Association of Metformin Use with Iron Deficiency Anemia in Urban Chinese Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Methods
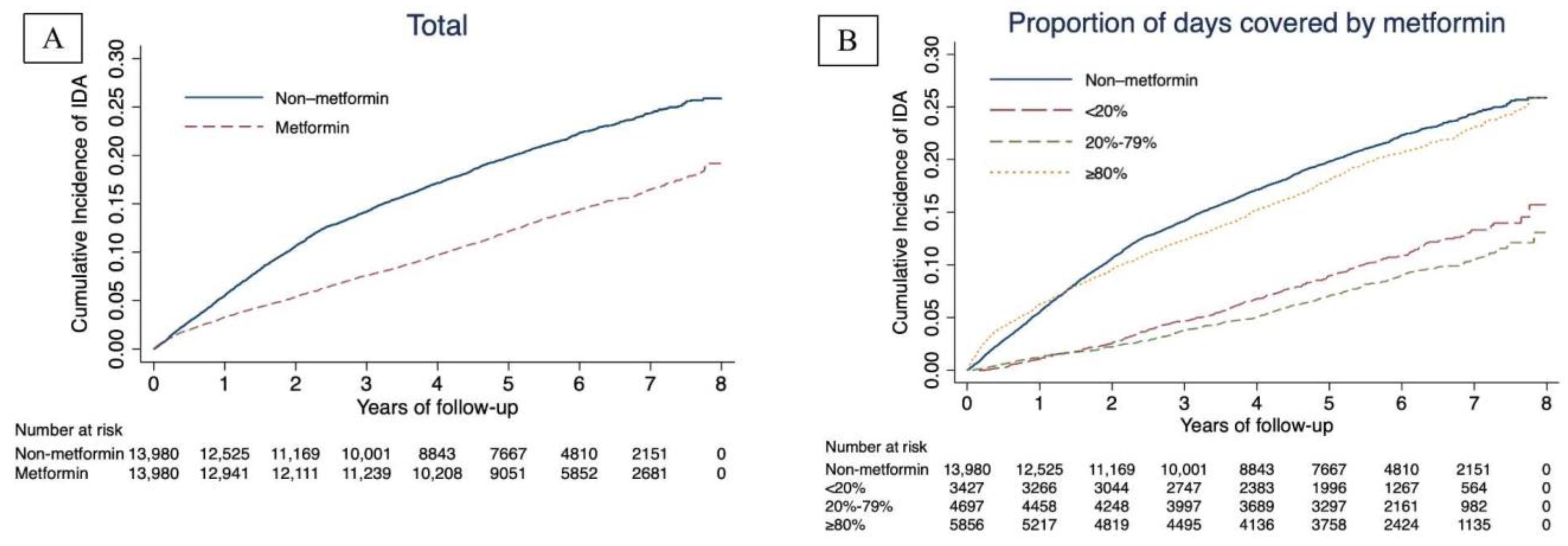
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Lachin, J.M.; Balasubramanyam, A.; Burch, H.B.; Buse, J.B.; Butera, N.M.; Cohen, R.M.; Crandall, J.P.; Kahn, S.E.; Krause-Steinrauf, H.; et al. Glycemia reduction in type 2 diabetes—Glycemic outcomes. N. Engl. J. Med. 2022, 387, 1063–1074. [Google Scholar] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Manco, M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. 2014, 2, 513–526. [Google Scholar] [CrossRef]
- AlDallal, S.M.; Jena, N. Prevalence of anemia in type 2 diabetic patients. J. Hematol. 2018, 7, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Arani, R.H.; Fakhri, F.; Tabiee, M.N.; Talebi, F.; Talebi, Z.; Rashidi, N.; Zahedi, M. Prevalence of anemia and its associated factors among patients with type 2 diabetes mellitus in a referral diabetic clinic in the north of iran. BMC Endocr. Disord. 2023, 23, 58. [Google Scholar]
- Farooqi, M.; Tahir, Y.; Rehan, B. Anemia in patients with type 2 diabetes: A common but neglected clinical finding. Acta Bio Med. 2022, 93, e2022044. [Google Scholar]
- Guo, W.; Zhou, Q.; Jia, Y.; Xu, J. Increased levels of glycated hemoglobin a1c and iron deficiency anemia: A review. Med. Sci. Monit. 2019, 25, 8371–8378. [Google Scholar] [CrossRef]
- Davis, R.E.; McCann, V.J.; Nicol, D.J. Influence of iron-deficiency anaemia on the glycosylated haemoglobin level in a patient with diabetes mellitus. Med. J. Aust. 1983, 1, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Soliman, N. Iron deficiency anemia and glucose metabolism. Acta Bio Med. 2017, 88, 112–118. [Google Scholar]
- Doyle-Delgado, K.; Chamberlain, J.J.; Shubrook, J.H.; Skolnik, N.; Trujillo, J. Pharmacologic approaches to glycemic treatment of type 2 diabetes: Synopsis of the 2020 american diabetes association’s standards of medical care in diabetes clinical guideline. Ann. Intern. Med. 2020, 173, 813–821. [Google Scholar] [CrossRef]
- Lipska, K.J.; Yao, X.; Herrin, J.; McCoy, R.G.; Ross, J.S.; Steinman, M.A.; Inzucchi, S.E.; Gill, T.M.; Krumholz, H.M.; Shah, N.D. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2017, 40, 468–475. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Cheen, M.H.H.; Goh, S.Y.; Bee, Y.M.; Lim, P.S.; Khee, G.Y.; Thumboo, J. Trends in medication utilization, glycemic control and outcomes among type 2 diabetes patients in a tertiary referral center in Singapore from 2007 to 2017. J. Diabetes 2019, 11, 573–581. [Google Scholar] [CrossRef]
- Yang, W.; Cai, X.; Wu, H.; Ji, L. Associations between metformin use and vitamin b12 levels, anemia, and neuropathy in patients with diabetes: A meta-analysis. J. Diabetes 2019, 11, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, O.R.; Mani, H.; Morgan, C.; Gill, G.V. Metformin and anaemia: Myth or reality? Pract. Diabetes Int. 2009, 26, 265–266. [Google Scholar] [CrossRef]
- Donnelly, L.A.; Dennis, J.M.; Coleman, R.L.; Sattar, N.; Hattersley, A.T.; Holman, R.R.; Pearson, E.R. Risk of anemia with metformin use in type 2 diabetes: A MASTERMIND study. Diabetes Care 2020, 43, 2493–2499. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Tang, W.; Deater, M.; Phan, N.; Marcogliese, A.N.; Li, H.; Al-Dhalimy, M.; Major, A.; Olson, S.; Monnat, R.J., Jr.; et al. Metformin improves defective hematopoiesis and delays tumor formation in fanconi anemia mice. Blood J. Am. Soc. Hematol. 2016, 128, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Xie, S.; Wang, J.; Li, Z.; Chen, L.; Mao, M.; Chen, C.; Huang, A.; Chen, Y.; et al. Metformin induces ferroptosis by inhibiting ufmylation of slc7a11 in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 206. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wu, J.; Wang, M.; Wang, X.; Wang, J.; Wu, T.; Wu, Y.; Hu, Y. Trends in prevalence and incidence of type 2 diabetes among adults in Beijing, China, from 2008 to 2017. Diabet. Med. 2021, 38, e14487. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Y.; Tian, Y.; Wu, Y.; Wang, M.; Wang, X.; Wang, Z.; Hu, Y. Association between ambient fine particulate matter and adult hospital admissions for pneumonia in Beijing, China. Atmos. Environ. 2020, 231, 117497. [Google Scholar] [CrossRef]
- Yang, R.; Yu, H.; Wu, J.; Chen, H.; Wang, M.; Wang, S.; Qin, X.; Wu, T.; Wu, Y.; Hu, Y. Metformin treatment and risk of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus in Beijing, China. Front. Endocrinol. 2023, 14, 1082720. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Wu, Y.; Wang, Z.J.; Wu, Y.Q.; Wu, T.; Wang, M.Y.; Wang, X.W.; Wang, S.Y.; Wang, J.T.; Yu, H.; et al. Healthcare costs associated with complications in patients with type 2 diabetes among 1.85 million adults in Beijing, China. Int. J. Environ. Res. Public Health 2021, 18, 3693. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Cunha, A.D., Jr.; Bragagnoli, A.C.; Costa, F.O.; Carvalheira, J.B.C. Repurposing metformin for the treatment of gastrointestinal cancer. World J. Gastroenterol. 2021, 27, 1883–1904. [Google Scholar] [CrossRef] [PubMed]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in human obesity and weight loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Liu, W.X.; Lu, K.; Pan, H.; Wang, T.; Yi, D.; Huang, J.; Zhao, L.; Ning, G.; et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 2020, 79, 635–645. [Google Scholar] [CrossRef]
- Yu, H.; Yang, R.; Wu, J.; Wang, S.; Qin, X.; Wu, T.; Hu, Y.; Wu, Y. Association of metformin and depression in patients with type 2 diabetes. J. Affect. Disord. 2022, 318, 380–385. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Mulherin, A.J.; Oh, A.H.; Kim, H.; Grieco, A.; Lauffer, L.M.; Brubaker, P.L. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology 2011, 152, 4610–4619. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Skuli, S.J.; Alomari, S.; Gaitsch, H.; Bakayoko, A.I.; Skuli, N.; Tyler, B.M. Metformin and cancer, an ambiguanidous relationship. Pharmaceuticals 2022, 15, 626. [Google Scholar] [CrossRef]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Kooy, A.; de Jager, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.; Stehouwer, C.D. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med. 2009, 169, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Pan, J.; Li, J.; Zeng, C.; Qi, W.; Shao, Y.; Liu, X.; Liu, L.; Xiao, G.; Zhang, H.; et al. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging 2020, 12, 1087. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Terkeltaub, R.; Lin, H.; Zhang, Y.; Zhou, B.; He, K.; Li, K.; Liu, Z.; Wei, J.; et al. Exploration of metformin as novel therapy for osteoarthritis: Preventing cartilage degeneration and reducing pain behavior. Arthritis Res. Ther. 2020, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Chen, H.; Yang, R.; Yu, H.; Wu, Y.; Hu, Y. Type 2 diabetes risk and lipid metabolism related to the pleiotropic effects of an abcb1 variant: A chinese family-based cohort study. Metabolites 2022, 12, 875. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Song, Y.; Chui, D. Aging Beijing: Challenges and strategies of health care for the elderly. Ageing Res. Rev. 2010, 9 (Suppl. 1), s2–s5. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]

| Variable | Metformin (N = 13,980) | Non-Metformin (N = 13,980) | SMD *, % | p | |
|---|---|---|---|---|---|
| Age, year | Unmatched | 58.41 | 64.71 | −49.3 | <0.001 |
| Matched | 64.73 | 64.02 | 5.6 | <0.001 | |
| Female, % | Unmatched | 43.71 | 40.41 | 6.7 | <0.001 |
| Matched | 40.37 | 41.40 | −2.1 | 0.057 | |
| Date of diagnosis of T2DM | Unmatched | 2011-05 | 2011-05 | −0.1 | 0.940 |
| Matched | 2011-05 | 2011-05 | −0.1 | 0.920 | |
| Comorbidity index | Unmatched | 1.11 | 1.31 | −16.9 | <0.001 |
| Matched | 1.28 | 1.25 | 2.5 | 0.022 | |
| Number of visits/year | Unmatched | 14.37 | 16.27 | −7.2 | <0.001 |
| Matched | 15.96 | 16.14 | −0.7 | 0.540 | |
| Gastric acid inhibitor, % | Unmatched | 31.43 | 43.13 | −24.4 | <0.001 |
| Matched | 43.35 | 41.41 | 4.0 | <0.001 | |
| Vitamin C, % | Unmatched | 34.58 | 43.90 | −19.2 | <0.001 |
| Matched | 44.06 | 42.50 | 3.2 | 0.004 |
| Non-Metformin | Proportion of Days Covered by Metformin | p | |||
|---|---|---|---|---|---|
| <20% | 20–79% | ≥80% | |||
| N | 13,980 | 3427 | 4697 | 5856 | |
| Age, year | 63.36 (13.17) | 65.54 (12.33) | 62.59 (12.57) | 64.25 (11.30) | <0.001 |
| Female, % | 5673 (40.58) | 1358 (39.63) | 1785 (38.00) | 2570 (43.89) | <0.001 |
| Comorbidity index | 1.20 (1.19) | 1.24 (1.12) | 1.23 (1.10) | 1.29 (1.12) | <0.001 |
| Number of visits/year | 16.43 (27.25) | 14.33 (24.35) | 14.15 (25.05) | 16.25 (25.67) | <0.001 |
| Gastric acid inhibitor, % | 5369 (38.40) | 1452 (42.37) | 1797 (38.26) | 2563 (43.77) | <0.001 |
| Vitamin C, % | 5639 (40.34) | 1539 (44.91) | 1845 (39.28) | 2604 (44.47) | <0.001 |
| Subgroups | Cases * | Total * | Non-Metformin (Ref) | Metformin | p-Int | Proportion of Days Covered by Metformin | p-Int | |||
|---|---|---|---|---|---|---|---|---|---|---|
| <20% | 20–79% | ≥80% | ||||||||
| Age, year | <65 | 1932 | 14,244 | 1.00 | 0.43 (0.39, 0.48) | <0.001 | 0.34 (0.28, 0.41) | 0.24 (0.20, 0.29) | 0.64 (0.57, 0.72) | <0.001 |
| ≥65 | 2696 | 13,716 | 1.00 | 0.73 (0.67, 0.78) | 0.47 (0.41, 0.54) | 0.48 (0.42, 0.55) | 1.12 (1.03, 1.23) | |||
| Sex | male | 2447 | 16,574 | 1.00 | 0.60 (0.55, 0.65) | 0.927 | 0.46 (0.39, 0.53) | 0.39 (0.34, 0.44) | 0.88 (0.80, 0.97) | 0.266 |
| female | 2181 | 11,386 | 1.00 | 0.62 (0.57, 0.67) | 0.39 (0.33, 0.47) | 0.36 (0.30, 0.42) | 0.94 (0.85, 1.03) | |||
| CI-index | 0 | 1270 | 8046 | 1.00 | 0.70 (0.62, 0.78) | 0.003 | 0.55 (0.45, 0.68) | 0.42 (0.34, 0.52) | 1.03 (0.90, 1.18) | 0.005 |
| 1 | 1574 | 10,742 | 1.00 | 0.61 (0.55, 0.68) | 0.42 (0.35, 0.52) | 0.35 (0.29, 0.42) | 0.95 (0.85, 1.07) | |||
| ≥2 | 1784 | 9172 | 1.00 | 0.55 (0.50, 0.60) | 0.36 (0.29, 0.43) | 0.37 (0.31, 0.44) | 0.80 (0.71, 0.89) | |||
| Number of visits/year | 0 | 1488 | 10,409 | 1.00 | 0.60 (0.54, 0.67) | 0.982 | 0.39 (0.32, 0.49) | 0.37 (0.31, 0.44) | 1.00 (0.88, 1.13) | 0.609 |
| 1–2 | 1017 | 5643 | 1.00 | 0.61 (0.54, 0.69) | 0.46 (0.37, 0.57) | 0.39 (0.31, 0.48) | 0.87 (0.76, 1.01) | |||
| >2 | 2123 | 11,908 | 1.00 | 0.60 (0.55, 0.66) | 0.43 (0.36, 0.51) | 0.37 (0.31, 0.44) | 0.87 (0.78, 0.97) | |||
| Gastric acid inhibitor | not use | 2374 | 16,779 | 1.00 | 0.65 (0.60, 0.71) | 0.005 | 0.46 (0.39, 0.54) | 0.42 (0.36, 0.48) | 0.98 (0.89, 1.09) | 0.013 |
| use | 2254 | 11,181 | 1.00 | 0.57 (0.52, 0.62) | 0.40 (0.34, 0.48) | 0.33 (0.28, 0.39) | 0.84 (0.76, 0.93) | |||
| Vitamin C | not use | 2465 | 16,333 | 1.00 | 0.58 (0.53, 0.63) | 0.213 | 0.45 (0.38, 0.53) | 0.35 (0.30, 0.40) | 0.87 (0.79, 0.95) | 0.186 |
| use | 2163 | 11,627 | 1.00 | 0.64 (0.59, 0.70) | 0.41 (0.35, 0.49) | 0.41 (0.35, 0.48) | 0.96 (0.87, 1.06) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Yang, R.; Yu, H.; Qin, X.; Wu, T.; Wu, Y.; Hu, Y. Association of Metformin Use with Iron Deficiency Anemia in Urban Chinese Patients with Type 2 Diabetes. Nutrients 2023, 15, 3081. https://doi.org/10.3390/nu15143081
Wu J, Yang R, Yu H, Qin X, Wu T, Wu Y, Hu Y. Association of Metformin Use with Iron Deficiency Anemia in Urban Chinese Patients with Type 2 Diabetes. Nutrients. 2023; 15(14):3081. https://doi.org/10.3390/nu15143081
Chicago/Turabian StyleWu, Junhui, Ruotong Yang, Huan Yu, Xueying Qin, Tao Wu, Yiqun Wu, and Yonghua Hu. 2023. "Association of Metformin Use with Iron Deficiency Anemia in Urban Chinese Patients with Type 2 Diabetes" Nutrients 15, no. 14: 3081. https://doi.org/10.3390/nu15143081
APA StyleWu, J., Yang, R., Yu, H., Qin, X., Wu, T., Wu, Y., & Hu, Y. (2023). Association of Metformin Use with Iron Deficiency Anemia in Urban Chinese Patients with Type 2 Diabetes. Nutrients, 15(14), 3081. https://doi.org/10.3390/nu15143081







