Dietary Strategies for Complementary Feeding between 6 and 24 Months of Age: The Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Included Topics
2.2. Methods for New Reviews and Review Updates
3. Results
3.1. Breastmilk Feeding during the CF Period
3.2. Animal Milk and Infant Formula Feeding
3.2.1. Animal Milk
3.2.2. Infant Formula
3.3. Frequency, Types, and Amount of Home Available Complementary Foods
3.3.1. Fruit and Vegetables (FV)
3.3.2. Nuts, Pulses, and Seeds (NPS)
3.3.3. Animal-Sourced Foods (ASF)
Eggs
Red Meats, Chicken, Fish, and Insect-Based Food Consumption
Other ASF Products
3.4. Provision of Complementary Food Interventions
3.4.1. Provision of Fortified Blended Foods, Locally and Commercially Produced Ready-to-Use Supplementary Foods, and Alternative Foods
3.4.2. Lipid-Based Nutrient Supplementation
3.4.3. Provision of Complementary Food Interventions by Food Security Status
3.4.4. Micronutrient Powders
3.5. Education to Promote Complementary Feeding
3.5.1. Complementary Feeding Education Interventions
3.5.2. Complementary Feeding Education Interventions by Food Security Status
3.5.3. Complementary Feeding Education Interventions in Undernourished Children
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewey, K.; Brown, K. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr. Bull. 2003, 24, 5–28. [Google Scholar] [CrossRef]
- UNICEF. Improving Young Children’s Diets during the Complementary Feeding Period; UNICEF Programming Guidance: New York, NY, USA, 2020. [Google Scholar]
- WHO. Complementary Feeding. Available online: https://www.who.int/health-topics/complementary-feeding#tab=tab_1 (accessed on 3 May 2022).
- Issaka, A.; Agho, K.; Page, A.; Burns, P.; Stevens, G.; Dibley, M. The problem of suboptimal complementary feeding practices in West Africa: What is the way forward? Matern. Child Nutr. 2015, 11 (Suppl. S1), 53–60. [Google Scholar] [CrossRef]
- Lartey, A. Matern and child nutrition in Sub-Saharan Africa: Challenges and interventions. Proc. Nutr. Soc. 2008, 67, 105–108. [Google Scholar] [CrossRef]
- Joshi, N.; Agho, K.; Dibley, M.; Senarath, U.; Tiwari, K. Determinants of inappropriate complementary feeding practices in young children in Nepal: Secondary data analysis of Demographic and Health Survey 2006. Matern. Child Nutr. 2012, 8, 45–59. [Google Scholar] [CrossRef]
- Kabir, I.; Khanam, M.; Agho, K.; Mihrshahi, S.; Dibley, M.; Roy, S. Determinants of inappropriate complementary feeding practices in infant and young children in Bangladesh: Secondary data analysis of Demographic Health Survey 2007. Matern. Child Nutr. 2012, 8, 11–27. [Google Scholar] [CrossRef]
- Menon, P. The crisis of poor complementary feeding in South Asia: Where next? Matern. Child Nutr. 2012, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- WHO. Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- WHO. Guiding Principles for Feeding Non-Breastfed Children 6-24 Months of Age; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- PAHO. Guiding Principles for Complementary Feeding of the Breastfed Child; PAHO: Washington, DC, USA, 2003. [Google Scholar]
- WHO. Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 3 May 2022).
- Carducci, B.; Keats, E.; Ruel, M.; Haddad, L.; Osendarp, S.; Bhutta, Z. Food systems, diets and nutrition in the wake of COVID-19. Nat. Food 2021, 2, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.; Catania, J.; Zaman, M.; Smith, E.; Smith, A.; Tsistinas, O.; Bhutta, Z.; Imdad, A. The Effect of Consumption of Animal Milk Compared to Infant Formula for Non-Breastfed/Mixed-Fed Infants 6–11 Months of Age: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Salam, R.; Hadi, Y.; Sadiq Sheikh, S.; Bhutta, A.; Weise Prinzo, Z.; Bhutta, Z. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst. Rev. 2019, 5, CD012611. [Google Scholar]
- Zohra, L.; Rind, F.; Irfan, O.; Hadi, R.; Das, J.; Bhutta, Z. Impact of Infant and Young Child Feeding (IYCF) Nutrition Interventions on Breastfeeding Practices, Growth and Mortality in Low- and Middle-Income Countries: Systematic Review. Nutrients 2020, 12, 722. [Google Scholar] [CrossRef]
- Salam, R.; MacPhail, C.; Das, J.; Bhutta, Z. Effectiveness of Micronutrient Powders (MNP) in women and children. BMC Public Health 2013, 13, 22. [Google Scholar] [CrossRef]
- Victora, C.; Bahl, R.; Barros, A.; França, G.; Horton, S.; Krasevec, J.; Murch, S.; Jeeva Sankar, M.; Walker, N.; Rollins, N.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Csölle, I.; Felső, R.; Szabó, É.; Metzendorf, M.; Schwingshackl, L.; Ferenci, T.; Lohner, S. Health outcomes associated with micronutrient-fortified complementary foods in infants and young children aged 6–23 months: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2022, 6, 533–544. [Google Scholar] [CrossRef]
- Kramer, M.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 8, CD003517. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escamilla, R.; Tomori, C.; Hernández-Cordero, S.; Baker, P.; Barros, A.; Bégin, F.; Chapman, D.; Grummer-Strawn, L.; McCoy, D.; Menon, P.; et al. Breastfeeding: Crucially important, but increasingly challenged in a market-driven world. Lancet 2023, 401, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Odom, E.; Li, R.; Scanlon, K.; Perrine, C.; Grummer-Strawn, L. Reasons for Earlier Than Desired Cessation of Breastfeeding. Pediatrics 2013, 131, e726–e732. [Google Scholar] [CrossRef]
- Dewey, K.; Cohen, R.; Rollins, N.; Informal Working Group on Feeding of Nonbreastfed Children. WHO technical background paper: Feeding of nonbreastfed children from 6 to 24 months of age in developing countries. Food Nutr. Bull. 2004, 25, 377–402. [Google Scholar] [CrossRef]
- Fomon, S.; Ziegler, E.; Nelson, S.; Edwards, B. Cow milk feeding in infancy: Gastrointestinal blood loss and iron nutritional status. J. Pediatrics 1981, 98, 540–545. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Thorisdottir, A. Whole cow’s milk in early life. Milk Milk Prod. Hum. Nutr. 2011, 67, 29–40. [Google Scholar]
- Ahern, G.; Hennessy, A.; Ryan, C.; Ross, R.; Stanton, C. Advances in Infant Formula Science. Annu. Rev. Food Sci. Technol. 2019, 10, 75–102. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. Available online: http://www.DietaryGuidelines.gov (accessed on 30 May 2023).
- WHO. International Code of Marketing of Breast-Milk Substitutes; World Health Organization: Geneva, Switzerland, 1981. [Google Scholar]
- Nebeling, L. Phytochemicals: The color of a healthy diet. J. Ped. Nutr. Dev. 2001, 98, 2–9. [Google Scholar]
- McGuire, S.U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 292–294. [Google Scholar] [CrossRef] [PubMed]
- WHO. Complementary Feeding: Family Foods for Breastfed Children, France; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- WHO. Anaemia. Available online: https://www.who.int/health-topics/anaemia#tab=tab_1 (accessed on 30 May 2023).
- WHO. Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Lutter, C.; Grummer-Strawn, L.; Rogers, L. Complementary feeding of infants and young children 6 to 23 months of age. Nutr. Rev. 2021, 79, 825–846. [Google Scholar] [CrossRef] [PubMed]
- Togias, A.; Cooper, S.; Acebal, M.; Assa’ad, A.; Baker, J.; Beck, L.; Block, J.; Byrd-Bredbenner, C.; Chan, E.; Eichenfield, L.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. Allergy Clin. Immunol. 2017, 139, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Allen, L. Nutritional importance of animal source foods. J. Nutr. 2003, 133, 3932S–3935S. [Google Scholar] [CrossRef]
- Eaton, J.; Rothpletz-Puglia, P.; Dreker, M.; Iannotti, L.; Lutter, C.; Kaganda, J.; Rayco-Solon, P. Effectiveness of provision of animal-source foods for supporting optimal growth and development in children 6 to 59 months of age. Cochrane Database Syst. Rev. 2019, 2, CD012818. [Google Scholar] [CrossRef]
- Dror, D.; Allen, L. The importance of milk and other animal-source foods for children in low-income countries. Food Nutr. Bull. 2011, 32, 227–243. [Google Scholar] [CrossRef]
- Iannotti, L.; Lutter, C.; Bunn, D.; Stewart, C. Eggs: The uncracked potential for improving maternal and young child nutrition among the world’s poor. Nutr. Rev. 2014, 72, 355–368. [Google Scholar] [CrossRef]
- WHO. WHO Child Growth Standards. Available online: https://www.who.int/publications/i/item/9789241547635 (accessed on 3 May 2022).
- Krasevec, J.; An, X.; Kumapley, R.; Begin, F.; Frongillo, E. Diet quality and risk of stunting among infants and young children in low- and middle-income countries. Matern. Child Nutr. 2017, 13, 10. [Google Scholar] [CrossRef]
- Headey, D.; Hirvonen, K.; Hoddinott, J. Animal Sourced Foods and Child Stunting. Am. J. Agric. Econ. 2018, 100, 1302–1319. [Google Scholar] [CrossRef]
- Tam, E.; Keats, E.; Rind, F.; Das, J.; Bhutta, Z. Micronutrient Supplementation and Fortification Interventions on Health and Development Outcomes among Children Under-Five in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef]
- Stewart, C.; Wessells, K.; Arnold, C.; Huybregts, L.; Ashorn, P.; Becquey, E.; Humphrey, J.; Dewey, K. Lipid-based nutrient supplements and all-cause mortality in children 6–24 months of age: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Pena-Rosas, J.; Boy-Mena, E.; Sachdev, H. Lipid based nutrient supplements (LNS) for treatment of children (6 months to 59 months) with moderate acute malnutrition (MAM): A systematic review. PLoS ONE 2017, 12, e0182096. [Google Scholar] [CrossRef]
- Chaparro, C.; Dewey, K. Use of lipid-based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub-groups in emergency settings. Matern. Child Nutr. 2009, 6, 1–69. [Google Scholar] [CrossRef]
- WHO. WHO Guideline: Use of Multiple Micronutrient Powders for Point-of-Use Fortification of Foods Consumed by Infants and Young Children Aged 6–23 Months and Children Aged 2–12 Years; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lassi, Z.; Das, J.; Zahid, G.; Imdad, A.; Bhutta, Z. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: A systematic review. BMC Public Health 2013, 13, S13. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Seyyedi, N.; Rahimi, B.; Eslamlou, H.; Afshar, H.; Spreco, A.; Timpka, T. Smartphone-based maternal education for the complementary feeding of undernourished children under 3 years of age in food-secure communities: Randomised controlled trial in Urmia, Iran. Nutrients 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- McFadden, A.; Mason, F.; Baker, J.; Begin, F.; Dykes, F.; Grummer-Strawn, L.; Kenney-Muir, N.; Whitford, H.; Zehner, E.; Renfrew, M. Spotlight on infant formula: Coordinated global action needed. Lancet 2016, 387, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Shea, S.; Basch, C.; Contento, I.; Zybert, P. Variability and tracking of nutrient intakes of preschool children based on multiple administrations of the 24-hour dietary recall. Am. J. Epidemiol. 1991, 134, 1427–1437. [Google Scholar] [CrossRef]
- Singer, M.; Moore, L.; Garrahie, E.; Ellison, R. The tracking of nutrient intake in young children: The Framingham Children’s Study. Am. J. Public Health 1995, 85, 1673–1677. [Google Scholar] [CrossRef]
- Robinson, S.; Marriott, L.; Poole, J.; Crozier, S.; Borland, S.; Lawrence, W.; Law, C.; Godfrey, K.; Cooper, C.; Inskip, H.; et al. Dietary patterns in infancy: The importance of maternal and family influences on feeding practice. Br. J. Nutr. 2007, 98, 1029–1037. [Google Scholar] [CrossRef]
- Madruga, S.; Araújo, C.; Bertoldi, A.; Neutzling, M. Tracking of dietary patterns from childhood to adolescence. Rev. Saude Publica 2012, 46, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Boulton, T.; Magarey, A.; Cockington, R. Tracking of serum lipids and dietary energy, fat and calcium intake from 1 to 15 years. Acta Paediatr. 1995, 84, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Latham, P. Edible Caterpilars and Their Food Plants in Bas-Congo Province, Democratic Republic of Congo, 2nd ed.; Department for International Development: London, UK, 2005. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Owino, V.; Kumwenda, C.; Ekesa, B.; Parker, M.; Ewoldt, L.; Roos, N.; Lee, W.; Tome, D. The impact of climate change on food systems, diet quality, nutrition, and health outcomes: A narrative review. Front. Clim. 2022, 4, 941842. [Google Scholar] [CrossRef]
- Padhani, Z.; Das, J.; Siddiqui, F.; Salam, R.; Lassi, Z.; Khan, D.; Abbasi, A.; Keats, E.; Soofi, S.; Black, R.; et al. Optimal timing of introduction of complementary feeding: A systematic review and meta-analysis. Nutr. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Keats, E.; Das, J.; Salam, R.; Lassi, Z.; Imdad, A.; Black, R.; Bhutta, Z. Effective interventions to address maternal and child malnutrition: An update of the evidence. Lancet Child Adolesc. Health 2021, 5, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Yakoob, M.; Bhutta, Z. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 2011, 11, S25. [Google Scholar] [CrossRef]





| Exposure/ Intervention | Location | Evidence Reviewed | Effect |
|---|---|---|---|
| Cow’s Milk vs. Infant Formula | United Kingdom; Iceland; United States (n = 2) | A Meta-Analysis of Two Randomized Controlled Trials (RCTs) and Two Cohort Studies. | Anemia [Cohort studies: Relative Risk (RR) = 2.26, 95% Confidence Interval (CI) 1.15–4.43, two studies, p = 0.02, I2 = 0%, Grade certainty: Low; RCTs: RR = 4.03, 95% CI 1.68–9.65, two studies, p = 0.002, I2 = 0%, Grade certainty: Low] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect/Association |
|---|---|---|---|
| Less Frequent vs. More Frequent Fruit and Vegetables (FV) | Norway and Nepal | Two Cohort Studies Ranging from 231–9490 Participants | Stability and change [Overall fruit consumption at 18 months was positively associated with overall fruit consumption at 36 months (Spearman’s rho = 0.36) and at 7 years of age (Spearman’s rho = 0.23), GRADE certainty = very low] |
| Stability and tracking [Moderate stability for the frequency of consumption of yellow fruits and vegetables and dark green leafy vegetable consumption using Generalized Estimating Equation (GEE) models (stability coefficient = 0.26, 95% CI: 0.18–0.35), GRADE certainty = very low] | |||
| Less Varied vs. More Varied FV | Germany and France | One Quasi-Experimental Study and its Associated Report with a Total of 254 Participants | Intake of new foods [The high vegetable variety produced the greatest increase in intake of new foods (p < 0.0001), GRADE certainty = very low] |
| Mean number of vegetables eaten [At follow up three (~67 months), children who had experienced a high variety of vegetables at weaning ate more of the new vegetables and familiar vegetables than those who had experienced low or no variety (14.1 g ± 1.5 vs. 4.3 g ± 1.5 and 3.2 g ± 1.4, p < 0.0001 for new vegetables, respectively; and 9.6 g ± 2.0 vs. 13.1 g ± 2.0 and 13.1 g ± 1.9, p = 0.03 for familiar vegetables, respectively, GRADE certainty = very low] | |||
| Australia | One Cohort Study with 333 Participants | Vegetable and fruit intake [A greater variety of vegetables tried at age 14 months was significantly associated with a higher fruit and vegetable intake score at age 3.7 years (Reg coefficient = 0.12, p = 0.05), GRADE certainty = very low] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect |
|---|---|---|---|
| Greater Amount vs. Lesser Amount of Nuts, Pulses, and Seeds (NPS) | Nigeria | One Randomized Controlled Trial (RCT) with 90 Participants | Length [Increasing measurements for length were seen for those who consumed maize/cowpea compared to those who did not (p < 0.05 between groups), GRADE certainty = very low] Weight [Increasing measurements for weight were seen for those who consumed maize/cowpea compared to those who did not (p < 0.05 between groups), GRADE certainty = very low] |
| Ethiopia | One RCT with 197 Participants | Nutrient intake [improved nutrient intakes were observed for protein, carbohydrate, and iron intake for those consuming greater amounts of broad bean (p < 0.05 between groups), GRADE certainty = very low] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect/Association |
|---|---|---|---|
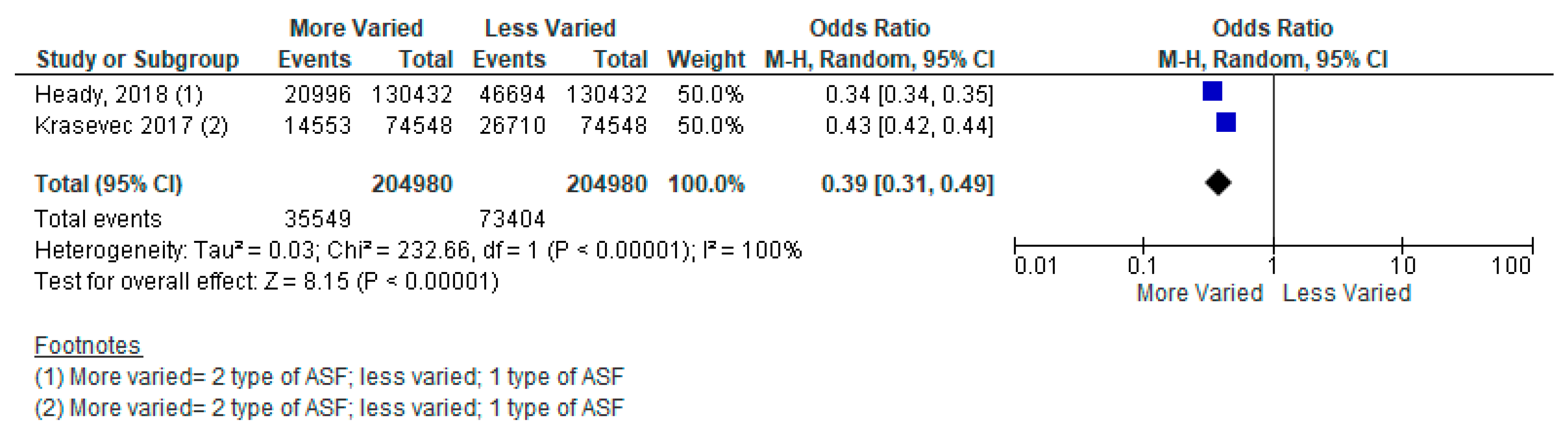
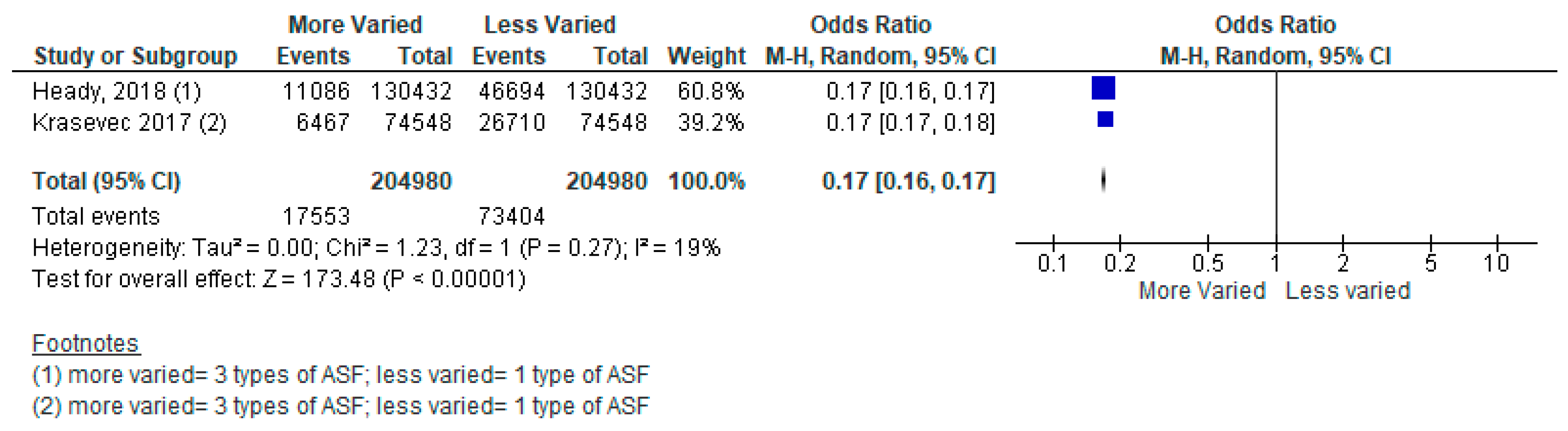
| More Varied vs. Less Varied Animal-sourced Foods (ASF) | 49 Low- and Middle-Income Countries from Across World Health Organization Regions | A Meta-Analysis of Two Cross-Sectional Studies | Three Types of ASF vs. Two Types Stunting [Odds Ratio (OR) = 0.44, 95% Confidence Interval (CI): 0.35–0.54, 409,960 participants, I2 = 99%, GRADE = very low] Two Types of ASF vs. One Type Stunting [OR = 0.39, 95% CI: 0.31–0.49, 409,960 participants, I2 = 100%, GRADE = very low] Three Types of ASF vs. One Type Stunting [OR = 0.17, 95% CI: 0.16–0.17, 409,960 participants, I2 = 19%, GRADE = very low] |
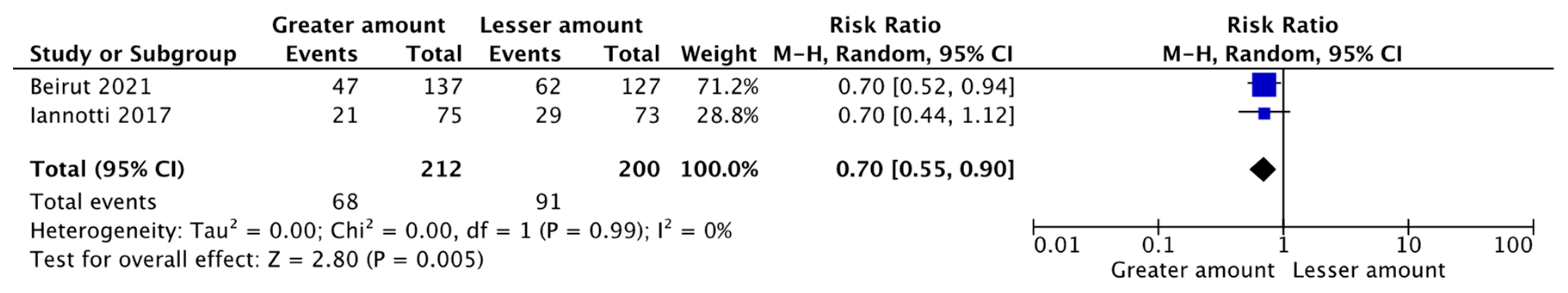
| Greater Amount vs. Lesser Amount of ASF | Malawi and Ecuador | A Meta-Analysis of Two Randomized Controlled Trials (RCTs) | Weight-for-age Z-score (WAZ) [Mean Difference (MD) = 0.15, 95% CI: 0.00–0.30, 743 participants, I2 = 0%, GRADE = moderate] Stunting [Relative Risk (RR) = 0.70, 95% CI: 0.55–0.90, 412 participants, I2 = 0%, GRADE = low] |
| Malawi (n = 2) and Ecuador | A Meta-Analysis of Three RCTs | Height-for-age Z-score (HAZ) [MD = 0.07, 95% CI: 0.07–0.20, 1017 participants, I2 = 0%, GRADE = low] Weight-for-height Z-score (WHZ) [MD = −0.09, 95% CI: 0.23–0.05, 1007 participants, I2 = 25%, GRADE = low] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect/Association |
|---|---|---|---|
| Provision of Complementary Foods vs. Control, SubGroups by Type of CF Provision | Malawi (n = 3), Ethiopia (n = 3), Niger (n = 2), India (n = 2), Zambia (n = 2), Ecuador (n = 2), DRC, Cambodia, Bangladesh, Ghana, Mali, Chad, Pakistan, Nigeria, South Africa, Vietnam, Guinea-Bissau, Brazil, Honduras, China | Updated Evidence from 14 RCTs, 11 cRCTs, and Three Non-RCTs | Height-for-age Z-score (HAZ) Fortified blended foods [Mean Difference (MD) = 0.25; 95% Confidence Interval (CI): 0.09–0.41, 5820 participants, I2 = 93%, GRADE = very low] Locally produced ready to use supplementary food [MD = 0.04; 95% CI: 0.02–0.06; 2906 participants; I2 = 0%, GRADE = low] |
| Weight-for-height Z-score (WHZ) Fortified blended foods [MD = 0.08; 95% CI: 0.01–0.15; 6966 participants, I2 = 56%, GRADE = very low] Locally produced ready to use supplementary food [MD = 0.02, 95% CI: 0.00–0.04, 2576 participants, I2 = 5%, GRADE = low] Commercially produced ready to use supplementary food [MD = 0.04, 95% CI: 0.01–0.07, 2581 participants, I2 = 0%, GRADE = low] | |||
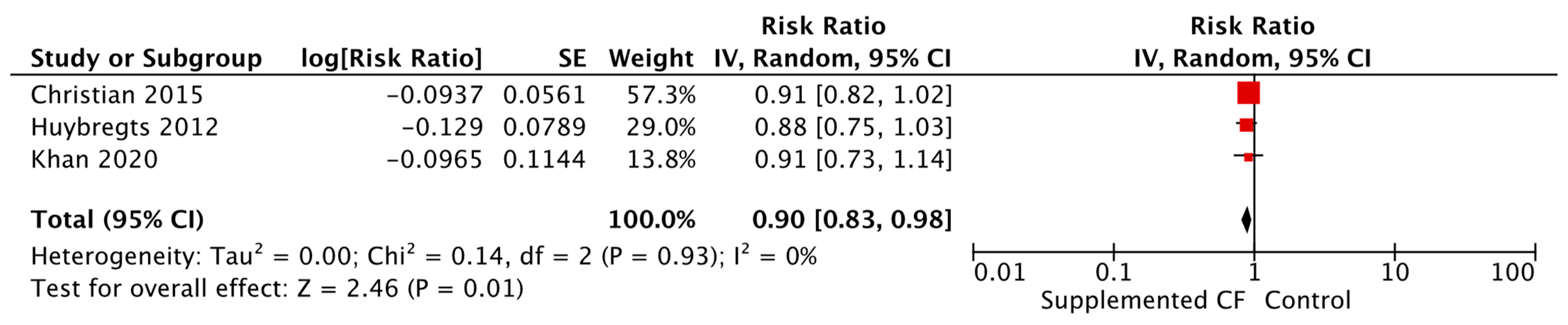
| Weight-for-age Z-score (WAZ) Fortified blended foods [MD = 0.16, 95% CI: 0.03–0.30, 5995 participants, I2 = 91%, GRADE = very low] Locally produced ready to use supplementary food [MD = 0.03, 95% CI: 0.01–0.05, 2576 participants, I2 = 0%, GRADE = low] Commercially produced ready to use supplementary food [Relative Risk (RR) = 0.90, 95% CI: 0.83–0.98, 2634 participants, I2 = 0%, GRADE = low] | |||
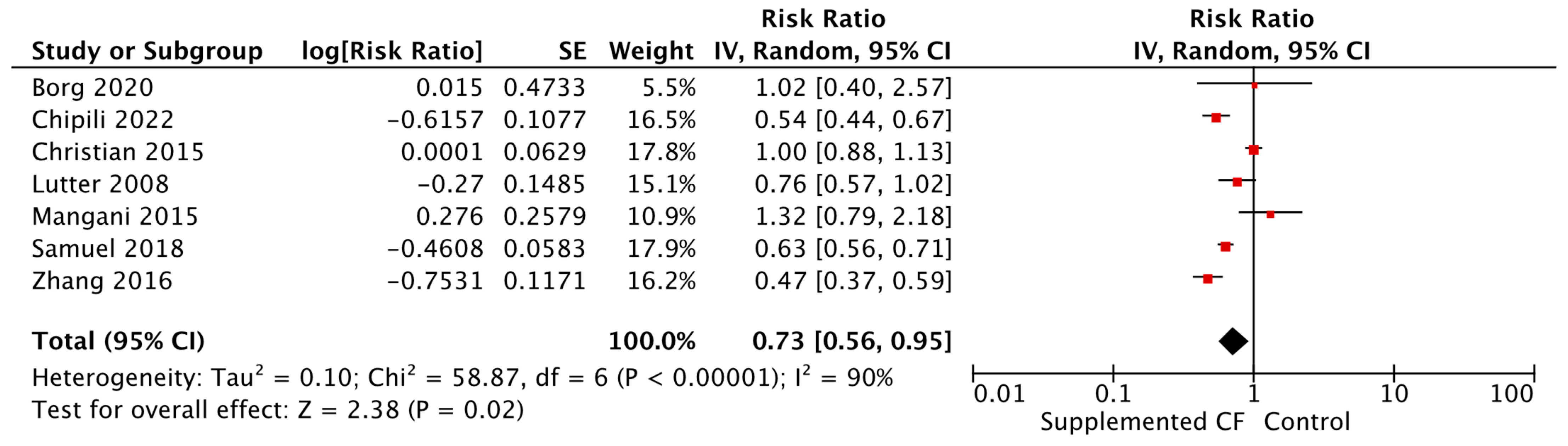
| Stunting Fortified blended foods [RR = 0.73, 95% CI: 0.56–0.95, 7358 participants, I2 = 90%, GRADE = very low] Commercially produced ready to use supplementary food [RR = 0.75, 95% CI: 0.61–0.92, 4762 participants, I2 = 52%, GRADE = very low] | |||
| Wasting Commercially produced ready to use supplementary food [RR = 0.75, 95% CI: 0.61–0.92, 4762 participants, I2 = 52%, GRADE = very low] | |||
| Change in Weight Locally produced ready to use supplementary food [MD = 0.03, 95% CI: 0.01–0.05, 2576 participants, I2 = 0%, GRADE = low] Commercially produced ready to use supplementary food [MD = 0.04, 95% CI: 0.01–0.07, 1911 participants, I2—0%, GRADE = very low] | |||
| Change in Height Locally produced ready to use supplementary food [MD = 0.08, 95% CI: 0.05–0.12, 2576 participants, I2 = 0%, GRADE = low] Commercially produced ready to use supplementary food [MD = 0.06, 95% CI: 0.00–0.11, 1911 participants, I2 = 0%, GRADE = low] | |||
| Mid-Upper Arm Circumference (MUAC) Commercially produced ready to use supplementary food [MD = 0.20, 95% CI: 0.02–0.38, 670 participants, GRADE = low] | |||
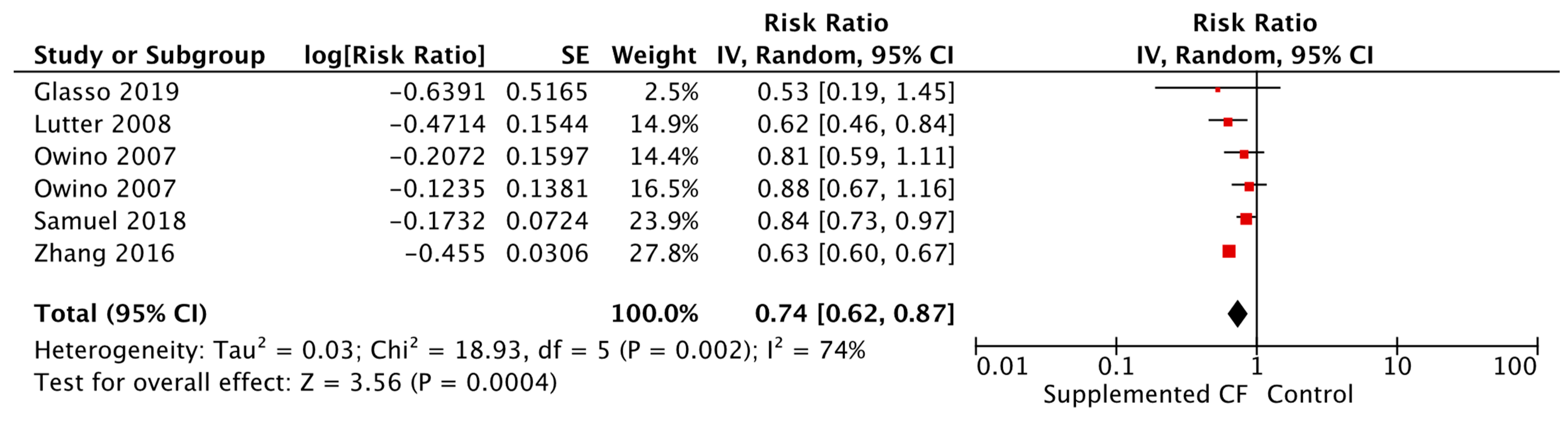
| Anemia Alternative food [RR = 0.52, 95% CI: 0.33–0.81, 62 participants, GRADE = very low] Fortified blended food [RR = 0.74, 95% CI: 0.62–0.87, 5511 participants I2 = 74%, GRADE = very low] | |||
| Hemoglobin Alternative food [Standardized Mean Difference (SMD) = 0.35, 95% CI: 0.02–0.69, 62 participants, GRADE = very low] Fortified blended food [SMD = 0.64, 95% CI: 0.29–1.00, 2727 participants, I2 = 61%, GRADE = very low] | |||
| Skin Conditions Alternative food [RR = 0.56, 95% CI: 0.32–0.98, 148 participants, GRADE = moderate] | |||
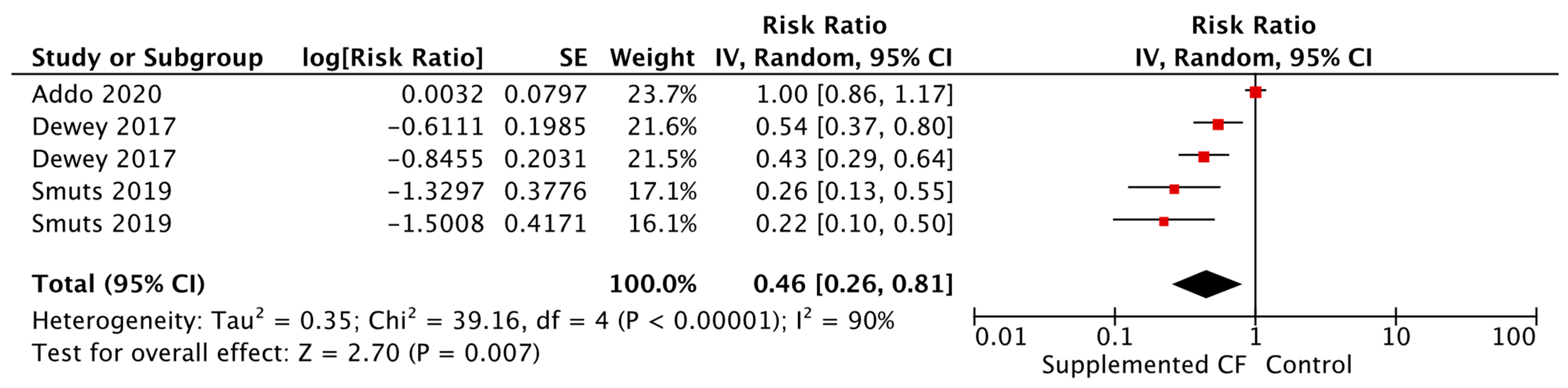
| Death Commercially produced ready to use supplementary food [RR = 0.43, 95% CI: 0.20–0.94, 7879 participants, GRADE = very low] | |||
| Provision of Complementary Foods vs. Control, SubGroups by Food Secure vs. Insecure Status | Malawi (n = 3), Ethiopia (n = 3), Niger (n = 2), India (n = 2), Zambia (n = 2), Ecuador (n = 2), DRC, Cambodia, Bangladesh, Ghana, Mali, Chad, Pakistan, Nigeria, South Africa, Vietnam, Guinea-Bissau, Brazil, Honduras, China | Updated Evidence from 14 RCTs, 11 cRCTs, and Three Non-RCTs | Stunting Food insecure [RR = 0.91, 95% CI: 0.83–1.00, 20 895 participants, I2 = 80%, GRADE = very low] Food secure [RR = 0.62, 95% CI: 0.44–0.88, 3363 participants, I2 = 83%, GRADE = very low] |
| Wasting Food insecure [RR = 0.87, 95% CI: 0.81–0.93, 27,987 participants, I2 = 12%, GRADE = very low] | |||
| HAZ Food insecure [MD = 0.18, 95% CI: 0.03–0.33, 20,287 participants, I2 = 100%, GRADE = very low] | |||
| WAZ Food insecure [MD = 0.09, 95% CI: 0.04–0.15, 22,239 participants, I2 = 93%, GRADE = very low] | |||
| Change in Height Food insecure [MD = 0.21, 95% CI: 0.07–0.35, 14,390 participants, I2 = 97%, GRADE = very low] | |||
| MUAC Food insecure [MD = 0.12, 95% CI: 0.05–0.18, 10,140 participants, I2 = 60%, GRADE = very low] | |||
| Hemoglobin Levels Food insecure [SMD = 0.59, 95% CI: 0.04–1.15, 7901 participants, I2 = 99%, GRADE = very low] Food secure [SMD = 0.49, 95% CI: 0.28–0.71, 863 participants, I2 = 0%, GRADE = low] | |||
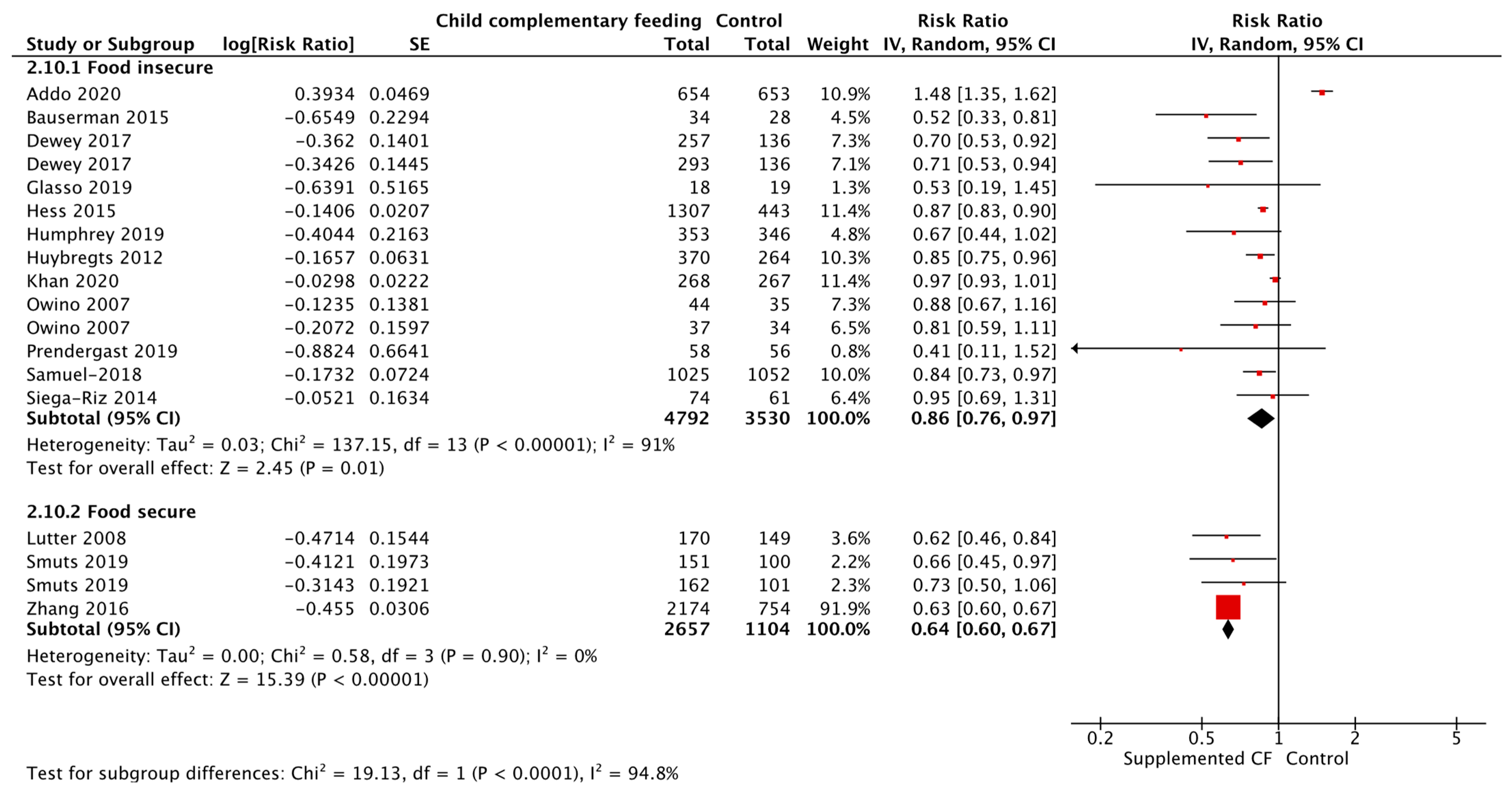
| Anemia Food insecure [RR = 0.86, 95% CI: 0.76–0.97, 8322 participants, I2 = 91%, GRADE = very low] Food secure [RR = 0.64, 95% CI: 0.60–0.67, 3761 participants, I2 = 0%, GRADE = very low] | |||
| Iron Deficiency Anemia Food secure [RR = 0.24, 95% CI: 0.14–0.42, 514 participants, GRADE = low] | |||
| Diarrhea Food secure [RR = 2.04, 95% CI: 1.07–3.87, 148 participants, GRADE = moderate] | |||
| Skin Conditions Food secure [RR = 0.56, 95% CI: 0.32–0.98, 148 participants, GRADE = moderate] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect/Association |
|---|---|---|---|
| Small-quantity lipid-based nutrient supplementation (SQ-LNS) vs. Control | Malawi (n = 3), Ghana (n = 2), Burkina Faso (n = 2), Bangladesh (n = 2), Republic of Congo, Madagascar, Zimbabwe, Haiti, Indonesia, Kenya, South Africa | Updated Evidence from Nine Cluster Randomized Controlled Trials (RCTs), Four RCTs, and Three Non-RCTs | Wasting [Relative Risk (RR): 0.90, 95% Confidence Interval (CI): 0.82–0.98, 16,976 participants, I2 = 0%, GRADE = very low] |
| Mid-upper Arm Circumference [Mean Difference (MD) = 0.10, 95% CI: 0.03–0.17, 9411 participants, I2 = 62% GRADE = very low] | |||
| Iron Deficiency Anemia [RR = 0.46, 95% CI: 0.26–0.81, 2643 participants, I2 = 90%, GRADE = very low] | |||
| Mean Diarrhea Episodes [MD = 0.05, 95% CI: 0.04–0.05, 2556 participants, GRADE = very low] | |||
| Upper Respiratory Tract Infection [RR = 0.87, 95% CI: 0.77–0.98, 2556 participants, GRADE = very low] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect |
|---|---|---|---|
| Micronutrient Powders (MNP) vs. No Intervention or Placebo | Bangladesh, Brazil, Burkina Faso, Cambodia, China, Colombia, Ethiopia, Ghana, Haiti, India, Indonesia, Kenya, Kyrgyzstan, Lao People’s Democratic Republic, Mali, Pakistan, Philippines, Uganda, Nepal | Updated Evidence from 19 Randomized Controlled Trials | Hemoglobin [Standardized Mean Difference (SMD) = 0.72, 95% Confidence Interval (CI): 0.22–1.22, 15 studies, 9089 participants, I2 = 99%, GRADE = low] |
| Weight-for-age Z-score [Mean Difference (MD) = 0.11, 95% CI: 0.02–0.20, 10 studies, 8253 participants, I2 = 86%, GRADE = low] | |||
| Weight-for-height Z-score [MD = 0.08, 95% CI: 0.03–0.14, 9 studies, 8065 participants, I2 = 67%, GRADE = low] |
| Exposure/ Intervention | Location | Evidence Reviewed | Effect |
|---|---|---|---|
| Complementary Feeding (CF) Education vs. Control, Healthy Children | India (n = 11), Bangladesh (n = 5), Ethiopia (n = 4), Indonesia (n = 3), Nepal (n = 3), Pakistan (n = 3), China (n = 2), Kenya (n = 2), Uganda (n = 2), Brazil (n = 2), Cambodia (n = 2), Malawi, Guatemala, Dominican Republic, Colombia, Peru, Iran, Somalia | Updated Evidence from 22 Cluster Randomized Controlled Trials (RCTs), 14 Non-RCTs, Nine RCTs, and One Quasi- RCT | Height-for-age Z-score (HAZ) [Mean Difference (MD) = 0.20, 95% Confidence Interval (CI): 0.12–0.28, 7457 participants, I2 = 85%, GRADE = very low] |
| Weight-for-age Z-score (WAZ) [MD = 0.18, 95% CI: 0.10–0.27, 5856 participants, I2 = 84%, GRADE = very low] | |||
| Weight-for-height Z-score (WHZ) [MD = 0.09, 95% CI: 0.01–0.17, 5260 participants, I2 = 84%, GRADE = very low] | |||
| Stunting [Relative Risk (RR) = 0.90, 95% CI: 0.84–0.96, 25,795 participants, I2 = 60%, GRADE = very low] | |||
| Underweight [RR = 0.87, 95% CI: 0.78–0.97, 23,176 participants, I2 = 73%, GRADE = very low] | |||
| Severe Underweight [RR = 0.39, 95% CI: 0.16–0.93, 816 participants, 1 study, GRADE = very low] | |||
| Severe Wasting [RR = 0.14, 95% CI: 0.03–0.74, 906 participants, I2 = 0%, GRADE = very low] | |||
| Change in Weight [MD =0.20, 95% CI: 0.07–0.34, 4176 participants, GRADE = very low] | |||
| Respiratory Illness [RR = 0.73, 95% CI: 0.60–0.90, 1588 participants, I2 = 17%, GRADE = very low] | |||
| Low Birth Weight [RR = 0.47, 95% CI: 0.26–0.85, 1049 participants, I2 = 0%, GRADE = very low] | |||
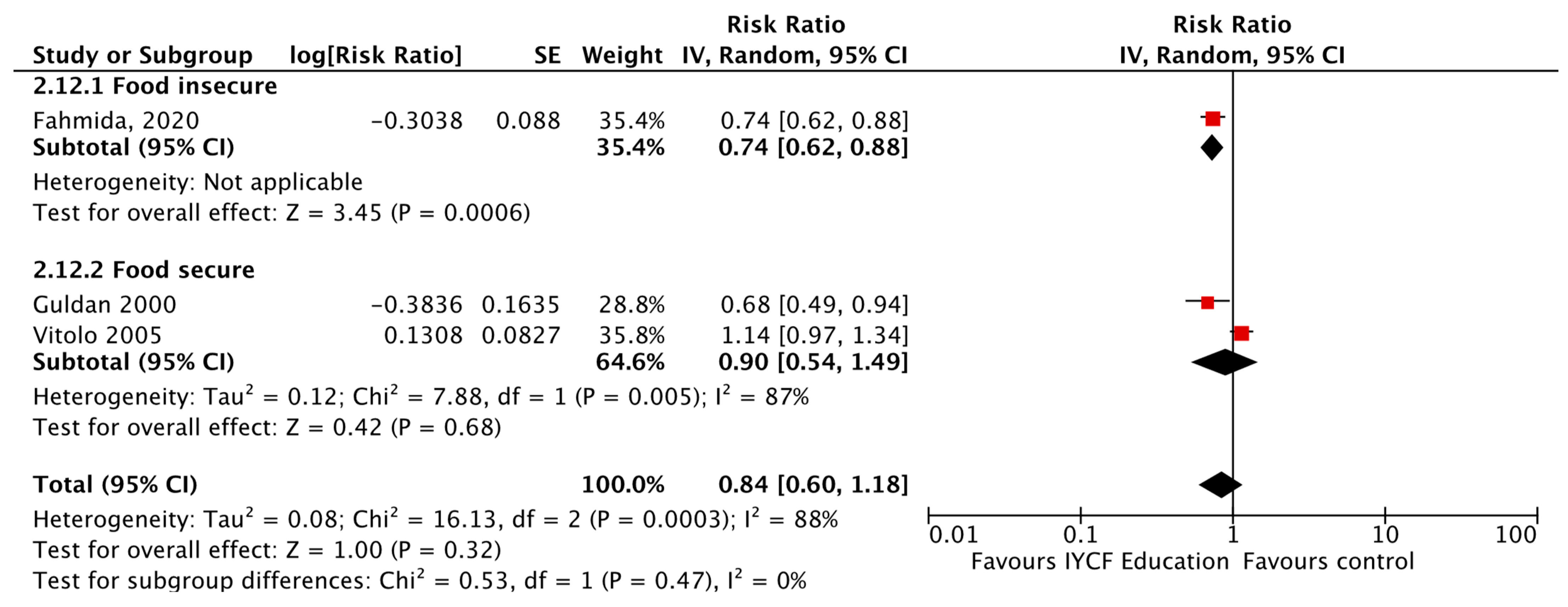
| CF Education vs. Control, Healthy Children, SubGroups by Food Secure vs. Insecure Status | India (n = 11), Bangladesh (n = 5), Ethiopia (n = 4), Indonesia (n = 3), Nepal (n = 3), Pakistan (n = 3), China (n = 2), Kenya (n = 2), Uganda (n = 2), Brazil (n = 2), Cambodia (n = 2), Malawi, Guatemala, Dominican Republic, Colombia, Peru, Iran, Somalia | Updated Evidence from 22 cRCTs, 14 Non-RCTs, Nine RCTs, and One Quasi- RCT | HAZ Food secure [MD = 0.39, 95% CI: 0.09–0.68, 828 participants, I2 = 79%, GRADE = very low] Food insecure [MD = 0.16, 95% CI: 0.08–0.24, 6629 participants, I2 = 84%, GRADE = very low] |
| WAZ Food secure [MD = 0.24, 95% CI: 0.09–0.38, 376 participants, I2 = 0%, GRADE = moderate] Food insecure [MD = 0.17, 95% CI: 0.09–0.26, 5480 participants, I2 = 86%, GRADE = very low] | |||
| Wasting Food secure [RR = 0.78, 95% CI: 0.62–0.98, 12,386 participants, I2 = 0%, GRADE = very low] | |||
| Stunting Food insecure [RR = 0.93, 95% CI: 0.89–0.98, 12,852 participants, I2 = 31%, GRADE = very low] | |||
| Underweight Food insecure [RR = 0.90, 95% CI: 0.81–1.00, 10,819 participants, I2 = 71%, GRADE = very low] Food secure [RR = 0.71, 95% CI: 0.53–0.95, 12,357 particpants, I2 = 30%, GRADE = very low] | |||
| Change in Weight Food insecure [MD = 0.20, 95% CI: 0.05–0.35, 3872 participants, I2 = 91%, GRADE = very low] | |||
| Change in Height Food secure [MD = 0.90, 95% CI: 0.26–1.55, 304 participants, I2 = 0%, GRADE = very low] | |||
| Anemia Food insecure [RR = 0.74, 95% CI: 0.62–0.88, 432 participants, GRADE = very low] | |||
| Diarrhea Food secure [RR = 0.67, 95% CI: 0.51–0.90, 397 participants, GRADE = very low] | |||
| Respiratory Illness Food secure [RR = 0.63, 95% CI: 0.46–0.85, 397 participants, GRADE = very low] | |||
| CF Education vs. Control, Undernourished Children | Iran | RCT | WHZ [MD = 0.34, 95% CI: 0.27–0.41, 100 participants, GRADE = very low] |
| WAZ [MD = 0.35, 95% CI: 0.29–0.41, 100 participants, GRADE = very low] | |||
| HAZ [RR = 0.35, 95% CI: 0.29–0.41, 100 participants, GRADE = very low] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, L.; Padhani, Z.; Salam, R.; Oh, C.; Rahim, K.; Maqsood, M.; Ali, A.; Charbonneau, K.; Keats, E.C.; Lassi, Z.S.; et al. Dietary Strategies for Complementary Feeding between 6 and 24 Months of Age: The Evidence. Nutrients 2023, 15, 3041. https://doi.org/10.3390/nu15133041
Harrison L, Padhani Z, Salam R, Oh C, Rahim K, Maqsood M, Ali A, Charbonneau K, Keats EC, Lassi ZS, et al. Dietary Strategies for Complementary Feeding between 6 and 24 Months of Age: The Evidence. Nutrients. 2023; 15(13):3041. https://doi.org/10.3390/nu15133041
Chicago/Turabian StyleHarrison, Leila, Zahra Padhani, Rehana Salam, Christina Oh, Komal Rahim, Maria Maqsood, Anna Ali, Kimberly Charbonneau, Emily C. Keats, Zohra S. Lassi, and et al. 2023. "Dietary Strategies for Complementary Feeding between 6 and 24 Months of Age: The Evidence" Nutrients 15, no. 13: 3041. https://doi.org/10.3390/nu15133041
APA StyleHarrison, L., Padhani, Z., Salam, R., Oh, C., Rahim, K., Maqsood, M., Ali, A., Charbonneau, K., Keats, E. C., Lassi, Z. S., Imdad, A., Owais, A., Das, J., & Bhutta, Z. A. (2023). Dietary Strategies for Complementary Feeding between 6 and 24 Months of Age: The Evidence. Nutrients, 15(13), 3041. https://doi.org/10.3390/nu15133041








