaughlin, J.; Bjourson, A.J.; Frati, E.; McGilligan, V.; Cheleschi, S.; et al. Exploring the Involvement of NLRP3 and IL-1β in Osteoarthritis of the Hand: Results from a Pilot Study. Mediat. Inflamm. 2019, 2019, 2363460. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zheng, Y.; Yu, Q.; Zeng, M.; Bai, L.; Yang, L.; Guo, M.; Jiang, X.; Gan, J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int. J. Mol. Med. 2023, 51, 35. [Google Scholar] [CrossRef]
- Ramirez-Perez, S.; Reyes-Perez, I.V.; Martinez-Fernandez, D.E.; Hernandez-Palma, L.A.; Bhattaram, P. Targeting inflammasome-dependent mechanisms as an emerging pharmacological approach for osteoarthritis therapy. iScience 2022, 25, 105548. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Wang, J.; Li, R.; Huang, Z.; Le Wang, L. Ketogenic diet ameliorates inflammation by inhibiting the NLRP3 inflammasome in osteoarthritis. Thromb. Haemost. 2022, 24, 113. [Google Scholar] [CrossRef] [PubMed]
- Camajani, E.; Feraco, A.; Basciani, S.; Gnessi, L.; Barrea, L.; Armani, A.; Caprio, M. VLCKD in Combination with Physical Exercise Preserves Skeletal Muscle Mass in Sarcopenic Obesity after Severe COVID-19 Disease: A Case Report. Healthcare 2022, 10, 573. [Google Scholar] [CrossRef]
- Camajani, E.; Feraco, A.; Proietti, S.; Basciani, S.; Barrea, L.; Armani, A.; Lombardo, M.; Gnessi, L.; Caprio, M. Very low calorie ketogenic diet combined with physical interval training for preserving muscle mass during weight loss in sarcopenic obesity: A pilot study. Front. Nutr. 2022, 9, 955024. [Google Scholar] [CrossRef]
- Vaishya, R.; Vijay, V.; Lama, P.; Agarwal, A. Does vitamin D deficiency influence the incidence and progression of knee osteoarthritis?—A literature review. J. Clin. Orthop. Trauma 2019, 10, 9–15. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- Haines, S.T.; Park SKVitamin, D. supplementation: What’s known, what to do, and what’s needed. Pharmacotherapy 2012, 32, 354–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; He, Y.; Peng, L.H.L.X.; Liu, M.; He, C.S.; Chen, J. Does vitamin D improve symptomatic and structural outcomes in knee osteoarthritis? A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 2393–2403. [Google Scholar] [CrossRef]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef]
- Szafors, P.; Che, H.; Barnetche, T.; Morel, J.; Gaujoux-Viala, C.; Combe, B.; Lukas, C. Risk of fracture and low bone mineral density in adults with inflammatory bowel diseases. A systematic literature review with meta-analysis. Osteoporos. Int. 2018, 29, 2389–2397. [Google Scholar] [CrossRef]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef]
- Jabbour, J.; Rahme, M.; Mahfoud, Z.R.; El-Hajj Fuleihan, G. Effect of high dose vitamin D supplementation on indices of sarcopenia and obesity assessed by DXA among older adults: A randomized controlled trial. Endocrine 2022, 76, 162–171. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Wages, P. Oxidative stress from environmental exposures. Curr. Opin. Toxicol. 2018, 7, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Gonzalez, A.; Simon, F.; Achiardi, O.; Vilos, C.; Cabrera, D.; Cabello-Verrugio, C. The Critical Role of Oxidative Stress in Sarcopenic Obesity. Oxid. Med. Cell. Longev. 2021, 2021, 4493817. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J. Sarcopenic Obesity: Involvement of Oxidative Stress and Beneficial Role of Antioxidant Flavonoids. Antioxidants 2023, 12, 1063. [Google Scholar] [CrossRef]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2016, 15, 1. [Google Scholar] [CrossRef]
- Hsiao, A.F.; Lien, Y.C.; Tzeng, I.S.; Liu, C.T.; Chou, S.H.; Horng, Y.S. The efficacy of high-and low-dose curcumin in knee osteoarthritis: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 63, 102775. [Google Scholar] [CrossRef]
- Nejadhosseinian, M.; Djalalinia, S.; Haerian, H.; Alikhani, M.; Mansour, A.; Mousavian, A.-H.; Mardani-Fard, H.A.; Kasaeian, A.; Faezi, S.T. The effects of antioxidants on knee osteoarthritis: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 1026450. [Google Scholar] [CrossRef]
- Rose, A.J. Amino Acid Nutrition and Metabolism in Health and Disease. Nutrients 2019, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P. Nonessential amino acid usage for protein replenishment in humans: A method of estimation. Am. J. Clin. Nutr. 2019, 110, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv. Nutr. Int. Rev. J. 2018, 9, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Zhai, G.; Wang-Sattler, R.; Hart, D.J.; Arden, N.K.; Hakim, A.J.; Illig, T.; Spector, T.D. Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1227–1231. [Google Scholar] [CrossRef]
- Nakajima, H.; Okada, H.; Kobayashi, A.; Takahashi, F.; Okamura, T.; Hashimoto, Y.; Nakanishi, N.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; et al. Leucine and Glutamic Acid as a Biomarker of Sarcopenic Risk in Japanese People with Type 2 Diabetes. Nutrients 2023, 15, 2400. [Google Scholar] [CrossRef]
- Mistry, D.; Gee, T.; Lee, P. Systematic Review for Protein and Creatine Supplements in Peri-operative Period in Elective Musculoskeletal Surgery-Knee and Hip Replacement. J. Arthritis 2022, 11, 6–10. [Google Scholar]
- Dreyer, H.C.; Strycker, L.A.; Senesac, H.A.; Hocker, A.D.; Smolkowski, K.; Shah, S.N.; Jewett, B.A. Essential amino acid supplementation in patients following total knee arthroplasty. J. Clin. Investig. 2013, 123, 4654–4666. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Owen, E.C.; Strycker, L.A.; Smolkowski, K.; Muyskens, J.B.; Kirkpatrick, T.K.; Christie, A.D.; Kuehl, K.S.; Lantz, B.A.; Shah, S.N.; et al. Essential Amino Acid Supplementation Mitigates Muscle Atrophy After Total Knee Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial. JBJS Open Access 2018, 3, e0006. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Handelsman, D.J.; Stanaway, F.; Waite, L.M.; Blyth, F.M.; Naganathan, V.; Cumming, R.G.; Hirani, V. Sarcopenic Obesity and Amino Acids: Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xing, B.; He, G.; Lyu, X.; Zeng, Y. The Effects of Electrical Acupuncture and Essential Amino Acid Supplementation on Sarcopenic Obesity in Male Older Adults: A Randomized Control Study. Obes. Facts 2018, 11, 327–334. [Google Scholar] [CrossRef]
- Takeuchi, F.; Takada, M.; Kobuna, Y.; Uchida, H.; Adachi, Y. Effects of Non-Essential Amino Acids on Knee Joint Conditions in Adults: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2022, 14, 3628. [Google Scholar] [CrossRef]
- Liao, C.D.; Liao, Y.H.; Liou, T.H.; Hsieh, C.Y.; Kuo, Y.C.; Chen, H.C. Effects of Protein-Rich Nutritional Composition Supplementation on Sarcopenia Indices and Physical Activity during Resistance Exercise Training in Older Women with Knee Osteoarthritis. Nutrients 2021, 13, 2487. [Google Scholar] [CrossRef]
- Liao, C.D.; Wu, Y.T.; Tsauo, J.Y.; Chen, P.R.; Tu, Y.K.; Chen, H.C.; Liou, T.H. Effects of Protein Supplementation Combined with Exercise Training on Muscle Mass and Function in Older Adults with Lower-Extremity Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Trials. Nutrients 2020, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lim, Y. Effects of Whey Peptide Supplementation on Sarcopenic Obesity in High-Fat Diet-Fed Mice. Nutrients 2022, 14, 4402. [Google Scholar] [CrossRef]
- Coker, R.H.; Miller, S.; Schutzler, S.; Deutz, N.; Wolfe, R.R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 2012, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.G.; Tomeleri, C.M.; Fernandes, R.R.; Sugihara Junior, P., Jr.; Cavalcante, E.F.; Cunha, P.M.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2019, 32, 88–95. [Google Scholar] [CrossRef]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008, 32, 211–222. [Google Scholar] [CrossRef]
- Shields, M.; Tremblay, M.S. Sedentary behaviour and obesity. Health Rep. 2008, 19, 19–30. [Google Scholar]
- Kreidieh, D.; Itani, L.; El Masri, D.; Tannir, H.; El Ghoch, M. Association Between Reduced Daily Steps and Sarcopenic Obesity in Treatment-Seeking Adults with Obesity. Front. Endocrinol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Felson, D.T.; Chaisson, C.E. 2 Understanding the relationship between body weight and osteoarthritis. Bailliere’s Clin. Rheumatol. 1997, 11, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Haas de Mello, A.; Costa, A.B.; Della Giustina Engel, J.; Tezza Rezin, G. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Central Eur. J. Immunol. 2020, 45, 469–475. [Google Scholar] [CrossRef] [PubMed]
- De Santi, M.; Annibalini, G.; Marano, G.; Biganzoli, G.; Venturelli, E.; Pellegrini, M.; Lucertini, F.; Brandi, G.; Biganzoli, E.; Barbieri, E.; et al. Association between metabolic syndrome, insulin resistance, and IGF-1 in breast cancer survivors of DIANA-5 study. J. Cancer Res. Clin. Oncol. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Karanth, S.D.; Washington, C.; Cheng, T.D.; Zhou, D.; Leeuwenburgh, C.; Braithwaite, D.; Zhang, D. Inflammation in Relation to Sarcopenia and Sarcopenic Obesity among Older Adults Living with Chronic Comorbidities: Results from the National Health and Nutrition Examination Survey 1999–2006. Nutrients 2021, 13, 3957. [Google Scholar] [CrossRef]
- Nelson, F.R.; Zvirbulis, R.A.; Zonca, B.; Li, K.W.; Turner, S.M.; Pasierb, M.; Wilton, P.; Martinez-Puig, D.; Wu, W. The effects of an oral preparation containing hyaluronic acid (Oralvisc®) on obese knee osteoarthritis patients determined by pain, function, bradykinin, leptin, inflammatory cytokines, and heavy water analyses. Rheumatol. Int. 2015, 35, 43–52. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, Y.H.; Huang, L.C. The effect of oral low molecular weight liquid hyaluronic acid combination with glucosamine and chondroitin on knee osteoarthritis patients with mild knee pain: An 8-week randomized double-blind placebo-controlled trial. Medicine 2021, 100, e24252. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Girolimetto, N.; Bentivenga, C.; Grandi, E.; Fogacci, F.; Borghi, C. Short-Term Effect of a New Oral Sodium Hyaluronate Formulation on Knee Osteoarthritis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Diseases 2020, 8, 26. [Google Scholar] [CrossRef]
- Stellavato, A.; Abate, L.; Vassallo, V.; Donniacuo, M.; Rinaldi, B.; Schiraldi, C. An in vitro study to assess the effect of hyaluronan-based gels on muscle-derived cells: Highlighting a new perspective in regenerative medicine. PLoS ONE 2020, 15, e0236164. [Google Scholar] [CrossRef]


| Sarcopenic Obesity in Individuals with KOA | ||||
|---|---|---|---|---|
| Tool | Cut-Off | |||
| Females | Males | |||
| Screening | Grip strength/BMI | 0.65 | 1.1 | |
| Diagnosis | ALM/BMI | 0.512 | 0.789 | |
| Kellgren and Lawrence KOA Classification System | ||||
| Grade 0 | No radiological findings of OA | |||
| Grade I | Doubtful joint space narrowing and possible osteophytic lipping | |||
| Grade II | Certain osteophytes and possible joint space narrowing | |||
| Grade III | Moderate multiple osteophytes, certain narrowing of joint space, some sclerosis and possible deformity of bone ends | |||
| Grade IV | Large osteophytes, marked narrowing of joint space, severe sclerosis, and certain deformity of bone ends | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zmerly, H.; El Ghoch, M.; Itani, L.; Kreidieh, D.; Yumuk, V.; Pellegrini, M. Personalized Nutritional Strategies to Reduce Knee Osteoarthritis Severity and Ameliorate Sarcopenic Obesity Indices: A Practical Guide in an Orthopedic Setting. Nutrients 2023, 15, 3085. https://doi.org/10.3390/nu15143085
Zmerly H, El Ghoch M, Itani L, Kreidieh D, Yumuk V, Pellegrini M. Personalized Nutritional Strategies to Reduce Knee Osteoarthritis Severity and Ameliorate Sarcopenic Obesity Indices: A Practical Guide in an Orthopedic Setting. Nutrients. 2023; 15(14):3085. https://doi.org/10.3390/nu15143085
Chicago/Turabian StyleZmerly, Hassan, Marwan El Ghoch, Leila Itani, Dima Kreidieh, Volkan Yumuk, and Massimo Pellegrini. 2023. "Personalized Nutritional Strategies to Reduce Knee Osteoarthritis Severity and Ameliorate Sarcopenic Obesity Indices: A Practical Guide in an Orthopedic Setting" Nutrients 15, no. 14: 3085. https://doi.org/10.3390/nu15143085
APA StyleZmerly, H., El Ghoch, M., Itani, L., Kreidieh, D., Yumuk, V., & Pellegrini, M. (2023). Personalized Nutritional Strategies to Reduce Knee Osteoarthritis Severity and Ameliorate Sarcopenic Obesity Indices: A Practical Guide in an Orthopedic Setting. Nutrients, 15(14), 3085. https://doi.org/10.3390/nu15143085






